Published online Nov 26, 2020. doi: 10.4330/wjc.v12.i11.550
Peer-review started: April 28, 2020
First decision: July 5, 2020
Revised: July 18, 2020
Accepted: October 30, 2020
Article in press: October 30, 2020
Published online: November 26, 2020
Processing time: 211 Days and 16.5 Hours
Given current evidence, the effect of left ventricular assist device (LVAD) implantation on pulmonary function tests remains controversial.
To better understand the factors contributing to the changes seen on pulmonary function testing and the correlation with pulmonary hemodynamics after LVAD implantation.
Electronic databases were queried to identify relevant articles. The summary effect size was estimated as a difference of overall means and standard deviation on a random-effects model.
A total of four studies comprising 219 patients were included. The overall mean forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and diffusion lung capacity of carbon monoxide (DLCO) after LVAD implantation were significantly lower by 0.23 L (95%CI: 0.11-0.34, P = 00002), 0.18 L (95%CI: 0.03-0.34, P = 0.02), and 3.16 mmol/min (95%CI: 2.17-4.14, P < 0.00001), respectively. The net post-LVAD mean value of the cardiac index was significantly higher by 0.49 L/min/m2 (95%CI: 0.31-0.66, P < 0.00001) compared to pre-LVAD value. The pulmonary capillary wedge pressure and pulmonary vascular resistance were significantly reduced after LVAD implantation by 8.56 mmHg (95%CI: 3.78-13.35, P = 0.0004), and 0.83 Woods U (95%CI: 0.11-1.55, P = 0.02), respectively. There was no significant difference observed in the right atrial pressure after LVAD implantation (0.61 mmHg, 95%CI: -2.00 to 3.32, P = 0.65). Overall findings appear to be driven by studies using HeartMateII devices.
LVAD implantation might be associated with a significant reduction of the spirometric measures, including FEV1, FVC, and DLCO, and an overall improvement of pulmonary hemodynamics.
Core Tip: Left ventricular assist device (LVAD) implantation can cause worsening of forced expiratory volume in one second, forced vital capacity, and diffusion lung capacity of carbon monoxide in post-LVAD patients. However, the benefits of LVAD implantation outweigh its risks in the context of pulmonary hemodynamics. More large scale studies are required to validate our findings.
- Citation: Ullah W, Meizinger C, Ali Z, Panchal A, Saeed R, Haas DC, Rame E. Effects of left ventricular assist device on pulmonary functions and pulmonary hemodynamics: A meta-analysis. World J Cardiol 2020; 12(11): 550-558
- URL: https://www.wjgnet.com/1949-8462/full/v12/i11/550.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i11.550
Since the advent of the left ventricular assist device (LVAD) in 1988, literature has appropriately focused on its survival benefits in heart failure patients[1,2]. Over the years, with an increase in its use, many LVAD related complications also started surfacing together. Arena et al[3] in 1999 were the first to present data, implicating a decrease in spirometric measures in patients with LVAD implantation. These observation was based on the comparison of pre and post-LVAD findings in a 58-year old gentleman[3]. Later on, more observational studies attempted to elucidate the complex interplay of pathophysiological dynamics between LVAD and pulmonary system, but had conflicting findings[4-7]. Until recently, there has been no concrete evidence in the form of a randomized controlled trial (RCT) or meta-analysis to identify the net clinical benefit of LVAD in the context of worsened pulmonary functions and improved pulmonary hemodynamics.
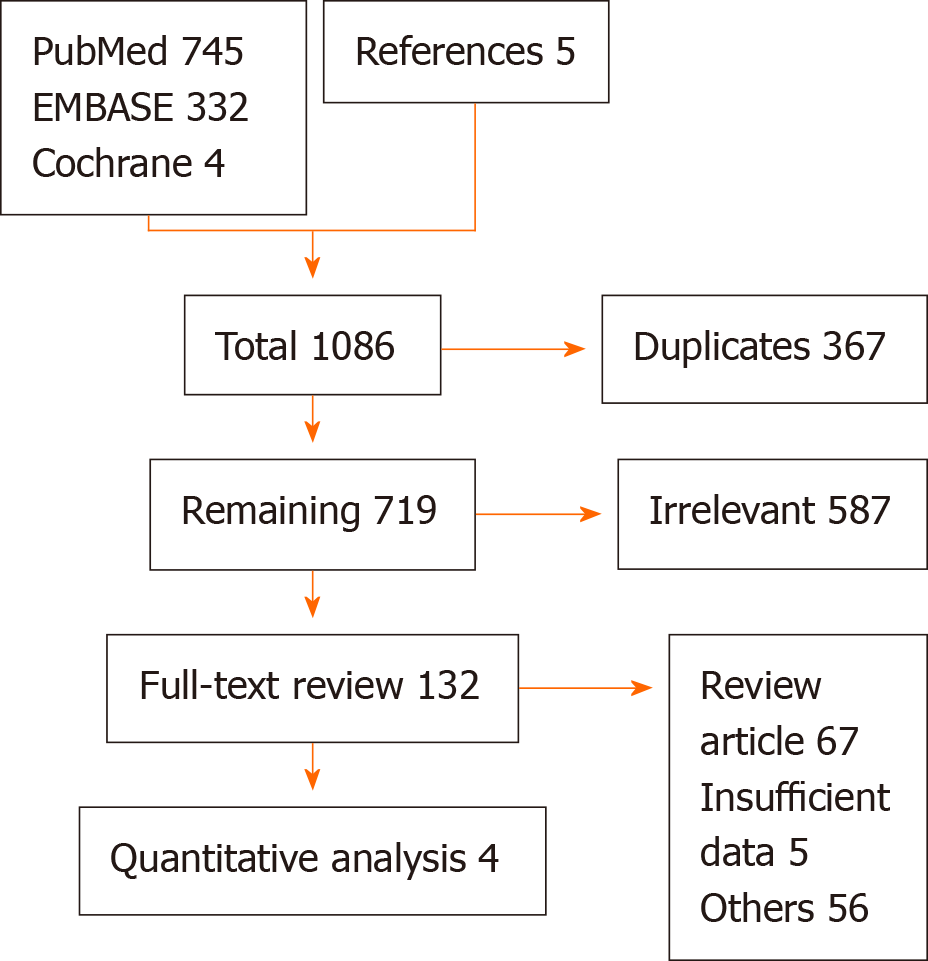
A literature search was performed up to December 2019, using PubMed, EMBASE, and Cochrane databases. There was no language or time restriction. The search strategies included various combinations of medical subject headings (MeSH) to generate two subsets of citations: One for LVAD and other for pulmonary function tests (PFTs). The terms from the two subsets were combined in 1:1 combination using boolean operators, and results from all possible combinations were screened for relevant articles. Based on our research question, articles from the reference lists pertinent to the clinical question were also evaluated by an independent author (backward snow bowling). Studies comparing changes in PFTs and pulmonary hemodynamics after LVAD implantation were included in the final analysis. Pulmonary hemodynamics included cardiac output (CO), right atrial pressure (RAP) and pulmonary capillary wedge pressures (PCWP) assessment; pulmonary vascular resistance (PVR) was calculated by the standard formula PVR = (mPAP - PCWP)/CO. The studies with insufficient data, case reports, review articles, and conference papers were excluded after detailed discussion (Figure 1).
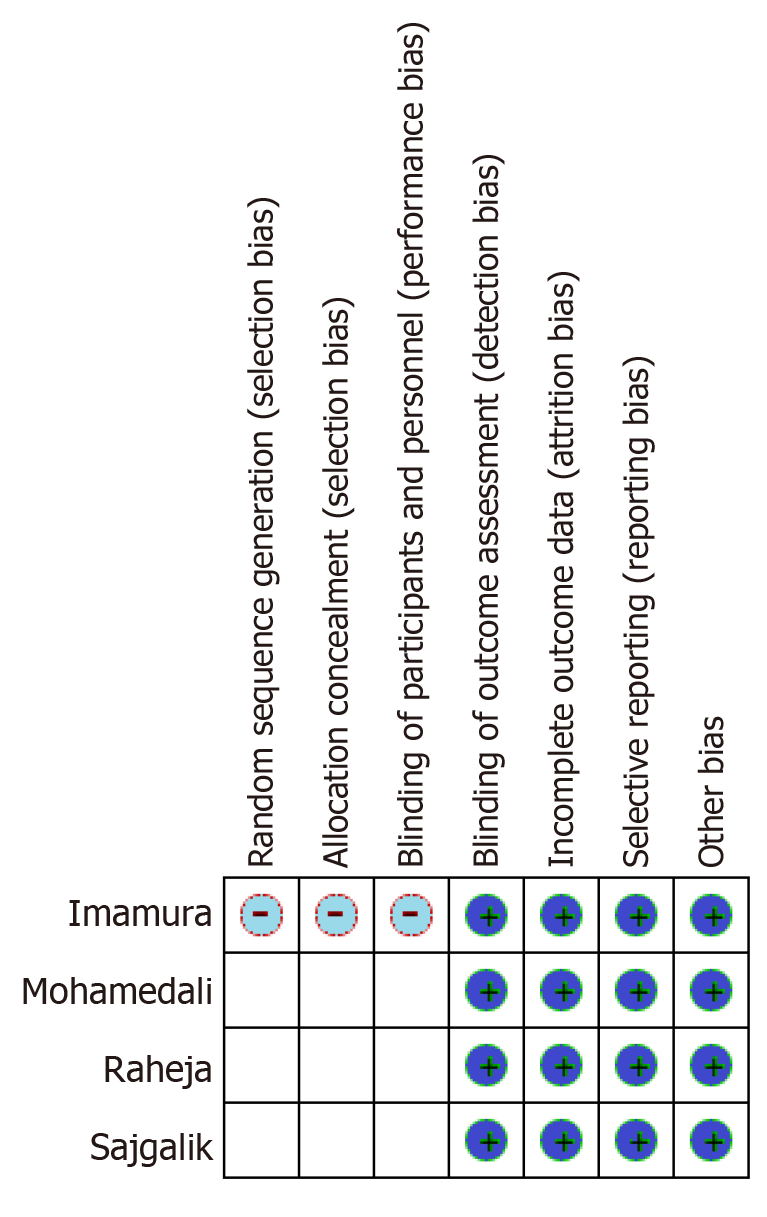
The overall methodological quality of the included studies was low. Due to the inclusion of single-center retrospective studies, the risk of selection bias could not be assessed. Similarly, randomization and allocation concealment of the subjects at the level of individual study could not be performed. Most studies, however, mentioned the baseline characteristics of the included population except by Imamura et al[5]. The risk of reporting bias across all studies was minimal due to adequate reporting of outcomes. Only patients who had post-LVAD spirometry measurements were included in the study, minimizing the risk of attrition bias. All included studies used a self-control model in which the same population was assessed before and after an intervention (LVAD implantation); hence, the risk for detection bias was negligible. The detailed and summary bias graphs are given in Figure 2.
The statistical analysis was performed using the random-effects model (inverse variance) to calculate the mean difference and SD for continuous variables. The probability value of P < 0.05 was considered statistically significant. The “test for overall effect” was reported as z value corroborating the inference from the 95%CI. Higgins I-squared (I2) statistic model was used to assess variations in outcomes of the included studies. I2 values of 50% or less corresponded to low to moderate, and 75% or higher indicated large amounts of heterogeneity. Publication bias was illustrated graphically using a funnel plot. The quality assessment of the included articles was performed using the Cochrane guidelines for the systematic review and meta-analysis, where each study was screened for five different types of bias (selection, performance, detection, attrition, and reporting bias). All statistical analysis was performed using the Cochrane Review Manager (RevMan) version 5.3.
The initial search on multiple databases revealed 1086 articles. After removal of irrelevant and duplicate items, 132 articles deemed relevant for full-text review. We further excluded 128 articles based on our selection criteria. Multiple attempts to authors to retrieve data were unsuccessful. Four articles qualified for final analysis[4-7]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is shown in Figure 1.
A total of 219 patients, with a mean age of 54 ± 11 years, were included. About 73% of the included subjects were males. Two thirds (74%) of the overall population had 2nd generation continuous flow devices (HeartMateII), while 26% of patients had a 3rd generation pulsatile flow device (HeartWare). The overall mean basal metabolic index was 26 ± 12 kg/m2. The mean left ventricular ejection fraction was 10%. The indications for LVAD implantation were non-ischemic and ischemic cardiomyopathy in 62% (136/219) and 36% (79/219) of patients, respectively. Sajgalik et al[6] were the only ones reporting hypertrophic cardiomyopathy in four patients. The functional status of the included population was defined either by a 6-min walk test (Raheja et al[7]) or by classification of the New York Heart Association (NYHA) (Sajgalik et al[6]). The mean brain nitric peptide level was 1695 pg/mL. Of the reported comorbidities, diabetes, chronic kidney disease, and hypertension were the most common. Approximately 22% (50/219) of the total population carried a diagnosis of chronic obstructive lung disease (COPD). The mean follow-up duration and timing of post-LVAD spirometry ranged from 6 mo to 12 mo. The detailed study characteristics are given in Supplementary Table 1.
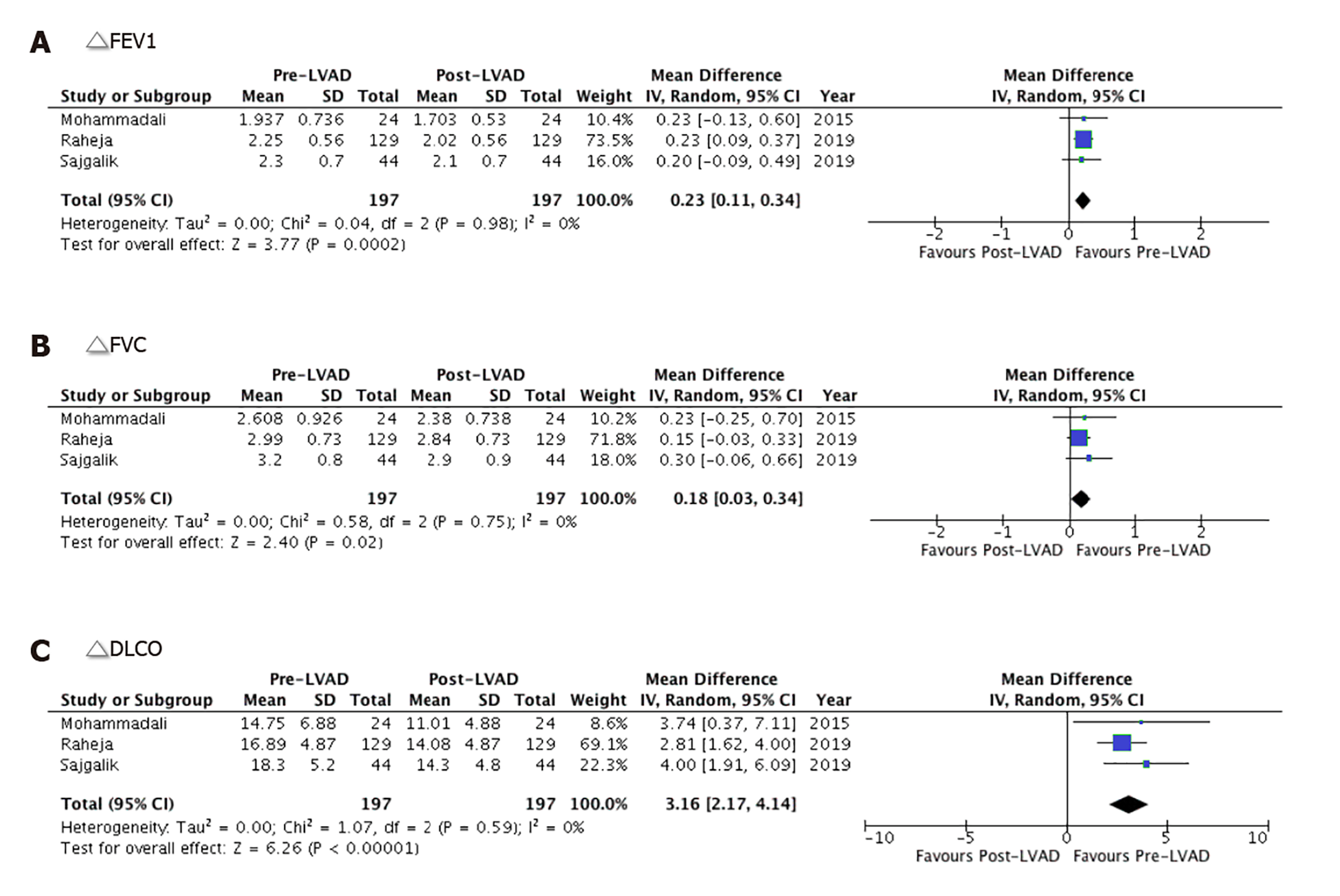
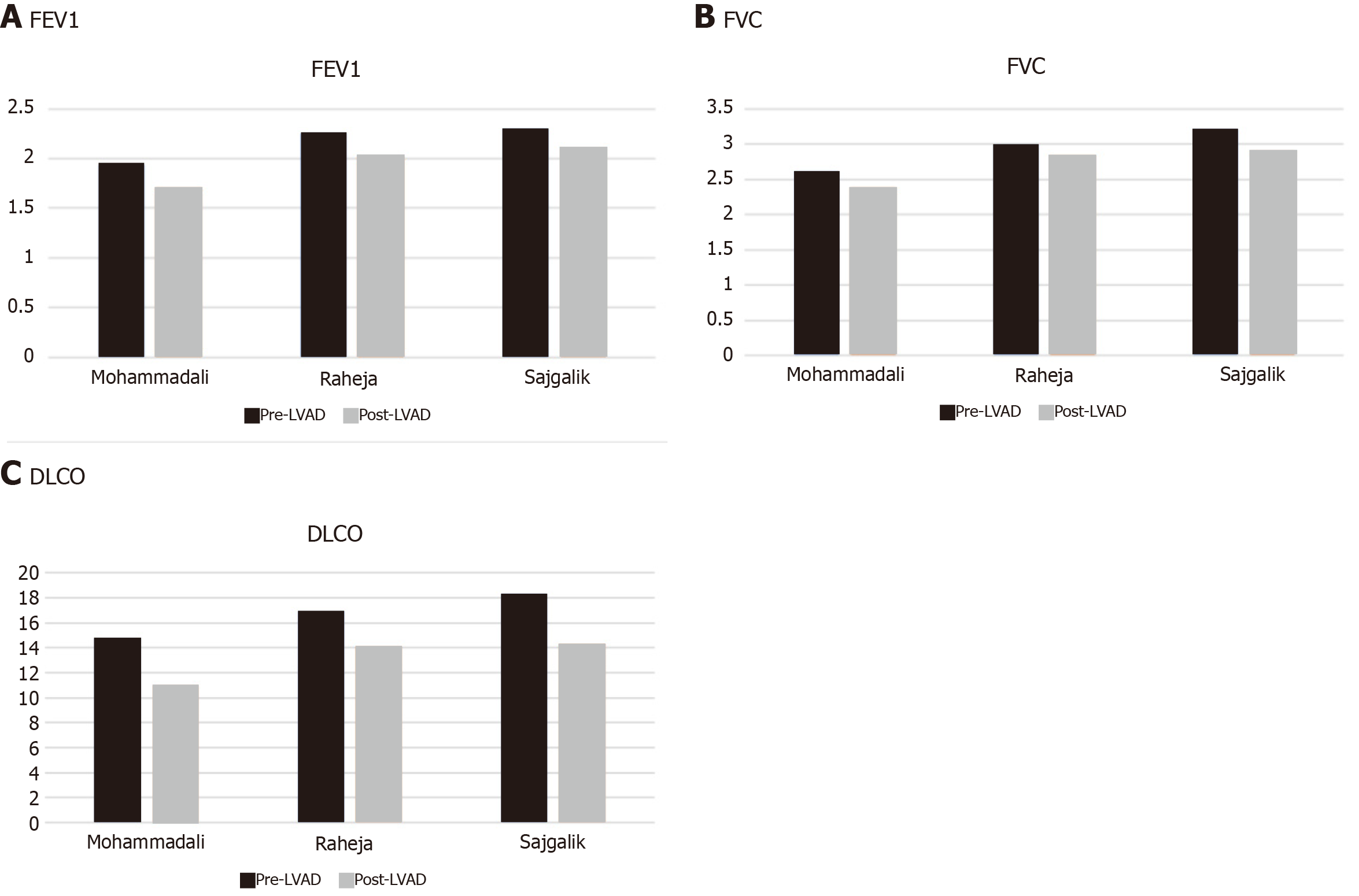
The overall mean forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) values were significantly lower after LVAD implantation by 0.23 L (95%CI: 0.11-0.34, P = 00002) and 0.18 L (95%CI: 0.03-0.34, P = 0.02), respectively. The heterogeneity among the outcomes of the included studies was minimal for both FEV1 and FVC with an I2 = 0% (P = 0.98 and P = 0.75, respectively) (Figure 3A and B). The overall mean value of ΔDLCO (diffusion lung capacity of carbon monoxide) was robustly reduced by 3.16 mmol/min (95%CI: 2.17-4.14, P < 0.00001) in the post-LVAD state. There was no heterogeneity among the outcomes of individual studies I2 = 0% (P = 0.59) (Figure 3C).
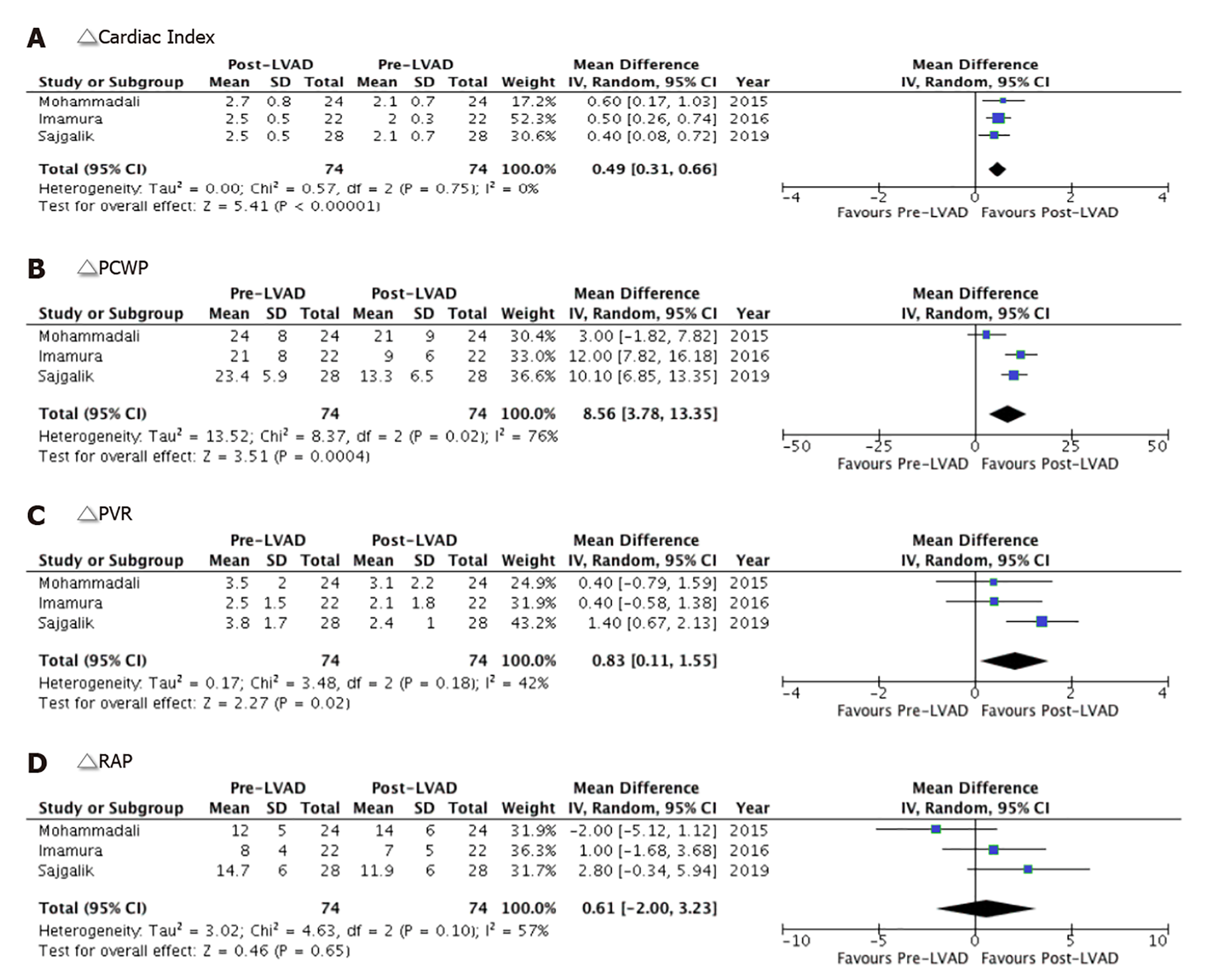
All indices of pulmonary hemodynamic observed an overall improvement with LVAD implantation. The net post-LVAD implantation mean cardiac index was significantly higher by 0.49 L/min/m2 (95%CI: 0.31-0.66, P < 0.00001) compared to pre-LVAD values with no heterogeneity (I2 = 0). Imamura et al[5] study contributed more than half to the overall effect size (52% weight) (Figure 4A). Compared to pre-LVAD values, the pooled estimate of PCWP and PVR was significantly reduced after post-LVAD implantation by 8.56 mmHg (95%CI: 3.78-13.35, P = 0.0004), and 0.83 Woods U (95%CI: 0.11-1.55, P = 0.02), respectively (Figure 4B and C). There was no significant difference observed in RAP in the post-LVAD cohort both at the study level and in the pooled analysis (0.61 mmHg, 95%CI: -2.00 to 3.32, P = 0.65). The heterogeneity among the included studies was moderate (I2 = 76%, I2 = 42%, I2 = 57%, respectively) (Figure 4D).
Our meta-analysis shows a substantial decrease in the overall mean spirometric measures (FEV1, FVC, FEV1/FVC, and DLCO) in post-LVAD patients. The pulmonary hemodynamics were, however, significantly improved with an overall mean decrease in the PCWP and PVR. As expected, post-LVAD patients observed a substantial improvement in the cardiac index. Together these changes represented a net decrease in left ventricular filling pressure and an improvement in the left ventricular function. There was no effect seen on the net difference of RAP in patients following LVAD implantation. The detailed pre- and post-LVAD spirometric metrics are given in Figure 5.
Many factors could be accounted for the restrictive pulmonary pattern seen in post-LVAD patients. The initial transient decline in PFTs (FVC and FEV1) could be attributed to anesthesia effects or respiratory muscle weakness due to the major cardiothoracic surgery[4]. Long term decrease in post-LVAD FVC can partly be explained by an LVAD patient now having an object that occupies approximately 50 mL (HeartWare) to 63 mL (HeartMateII) of intrathoracic space[4]. In addition to the anatomical limitations, a constellation of mechanical and physiological mechanisms compromises the diaphragmatic functions, such as surgical implantation of HeartMateII below the diaphragm, sternotomy, thoracic scaring and respiratory muscle weakness due to cardiac cachexia[7]. Moreover, heart failure induced reactive fibrosis, pleural effusion, and cardiomegaly can worsen the post-LVAD spirometric measures by mechanically impeding the lungs from expanding[6]. Heart failure can also incite bronchial hypersensitivity or can cause interstitial edema, and compressive atelectasis, further compromising the spirometric metrics of post-LVAD patients. Some post-LVAD patients are at high risk of decline in spirometric measures if they have a compelling indication for pneumotoxic medications such as amiodarone[4-7].
The literature on the post-LVAD pulmonary dynamics is scarce. Our extensive literature search identified four studies for quantitative analysis[4-7]. All these studies unanimously agreed on the decline of pulmonary functions and the improvement of pulmonary hemodynamics in post-LVAD patients. These studies, however, should be interpreted in the context of its methodological limitations. Most studies used older generation devices (HeartMateII), while more contemporary studies used the newer generation pulsatile LVAD devices (HeartWare). This potentially skewed the overall results of the studies. Mohamedali et al[4] in 2015, were the first to recognize that a decrease in the spirometric measures (FEV1, FVC, and DLCO) was actually driven by the HeartMateII devices, rather than HeartWare. They attributed these findings to the surgical resection related paralysis of the diaphragm, post-procedure lung fibrosis, and restriction of the left hemidiaphragm by a relatively larger device size in the former[4]. Raheja et al[7] subsequently validated the overall results of the prior study. They also quantified the effect of LVAD on the functional status of heart failure patients using a six-minute walk distance (MWD). A significant correlation between the MWD and LVAD induced change in FEV1 (P = 0.0466), and DLCO (P = 0.0032) was demonstrated; however, these findings were never adjusted for the duration of follow up[7]. Sajgalik et al[6] hurdled these limitations by performing a stratified analysis based on different follow-up durations. Although a substantial amount of decline in FEV1 (P = 0.04) and FVC (P = 0.01) was observed at 12-mo, there was no significant decrease in PFTs on a shorter follow-up duration of 6-mo (FVC P = 0.07, FEV1 P = 0.27). Similarly, the observed predictive effect of survival with the change of DLCO at 6-mo (P = 0.03) was attenuated at an extended follow-up duration of 12-mo (P = 0.22)[6].
Mohamedali et al[4] exclusively used the 2nd generation (HeartMateII) devices, while about 77% LVADs in the Sajgalik et al[6] and 88% of devices used by Raheja et al[7] study were HeartMateII devices. Despite a higher percentage of HeartMateII devices in the later studies, the overall outcomes were never stratified based on the device type[5-7]. Therefore, a clear distinction between the relative safety of HeartMateII and HeartWare could not be ascertained. This, along with the fact that all studies had substantial limitations due to non-randomized data, call into question the generalizability of individual results.
One can also argue that spirometric changes observed in post-LVAD patients could be a transient decline of the pre-existing lung disease, and might not have long-term consequences. As claimed by Imamura et al[5] in his analysis, that the post-LVAD PFTs worsening is a reversible phenomenon with heart transplantation. However, more studies are needed to shed light on the long-term impacts of LVAD implantation on pulmonary dynamics. It should also be noted that, approximately 36% of patients from Sajgalik et al[6] study had obstructive sleep apnea, while 29% of patients in Mohamedali et al[4], and 22% of Raheja et al[7] patients had COPD. It is unclear if the decrease of post-LVAD spirometric measures in the mentioned cohorts was an actual device effect or was merely worsening of their underlying lung condition.
Given apparent differences in the outcomes and significant effect of covariates on the results, one should be cautious about the clinical interpretations of individual results. This, along with the fact that previous small studies were either underpowered to detect clinical outcomes, or had conflicting findings, prompted us to integrate the findings of these studies.
In contrast to individual data, our meta-analysis provides strong evidence that LVAD related beneficial effects outweigh its potential spirometric decline in heart failure patients. A minimal mean decrease in post-LVAD measures of FEV1 and FCV by only 0.23 L and 0.18 L respectively is massively offset by a significantly higher increase in the cardiac index by 0.49 L/min/m2, and improvement of PCWP by 8.56 mmHg and PVR by 0.83 Woods U, respectively. Our meta-analysis also illustrates the paucity of randomized data in post-LVAD patients and highlights the limits on our ability to draw definitive conclusions due to the retrospective nature of data.
Our study is constrained by the limitations of the available data. A significant barrier was our inability to perform a stratified subgroup analysis based on different LVAD types and variable follow up durations. The inherent heterogeneity in the inclusion criteria of studies could not be accounted for in the pooled analysis. This, in addition to the biases and confounding factors inherent in observational non-randomized studies, call for caution when interpreting the results of this meta-analysis.
To determine the actual effect of LVAD implantation on spirometric metrics, it is imperative to account for all the confounding variables in a controlled population. One way would be to determine the causation of lung findings on a prospective scale or conduction of a RCT. This will enable the researchers not only to assess the risk of post-LVAD spirometric changes but can also help in the identification of instantaneous event rates such as mortality and survival benefits.
LVAD implantation might be associated with worsening of FEV1, FVC, and DLCO in post-LVAD patients. However, the overall benefits of LVAD in the context of pulmonary hemodynamics outweigh the presumed harm to spirometric metrics. More large scale studies are needed to validate our findings.
Recent studies suggest that left ventricular assist device (LVAD) implantation has not been associated with an improvement in pulmonary function tests. However, the improvement seen in post-LVAD pulmonary hemodynamics outweighs the observed decrease in spirometry.
The studies investigating these parameters are not expansive and the overall methodological quality of the studies available is low. Further inquiry into the effects of the LVAD implantation on pulmonary hemodynamics, objective pulmonary function testing and on the observed clinical outcomes is needed.
This meta-analysis aims to stratify the observed outcomes in studies assessing these parameters, in order to better understand the factors contributing to the changes seen on pulmonary function testing and the correlation with pulmonary hemodynamics.
Our study literature search was performed on published data until December 2019, using PubMed, EMBASE, and Cochrane databases. After screening the studies 132 articles deemed relevant were reviewed. 128 articles were excluded based on our selection criteria. Four studies were analysed and included in this meta-analysis.
A total of four studies comprising 219 patients were included. The overall mean forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and diffusion lung capacity of carbon monoxide (DLCO) after LVAD implantation were significantly lower by 0.23 L (95%CI: 0.11-0.34, P = 00002), 0.18 L (95%CI: 0.03-0.34, P = 0.02), and 3.16 mmol/min (95%CI: 2.17-4.14, P < 0.00001), respectively. The pulmonary capillary wedge pressure and pulmonary vascular resistance were significantly reduced after LVAD implantation by 8.56 mmHg (95%CI: 3.78-13.35, P = 0.0004), and 0.83 Woods U (95%CI: 0.11-1.55, P = 0.02), respectively. There was no significant difference observed in the right atrial pressure after LVAD implantation (0.61 mmHg, 95%CI: -2.00 to 3.32, P = 0.65).
LVAD implantation might be associated with a significant reduction of the spirometric measures, including FEV1, FVC, and DLCO, and an overall improvement of pulmonary hemodynamics.
The short term and long-term effects of LVAD on the pulmonary hemodynamics on and pulmonary function tests need to be expanded and are essential in order to better assess outcomes. The need for randomized control trials exists to identify confounding factors that may affect the outcomes seen in the studies analyzed. Also, further studies with extended follow-up are needed to assess the clinical outcomes of the changes seen on PFTs and hemodynamics.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Caceres-Loriga FM, Soriano-Ursúa MA, Teragawa H S-Editor: Huang P L-Editor: A P-Editor: Wang LYT
| 1. | Frazier OH, Macris MP, Myers TJ, Duncan JM, Radovancević B, Parnis SM, Cooley DA. Improved survival after extended bridge to cardiac transplantation. Ann Thorac Surg. 1994;57:1416-1422; discussion 1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Foray A, Williams D, Reemtsma K, Oz M, Mancini D. Assessment of submaximal exercise capacity in patients with left ventricular assist devices. Circulation. 1996;94:II222-II226. [PubMed] |
| 3. | Arena R, Humphrey R, McCall R. Altered exercise pulmonary function after left ventricular assist device implantation. J Cardiopulm Rehabil. 1999;19:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Mohamedali B, Bhat G, Yost G, Tatooles A. Changes in Spirometry After Left Ventricular Assist Device Implantation. Artif Organs. 2015;39:1046-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Imamura T, Kinugawa K, Kinoshita O, Nawata K, Ono M. Reversible decline in pulmonary function during left ventricular assist device therapy. J Artif Organs. 2016;19:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Sajgalik P, Kim CH, Stulak JM, Kushwaha SS, Maltais S, Joyce DL, Joyce LD, Johnson BD, Schirger JA. Pulmonary function assessment post-left ventricular assist device implantation. ESC Heart Fail. 2019;6:53-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Raheja S, Nemeh H, Williams C, Tita C, Selektor Y, Chamogeorgiakis T, Lanfear D. Pulmonary Function Testing and Outcomes after Left Ventricular Assist Device Implantation. Heart Surg Forum. 2019;22:E202-E206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |