Published online Aug 26, 2020. doi: 10.4330/wjc.v12.i8.368
Peer-review started: January 31, 2020
First decision: April 29, 2020
Revised: June 5, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: August 26, 2020
Processing time: 199 Days and 11.8 Hours
Ehlers-Danlos syndrome (EDS) is a heterogeneous group of connective tissue disorders comprised of several types. Classic EDS is an autosomal dominant disorder with stretchable skin, delayed wound healing with poor scarring, joint hypermobility with subluxations or dislocations, easy bruisability, hernias, aneurysms and cardiac abnormalities. Advances in genomics technology using next-generation sequencing has led to the discovery of causative genes for connective tissue disorders, hereditary cardiomyopathies and cardiovascular diseases including several genes for connective tissue disorders. A 55 year-old male exhibited thin stretchable skin, atrophic scars, easy bruising, joint pain and dislocations requiring multiple knee surgeries and a Beighton hyperflexibility score of 6 out of 7. He was found to have a heterozygous missense COL5A1 gene variant involving exon 3 at nucleotide c:305T>A with an amino acid position change at p.lle102Asn consistent with classic EDS. He had a heart transplant at 43 years of age due to cardiac failure of unknown cause. This patient with classic EDS is brought to medical attention and should be of interest to cardiologists, heart transplant specialists and surgeons, particularly in individuals with unexplained cardiac failure and then diagnosed prior to surgical intervention to avoid poor wound healing, scarring and other tissue involvement (e.g., vascular anomalies, blood pressure instability, aneurysms) as components of EDS.
Core tip: Ehlers-Danlos syndrome (EDS) consists of a group of connective tissue disorders involving both autosomal dominant and recessive inheritance patterns often including collagen genes with variants readily detectable using disease-specific gene panels with next-generation sequencing. A 55 year-old male is reported with features of a connective tissue disorder. He had a heart transplant at 43 years of age. He was found to have a COL5A1 gene variant (c:605T>A; p.I1e102Asn) causing classic EDS. He is brought to medical attention for consideration of a genetic cause of cardiac failure including EDS in other patients and complications of surgery which may occur.
- Citation: Butler MG. Classic Ehlers-Danlos syndrome and cardiac transplantation - Is there a connection? World J Cardiol 2020; 12(8): 368-372
- URL: https://www.wjgnet.com/1949-8462/full/v12/i8/368.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i8.368
Ehlers-Danlos syndrome (EDS) is a connective tissue disorder comprised of several types due to mutations in genes encoding proteins (e.g., collagen) such as COL5A1 and COL5A2 accounting for 50% of patients with a clinical diagnosis of classic EDS[1]. Classic EDS is an autosomal dominant disorder identified in one in 20000 individuals with stretchable skin, delayed wound healing with poor scarring, joint hypermobility with subluxations or dislocations, pes planus, easy bruisability, hernias, aneurysms, and cardiac abnormalities[2-4]. Historically, EDS is grouped into six categories (classic, hypermobile, vascular, kyphoscoliosis, arthrochalasia and dermatosparaxis) with different genetic causes and inheritance patterns[4,5]. In 2017, EDS was assigned into 13 heritable disorders that affects an estimated 10 million people worldwide. These disorders are: Autosomal dominant classic (cEDS); autosomal recessive classic - like (clEDS); autosomal recessive cardiac-valvular (cvEDS), autosomal dominant vascular (vEDS), autosomal dominant hypermobile (hEDS), autosomal dominant arthrochalasia (aEDS), autosomal recessive dermatosparaxis (dEDS), autosomal recessive kyphoscoliosis (kEDS), autosomal recessive brittle cornea syndrome (BCS), autosomal recessive spondylodysplastic (spEDS), autosomal recessive musculocontractural (mcEDS), autosomal dominant or recessive myopathic (mEDS), and autosomal dominant periodontal[6].
Advances in genomics technology using next-generation sequencing (NGS) has led to the discovery of causative genes along with candidate gene approaches, disease specific panels or whole- exome analysis in patients presenting with features of a connective tissue disorder with newer classifications. Applying genomics to the field of cardiovascular-related disorders has identified over 80 genes causing connective tissue disorders using available comprehensive NGS gene testing panels. Over 150 genes have been identified playing a role in hereditary cardiomyopathies including hypertrophic, dilated or left ventricular non-compaction cardiomyopathy and hereditary arrhythmogenic right ventricular cardiomyopathy but do not include collagen genes. In addition, over 250 genes are found on commercially available comprehensive cardiovascular disease NGS panels with at least three collagen (i.e., COL3A1, COL5A1, COL5A2) genes (e.g., Fulgent Diagnostics, Irvine, CA, United States) and advanced genetic testing should be applied to interrogate gene panels for cardiology services including for heart transplantation[7].
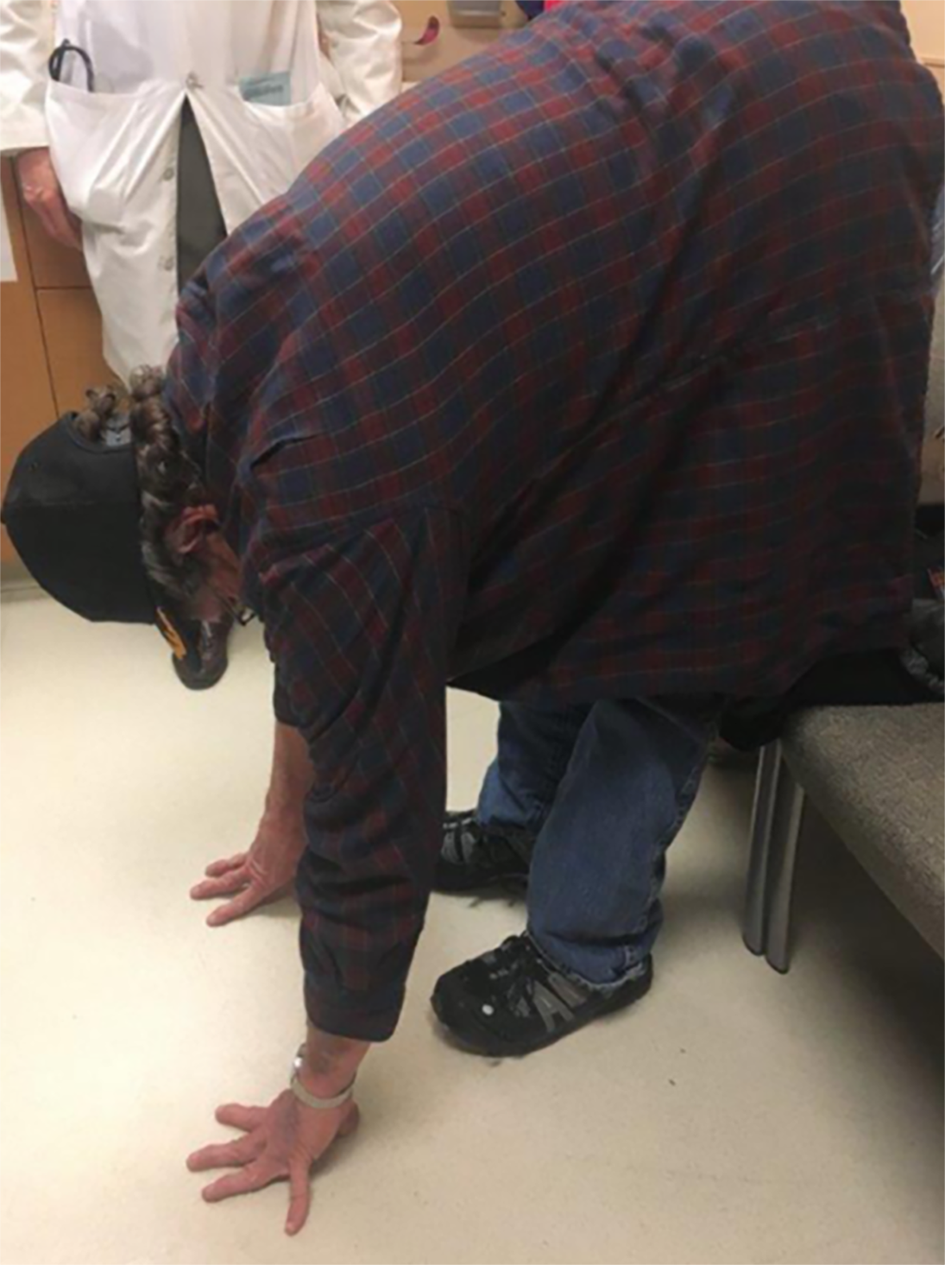
A 13-year-old son with features of a connective tissue disorder was identified previously based on a physical examination with hypermobility assessed using the Beighton scale[8,9]. His numerical rating score was high at 8 out of 9 with scores greater than 5 indicative of a connective tissue disorder. The score comprised passive dorsiflexion of the fifth finger beyond 90° (one point), passive bilateral apposition of both thumbs to the flexor aspects of forearms (two points), hyperextension of the elbows beyond 180° (two points), hyperextension of the knees beyond 180° (two points), and forward flexion of the trunk with palms of hands resting on the floor (one point). He had no heart murmur and a previous echocardiogram showed normal intra-cardiac anatomy and size, but his aortic root was dilated. A comprehensive connective tissue disorder NGS gene panel consisting of 50 genes was ordered and performed at the University of Nebraska Medical Center (Omaha, NE, United States). A heterozygous missense COL5A1 gene variant was found involving exon 3 at nucleotide c:305T>A with an amino acid position change at p.Ile102Asn. This gene variant was also found in his 55-year-old father exhibiting similar clinical features of thin stretchable skin with poor atrophic scars, hypermobility, joint pain and easy bruising with increased pigment on the anterior surface of both lower legs. Due to multiple knee surgeries in the past, bilateral knee movement or range could not be assessed, none-the-less his Beighton hyperflexibility score was 6 out of 7 (excluding knee mobility measures) (see Figure 1). Interestingly, his father had a heart transplant at 43 years of age due to cardiac failure with no known cause identified including infections, anatomic defects or metabolic problems. There was also no evidence of a spontaneous dissection or closure of a main coronary vessel causing infarction and subsequent heart failure.
The COL5A1 gene encodes one of the low abundant fibrillar collagens related to connective tissue abnormalities and when disturbed leads to autosomal dominant classic EDS[6]. The gene variant seen in the father and his son has not been described previously and the amino acid substitution was considered harmful by computer in silica prediction programs impacting protein structure.
The father’s cardiac failure was of unknown cause and required a heart transplant, potentially attributable to a connective tissue disorder that should be brought to medical attention as congestive heart failure affects 23 million people worldwide including 7.5 million in North America. It has a prevalence of 2.6% in the United States population in those greater than 20 years of age[10]. It is estimated that about 90000 heart transplants have occurred worldwide since 1983 with a current median survival rate of 50% at 12 years[11,12].
The clinical presentation and autosomal dominant inheritance pattern in this family involving a disturbed connective tissue gene leading to classic Ehlers-Danlos syndrome should be of interest to cardiologists, heart transplant specialists and surgeons with a possible relationship to cardiac involvement and heart failure requiring transplantation. Complications of connective tissue disorders should be recognized early and avoided in those patients due to their poor wound healing, scarring and other tissue involvement (e.g., vascular anomalies, blood pressure instability, aneurysms) and taken into consideration prior to surgical intervention.
Patients with unexplained heart failure should be checked for hypermobility (e.g., use of the Beighton scale) and genetically tested for connective tissue disorders using readily available comprehensive NGS gene panels prior to seeking heart transplantation. Evaluating and reporting of other similarly affected patients with genetic testing is encouraged to further elucidate whether connective tissue disorders may play a role in a subset with heart failure requiring transplantation as seen in this patient. In addition, complications of those having connective tissue disorders such as Ehlers-Danlos syndrome may necessitate closer surveillance and monitoring during and after surgical intervention with prolonged recovery and healing, as well as counseling of at-risk family members requiring screening and advanced genetic testing.
The author recognizes Waheeda Hossain, MD for expert preparation of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Erkut B S-Editor: Gong ZM L-Editor: A P-Editor: Li X
| 1. | Malfait F, Wenstrup RJ, De Paepe A. Clinical and genetic aspects of Ehlers-Danlos syndrome, classic type. Genet Med. 2010;12:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Ritelli M, Dordoni C, Venturini M, Chiarelli N, Quinzani S, Traversa M, Zoppi N, Vascellaro A, Wischmeijer A, Manfredini E, Garavelli L, Calzavara-Pinton P, Colombi M. Clinical and molecular characterization of 40 patients with classic Ehlers-Danlos syndrome: identification of 18 COL5A1 and 2 COL5A2 novel mutations. Orphanet J Rare Dis. 2013;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Ehlers E. Cutis Iaxa, neigong zu harmorrhagien in der haut, lockerun mehrer Artikulationen. Dermat Ztschr. 1901;8:173. |
| 4. | Jones KL, Jones MC, del Campo M. Smith’s Recognizable Patterns of Human Malformation. 7th ed. Philadelphia, PA: Elsevier Saunders; 2013. |
| 5. | Kaufman CS, Butler MG. Mutation in TNXB gene causes moderate to severe Ehlers-Danlos syndrome. World J Med Genet. 2016;6:17-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, Castori M, Cohen H, Colombi M, Demirdas S, De Backer J, De Paepe A, Fournel-Gigleux S, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavallee ME, Levy H, Mendoza-Londono R, Pepin M, Pope FM, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GJ, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Wheeldon N, Zschocke J, Tinkle B. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 1156] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 7. | Keating BJ, Pereira AC, Snyder M, Piening BD. Applying genomics in heart transplantation. Transpl Int. 2018;31:278-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Sen P, Butler MG. Classic Ehlers-Danlos Syndrome in a Son and Father with a Heart Transplant Performed in the Father. J Pediatr Genet. 2019;8:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51:444-453. [PubMed] |
| 10. | Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3702] [Cited by in RCA: 3717] [Article Influence: 265.5] [Reference Citation Analysis (0)] |
| 11. | Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant. 2011;30:1078-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 12. | Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis. 2014;6:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |