Published online Oct 26, 2020. doi: 10.4330/wjc.v12.i10.501
Peer-review started: May 7, 2020
First decision: May 24, 2020
Revised: May 31, 2020
Accepted: September 11, 2020
Article in press: September 11, 2020
Published online: October 26, 2020
Processing time: 168 Days and 14.8 Hours
The utility of novel oral soluble guanylate cyclase (sGC) stimulators (vericiguat and riociguat), in patients with reduced or preserved ejection fraction heart failure (HFrEF/HFpEF) is currently unclear.
To determine the efficacy and safety of sGC stimulators in HF patients.
Multiple databases were searched to identify relevant randomized controlled trials (RCTs). Data on the safety and efficacy of sGC stimulators were compared using relative risk ratio (RR) on a random effect model.
Six RCTs, comprising 5604 patients (2801 in sGC stimulator group and 2803 placebo group) were included. The primary endpoint (a composite of cardiovascular mortality and first HF-related hospitalization) was significantly reduced in patients receiving sGC stimulators compared to placebo [RR 0.92, 95% confidence interval (CI): 0.85-0.99, P = 0.02]. The incidence of total HF-related hospitalizations were also lower in sGC group (RR 0.91, 95%CI: 0.86-0.96, P = 0.0009), however, sGC stimulators had no impact on all-cause mortality (RR 0.96, 95%CI: 0.86-1.07, P = 0.45) or cardiovascular mortality (RR 0.94, 95%CI: 0.83-1.06,
The use of vericiguat and riociguat in conjunction with standard HF therapy, shows no benefit in terms of decreasing HF-related hospitalizations or mortality.
Core Tip: Recently soluble guanylate cyclase (sGC) stimulators (vericiguat and riociguat) have emerged as a novel treatment for heart failure with reduced or preserved ejection fraction. Data published in literature revealed no additional benefits to guideline-based medical therapy in reducing incidence of heart failure hospitalization and mortality with sGC stimulator use. Large scale studies are required to determine the efficacy of sGC stimulators in patients with heart failure.
- Citation: Ullah W, Mukhtar M, Al-Mukhtar A, Saeed R, Boigon M, Haas D, Rame E. Safety and efficacy of soluble guanylate cyclase stimulators in patients with heart failure: A systematic review and meta-analysis. World J Cardiol 2020; 12(10): 501-512
- URL: https://www.wjgnet.com/1949-8462/full/v12/i10/501.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i10.501
There are an estimated 6.5 million adults in the United States suffering from heart failure (HF) with the disease accounting for nearly 1 in every 8 deaths[1] Approximately 1 million[2,3] HF-related hospitalizations (HHF) occur annually, accounting[4] for over 6.5 million hospital days and $37.2 billion in costs every year[1]. This economic burden has risen dramatically over the past two decades, with the increasing prevalence of risk factors for HF adding to new cases and better therapies adding to increased life expectancy among HF patients[1].
Despite traditional pharmacologic management with beta-blockers, angiotensin-converting-enzyme inhibitors (ACEI) and mineralocorticoid receptor antagonists to reduce HF exacerbations and mitigate clinical progression, overall prognosis remains dismal[2]. This has led researchers to target alternative pathways involved in the pathogenesis of HF, with promising research focusing on soluble guanylate cyclase (sGC) and the natriuretic peptide system (NPS)[3,4].
Both pathways influence myocardial perfusion and ventricular function through their common second messenger: Cyclic guanosine monophosphate (cGMP). The therapeutic augmentation of NPS with a combination angiotensin receptor–neprilysin inhibitor (sacubitril) has proven to be immensely beneficial, to the extent that the pioneering PARADIGM trial was halted early given the clear benefits in terms reducing mortality [risk ratio (RR) 0.84, 95% confidence interval (CI): 0.76-0.93, P < 0.001] and hospitalization (by 21%, P < 0.001) compared to ACEI alone[5]. However, the use of conventional vasodilators (nitrites and nitrates) to achieve soluble GC activation has met with more mixed results, with the development of tolerance, hypotension and failure of treatment being reported[6]. These discouraging findings have been attributed to a relative deficiency of sGC due to reduced nitric oxide (NO) bioavailability and endothelial dysfunction in HF leading to impaired cyclic GMP generation[7].
Novel sGC stimulators (vericiguat and riociguat) have shown advances over traditional vasodilators[6], by augmenting the cGMP signaling pathway, independent of NO and enhancing the effect of endogenous NO[2,8]. In contrast to the conventional therapeutic approach of antagonizing counterregulatory neurohormonal pathways, such as by phosphodiesterase inhibitors, or by addition of exogenous NO, sGC stimulators sensitize soluble GC to endogenous NO, thereby potentially having more efficacy for HF treatment[9].
In this regard, multiple clinical trials have attempted to explore the utility of vericiguat and riociguat in patients with HF[2,8]. The VICTORIA (vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial recently established that vericiguat in patients with HFrEF can reduce the risk of cardiovascular mortality and HF-related hospitalizations[2]. These findings, however, stand in contrast to previous trials that have not shown any consistent benefit with sGC stimulators. The ambiguity of current literature and the absence of any definite large-scale studies to determine the true merits of sGC stimulators in patients with HF, motivated us to perform this meta-analysis.
The MEDLINE (PubMed, Ovid), Embase, Clinicaltrials.org and Cochrane databases were queried with various combinations of medical subject headings (MeSH) to identify relevant articles. There were no language or time restrictions placed. Backward snowballing was performed to retrieve unidentified studies that were missed on the initial search. The MeSH used included two subsets: One for HF using the terms like “heart failure,” “HFrEF,” “HFpEF,” “CHF,” “cardiac failure,” and the other for sGC using “guanylate cyclase stimulators,” “sGC,” “vericiguat,” and “riociguat.” The two subsets of MeSH were combined in a 1:1 combination using Boolean operators. Results from all possible combinations were downloaded into an EndNote library. All randomized control trials (RCT) until March 31, 2020, comparing the safety and efficacy of sGC in HF were evaluated for inclusion.
Patients with HFrEF and HFpEF [New York Heart Association (NYHA) class II-IV], on optimal guideline-based medical therapy requiring hospitalization or outpatient intravenous (IV) diuretics, were included in this study. Patients requiring IV inotropic support, in acute decompensated HF or requiring mechanical device support were excluded, so were patients using suboptimal doses of vericiguat (< 10 mg daily) or riociguat (< 2 mg daily), nitrates, alternative sGC stimulators or PDE inhibitors.
The primary efficacy endpoint was a composite of the first hospitalization for HF and death from cardiovascular causes. The secondary efficacy endpoints were the components of the primary outcome, total HF-related hospitalizations, cardiovascular and all-cause mortality. Safety endpoints included anemia, hypotension, syncope, and a composite of the later two. A detailed search map and definition of outcomes are given in the Supplementary Appendix.
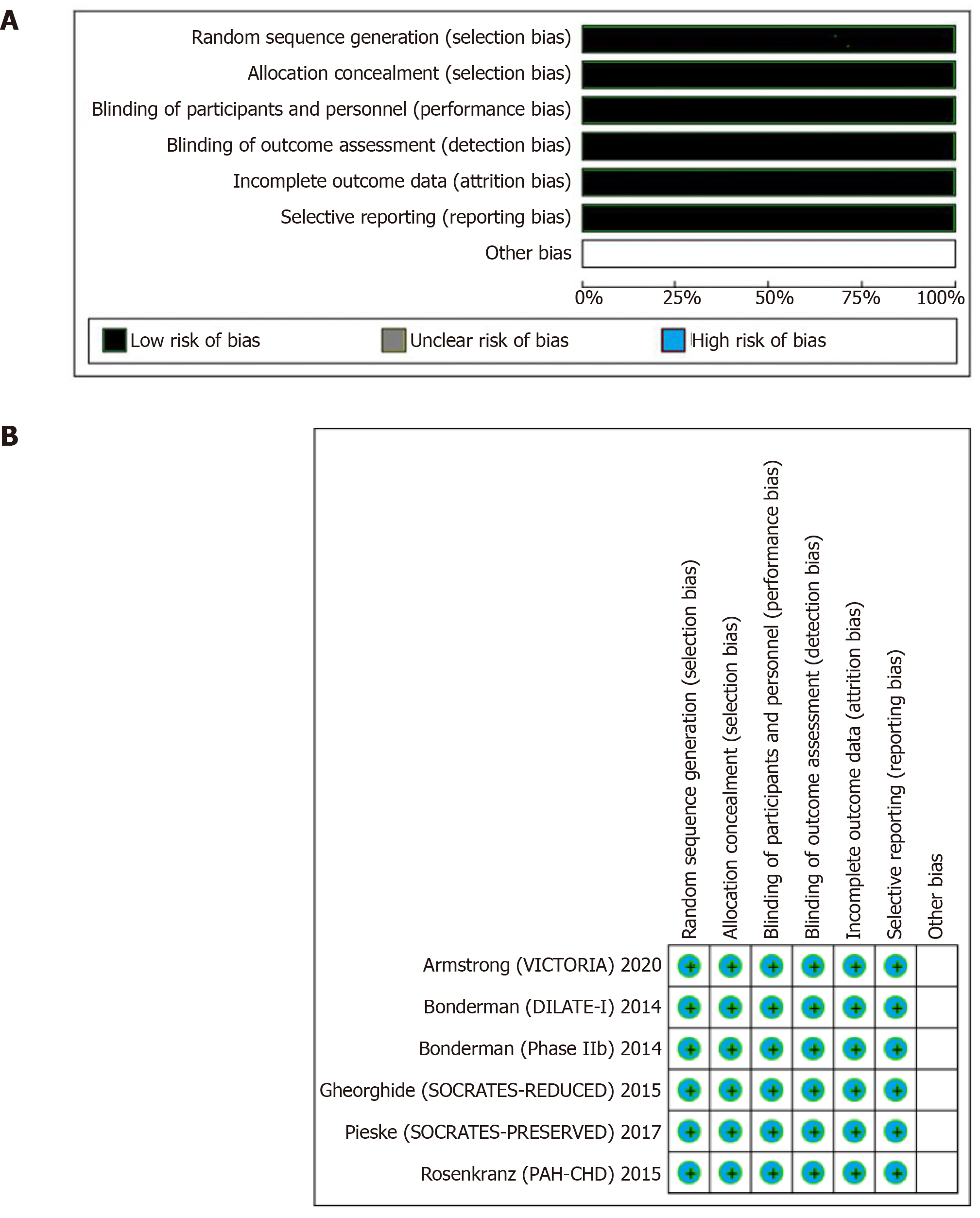
The statistical analysis was performed using the Cochran–Mantel–Haenszel test on a random-effect model to calculate RR for the dichotomous outcomes of RCTs. The probability value of P < 0.05 was considered statistically significant. The “test for overall effect” was reported as the z value corroborating the inference from the 95%CI. Subgroup analysis based on the choice of sGC stimulator and type of HF was also performed. Higgins I-squared (I2) statistical model was used to assess variations in outcomes of the included studies. I2 less than 40% corresponded to low heterogeneity. Depending upon the strength of evidence for heterogeneity (P value from the chi-square χ2 analysis), I2 of 41 to 74% indicated moderate (P ≥ 0.05) or moderate to severe (P ≤ 0.05) and I2 of 75% or higher suggested substantial heterogeneity. Publication bias was illustrated graphically using a funnel plot. The methodological quality assessment of the included RCTs was performed using the Cochrane collaboration tool for the systematic review and meta-analysis, where each study was screened for five different types of bias (selection, performance, detection, attrition, and reporting bias). All statistical analysis was performed using the Cochrane Review Manager (RevMan) version 5.3.
The overall quality of the included RCTs was high (Figure 1). Due to adequate randomization and allocation concealment, the risk of selection bias was low. The risk of performance and detection bias were reduced with appropriate blinding of participants and outcomes, respectively. Similarly, reporting bias across all studies was decreased due to an adequate description of the study results. The fact that most RCTs used an “intention to treat model” or had a minimal loss at follow-up, the risk of attrition bias was low.
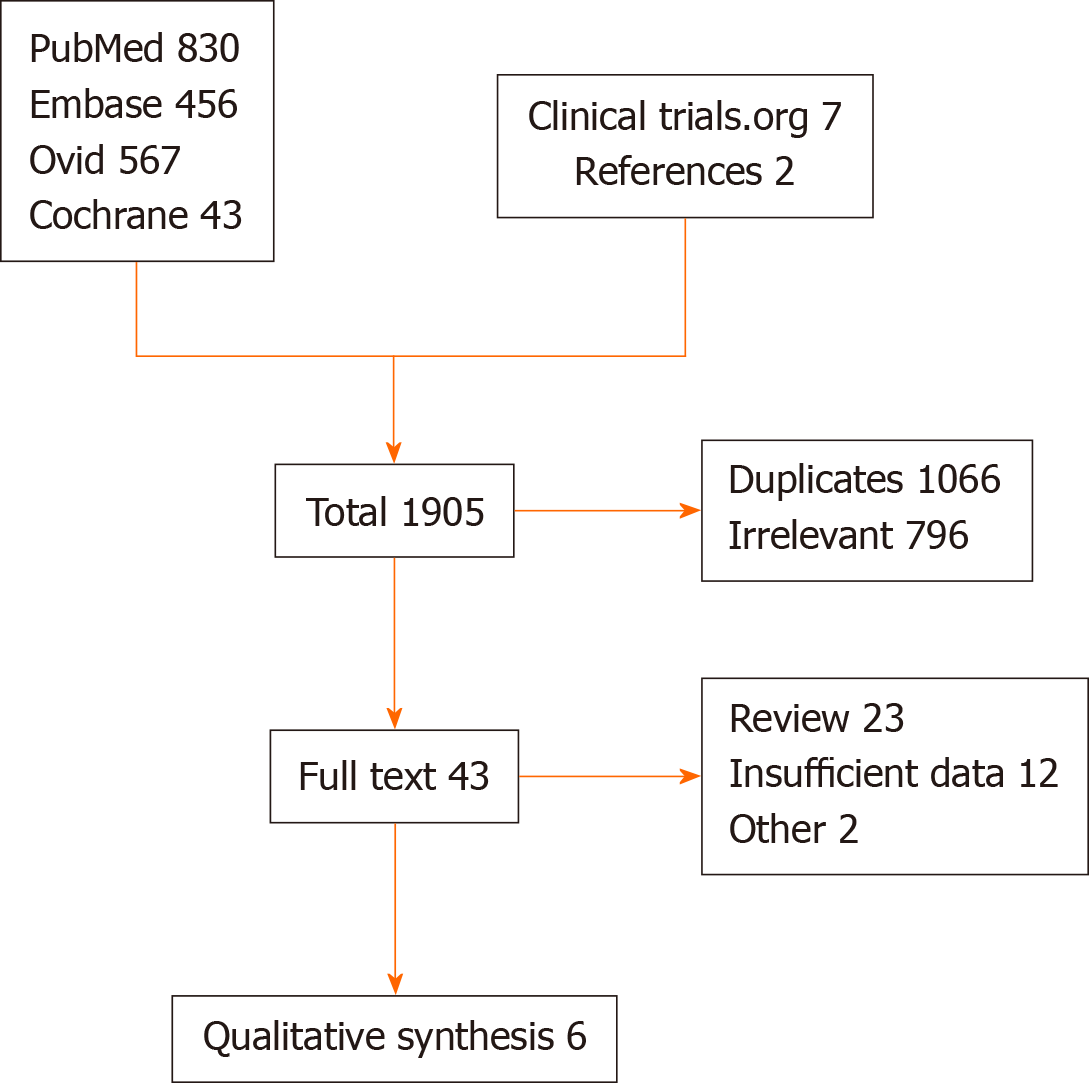
The initial search revealed 1905 articles. After the removal of irrelevant and duplicate items, 43 studies were selected for full-text review. Of these, 37 articles were excluded based on our selection criteria, 6 articles (all RCTs) qualified for quantitative analysis[2,8,10-13]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is shown in Figure 2.
A total of 5604 patients, 2801 in the sGC stimulator group, and 2803 in the placebo group were included. The mean age of patients receiving sGC stimulator was 64 and for the placebo group 62 years; comprising 59% and 57% male patients, respectively. Three of the included trials used vericiguat (1.25, 2.5, 5, or 10 mg), and three RCTs used riociguat (0.5, 1, or 2 mg) in the experimental arm. Baseline characteristics of treatment and placebo groups were comparable. Use of the concomitant guideline-directed HF medical therapy was also balanced between the two groups. The VICTORIA and the (soluble guanylate cyclase stimulator in heart failure patients with preserved and reduced EF) SOCRATES trials used vericiguat in HFrEF patients. These patients had a mean EF of 29% and NYHA class III-IV. The median baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were 2816 pg/mL and 3076 pg/mL, respectively. The SOCRATES-PRESERVED trial used the Kansas City cardiomyopathy questionnaire-clinical summary score to gauge symptomatic improvement in the HFpEF population. The DILATE-1 (acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure) investigated riociguat in the HFpEF population. The PAH-CHD (pulmonary arterial hypertension after correction of congenital heart disease) trial included younger patients with a mean 38 ± 15 years. The PAH-CHD and the LEPHT (left ventricular systolic dysfunction associated with pulmonary hypertension) had 100% and 97% of HF patients with NYHA II-III, respectively. The overall follow-up duration ranged from 12-43 wk, with a mean follow up of 19 wk. The detailed baseline characteristics, inclusion criteria, and definitions of outcomes are given in Supplementary Tables 1-3, respectively.
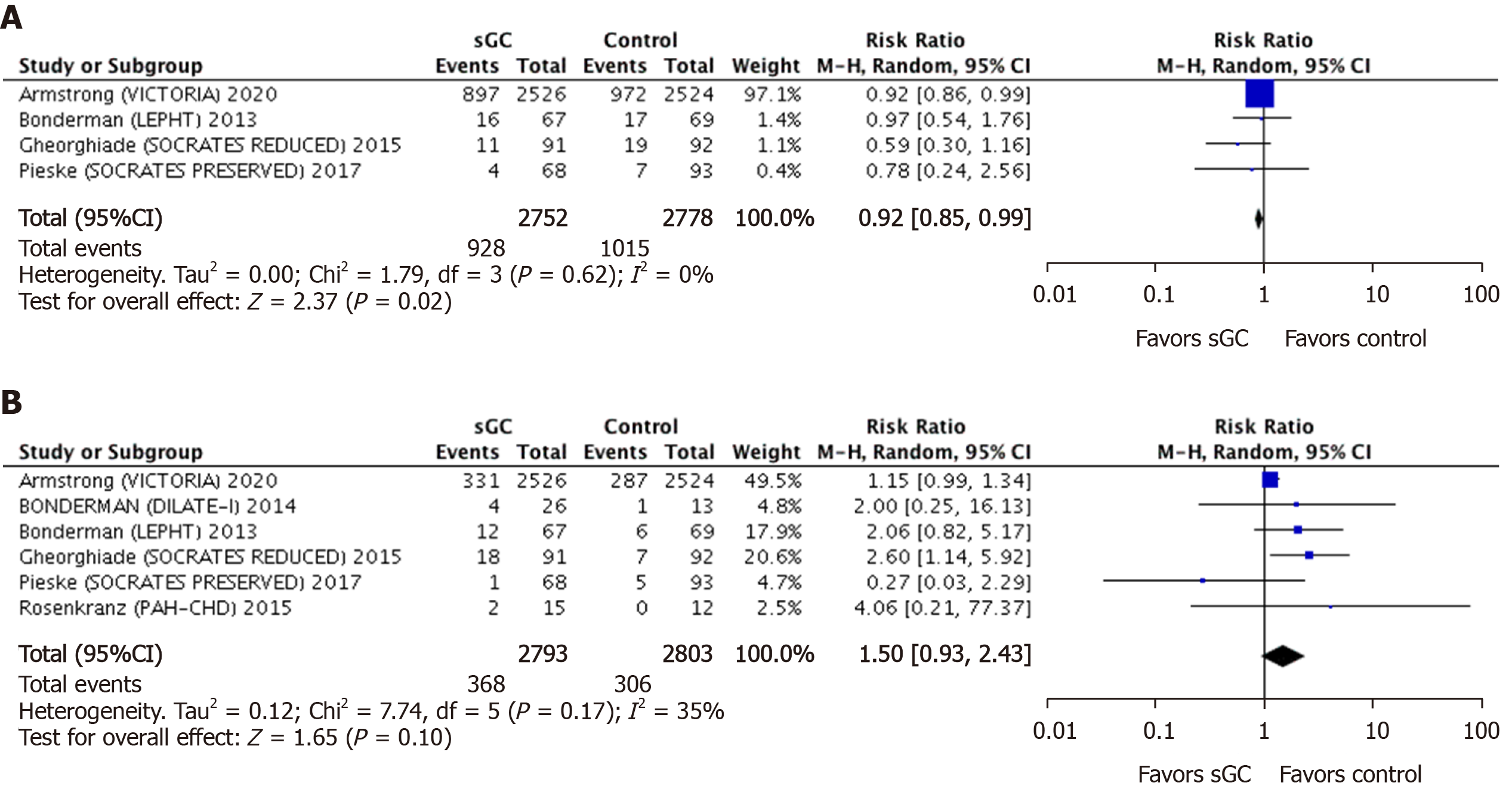
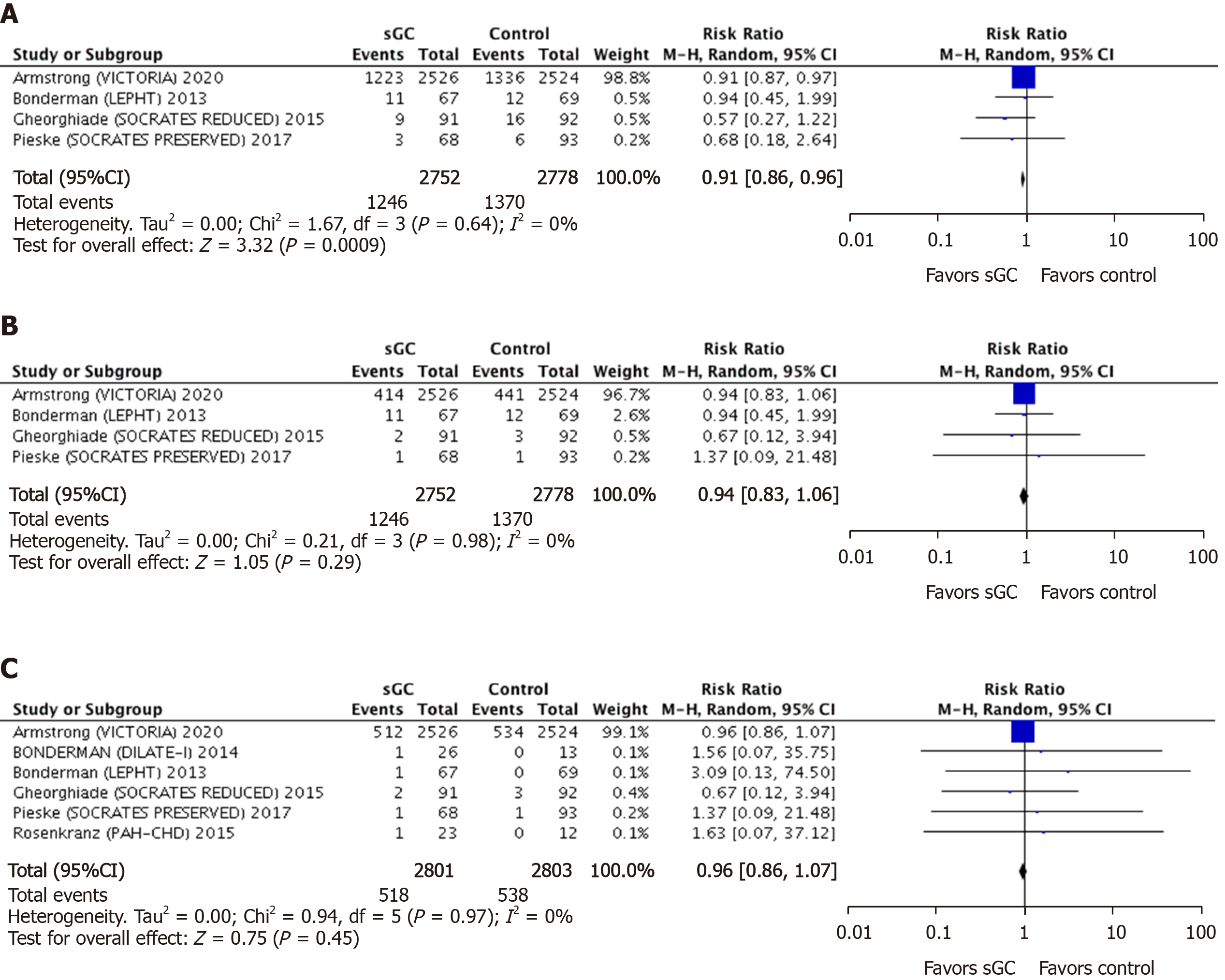
Pooled efficacy endpoints: Four studies comprising 5530 patients (2752 sGC stimulator and 2778 placebo) compared the primary composite endpoint (cardiovascular mortality plus first-time hospitalization) between the sGC stimulators and the control group. At a mean follow-up of 21-wk, a significantly lower rate of the primary endpoint was obtained with the use of sGC stimulator in HF patients (RR 0.92, 95%CI: 0.85-0.99, P = 0.02) (Figure 3A). Similarly, compared to placebo, the rate of total HF-related hospitalizations was significantly lower (RR 0.91, 95%CI: 0.86-0.96, P = 0.000.9) in patients on sGC stimulators. However, the incidence of cardiovascular and all-cause mortality remained identical in both groups at a mean follow up of 19-mo (RR 0.94, 95%CI: 0.83-1.06, P = 0.29 and RR 0.96, 95%CI: 0.86-1.07, P = 0.45, respectively) (Figure 3B). Six studies consisting of 5604 patients (2801 sGC stimulator and 2803 placebo) contributed to the later comparison (Figure 4). There was no heterogeneity among the outcomes of the included studies (I2 = 0%).
Pooled safety endpoints: Six studies comprising 5596 patients (2793 sGC stimulator and 2803 placebo) were used to calculate the incidence of net adverse events of clinical interest (NAECI) (a composite of hypotension and syncope). The rate of NAECI was 1.5 times higher but statistically non-significant in patients receiving sGC stimulators compared to placebo (RR 1.50, 95%CI: 0.93-2.41, P = 0.10) (Figure 3B). The incidence of hypotension (RR 1.47, 95%CI: 0.93-2.33, P = 0.10) and syncope (RR 1.18, 95 CI: 0.90-1.55, P = 0.24) were also numerically higher with the use of sGC stimulator use by 47% and 18% respectively; however, none of these differences reach the level of statistical significance. The incidence of anemia was significantly higher in sGC group (RR 1.33, 95%CI: 1.08-1.64, P = 0.007). There was minimal heterogeneity among the studies comparing NAECI and hypotension (I2 = 35% and I2 = 20%, respectively) .
Net clinical benefit: The overall number needed to treat (NNT) for the primary composite endpoint by adding vericiguat to the standard guideline-directed HF therapy was 35 (95%CI: 18.7-332.2). The overall number needed to harm (NNH) for NAECI was 44 (95%CI: 25.2-180). The net clinical benefit (NCB) was 9, indicating futility. The overall NNT to prevent one death due to any-cause was 142 (95%CI: 36.3-74.2) and to prevent one death from cardiovascular cause was 111 (95%CI: 35.3-96.7). None of the NNT values was statistically significant, as evidenced by the cross-over of its CI with the NNH.
A stratified analysis of prespecified subgroups adjusted on the type of HF (HFrEF and HFpEF) and choice of experimental regimen (vericiguat and riociguat) showed significant deviation from the pooled results. Two studies comprising 5233 patients (2616 sGC stimulator and 2617 placebo) contributed to the comparison of vericiguat and placebo agents in HFrEF patients. In contrast to the pooled results, there was no significant difference in the incidence of primary composite endpoint between patients receiving vericiguat and placebo for HFrEF (RR 0.84, 95%CI: 0.58-1.21, P = 0.24). Similarly, the rate of primary composite endpoint remained identical across patients on placebo and those receiving riociguat for HFrEF (RR 0.97, 95%CI: 0.54-1.76, P = 0.92) or HFpEF (RR 0.78, 95%CI: 0.24-2.56, P = 0.68) (Supplementary Figure 1). Compared to placebo, there was no significant difference in the rate of total HF-related hospitalizations across HFrEF patients receiving vericiguat (RR 0.84, 95%CI: 0.60-1.20, P = 0.34) or riociguat (RR 0.97, 95%CI: 0.54-1.76, P = 0.92) and HFpEF patients on riociguat (RR 0.68, 95%CI: 0.18-2.64, P = 0.58) (Supplementary Figure 2).
The incidence of all-cause mortality stratified by the type of sGC stimulators or type of HF mirrored the overall results. A similar rate of mortality was obtained between patients on placebo vs those on vericiguat (RR 0.96, 95%CI: 0.86-1.07, P = 0.43) or riociguat (RR 1.97, 95%CI: 0.32-12.16, P = 0.46) (Supplementary Figure 3). Both HFrEF (5369 patients, 2648 sGC stimulator and 2685 placebo) and HFpEF (200 patients, 94 sGC stimulator and 106 placebo group) followed the pooled results of all-cause mortality, showing a similar incidence of mortality between the two groups (RR 0.96, 95%CI: 0.86-1.07, P = 0.44 and RR 1.45, 95%CI: 018-11.54, P = 0.73, respectively) (Supplementary Figure 4). Sensitivity analysis by the exclusion of PAH-CHD study also did not alter the results of pooled analysis (RR 0.96, 95%CI: 0.86-1.07, P = 0.45) (Supplementary Figure 5).
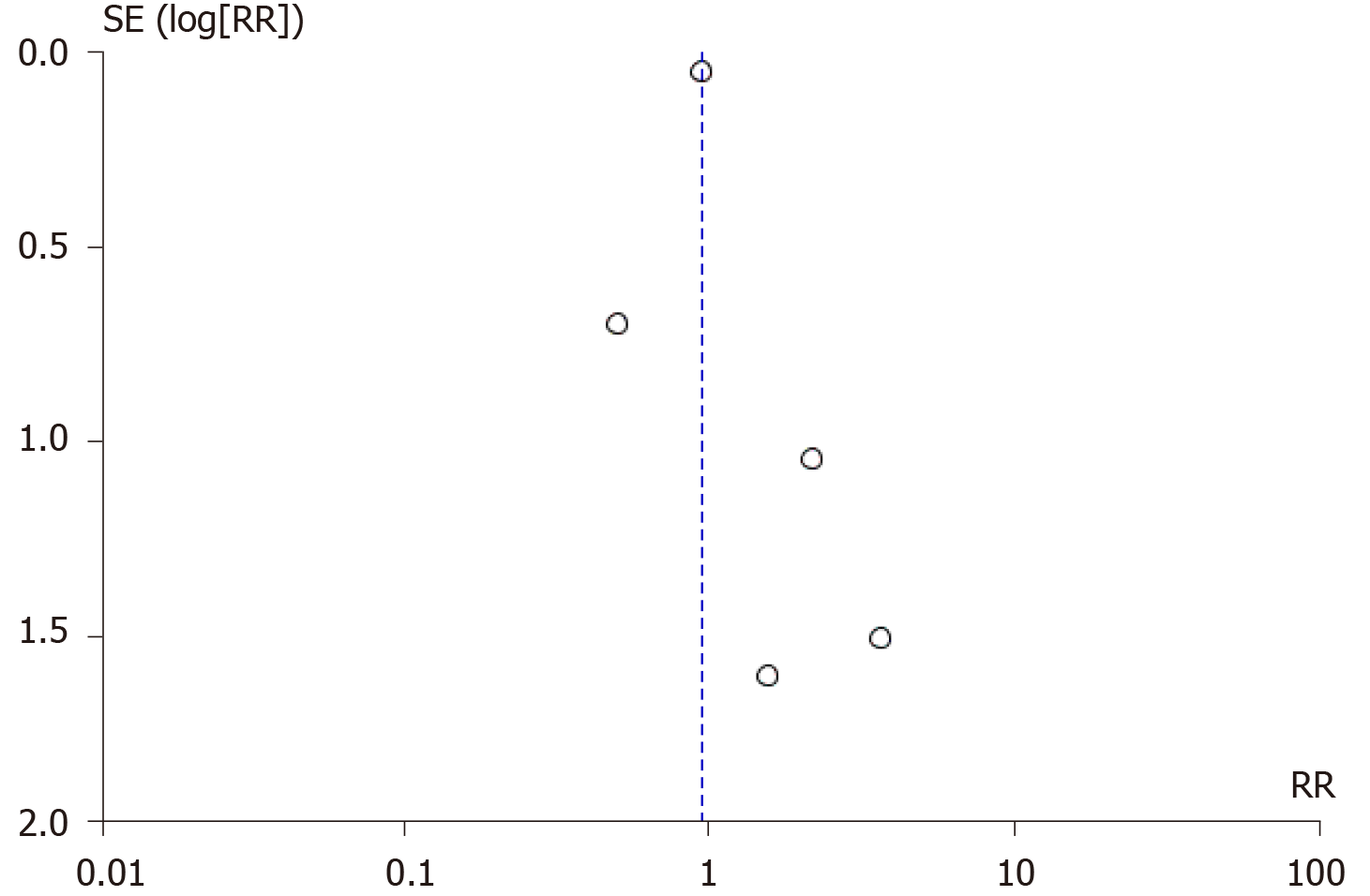
The funnel plot showed asymmetry, indicating the possibility of publication bias. (Figure 5) The vertical axis of the plot used standard error to estimate the sample size of the study, plotting large population studies on top and smaller at the bottom. The horizontal spread reflected the power and effect size of the included studies. One can argue that it is difficult to differentiate between “findings by chance” and “real asymmetry,” as only six articles were assessed for potential publication bias. As pointed by Sterne et al[14]. in a study of fewer than ten articles, it is difficult to ascertain publication bias.
To our knowledge, this is the largest study performed to assess the safety and efficacy of novel sGC stimulators (vericiguat and riociguat) in patients with HF. The results were drawn from 6 RCTs, comprising 5604 patients. In the combined analysis, among patients with high-risk HF (NYHA class II-IV), the addition of sGC stimulators to current guideline-based medical therapy showed a modest decrease in risk of the primary composite endpoint (first HF hospitalization plus cardiovascular death) by 8%. With a similar decrease in HF-related total hospitalization by 9%. However, these benefits were attenuated when the pooled results were matched based on the type of HF and choice of sGC stimulator. Neither vericiguat nor riociguat groups reached the threshold of statistical significance when the efficacy endpoints (hospitalization and death) were stratified by HFrEF and HFpEF. Similarly, compared to the control group, both vericiguat and riociguat failed to lower the incidence of cardiovascular or all-cause mortality, irrespective of the type of HF or duration of follow-up. Moreover, the incidence of NAECI and its components (hypotension and syncope) in the intervention group were 1.5 times higher than the placebo group. Briefly, our analysis did not show the same positive findings seen in the recent VICTORIA trial and highlight the ambiguity in the use of sGC stimulators, until more definitive evidence is available (Supplementary Figure 6).
It is interesting to compare our combined results with all included RCTs. The SOCRATES-PRESERVED and the SOCRATES-REDUCED trials used vericiguat in patients with HFpEF and HFrEF, respectively[10,11]. These trials were primarily designed to determine the optimal dose and tolerability of vericiguat. While on pooled analysis, the relative difference in the NT-proBNP levels was identical, the SOCRATES-REDUCED did show a significant dose-response relationship. Compared to placebo, a higher vericiguat dose of 10 mg was associated with greater reductions in NT-proBNP (P = 0.02) and significant improvement in LVEF (+1.5% vs +3.7%, P = 0.02) at 3 mo follow-up[11]. Similarly, the SOCRATES-PRESERVED trial showed a substantial improvement in the functional and symptomatic status of the HFpEF patients (increase in KCCQ-CSS by more than 5 points) on the 10 mg dose of vericiguat at 3 mo[10]. Both SOCRATES-PRESERVED and REDUCED trials used surrogate markers of disease severity and were relatively underpowered to gauge hard clinical outcomes (mortality and hospitalizations).
Three of the included RCTs compared the merits of riociguat against a placebo in both HFrEF and HFpEF patients. The PATENT-1 (the pulmonary arterial hypertension sGC-stimulator trial-1) and its long-term extension study PATENT-2 were unique in terms of inclusion criteria and assessment of outcomes. Riociguat was found to be associated with a decrease in the mean NT-proBNP levels, improved 6-minute walking distance (6MWD) and pulmonary vascular resistance (PVR) in patients with PAH-CHD[12]. Both LEPHT and DILATE-1 trials demonstrated a significant increase in the stroke volume (P = 0.001 and P = 0.04) in their respective HFrEF and HFpEF patient populations. However, there was no significant decrease in the mean pulmonary arterial pressure (PAP), the primary endpoint, even at the maximally tolerated dose of riociguat (2 mg)[8,13]. These trials were also underpowered to assess major clinical endpoints and had variability in dose-response outcomes, limiting their utility.
The more contemporary VICTORIA trial was adequately powered and specifically designed to measure clinically relevant outcomes[2]. The trial used a cox-regression-model to calculate a sample size of 5050 patients, who were hospitalized with the diagnosis of HFrEF. Both vericiguat and placebo groups in the trial were appropriately matched, minimizing ascertainment bias, but had a high rate of noncompliance and loss to follow-up. At a median follow-up of 10 mo, 24% of the vericiguat and 22% of the placebo arm had discontinued the trial regimen. While this amount of nonadherence was anticipated and an “intention to treat model” was used to reduce its impact on the overall results, the pooled difference in the primary composite endpoint was modest (RR 0.90, 95%CI: 0.82-0.98, P = 0.02). The incidence of cardiovascular mortality was near identical in the vericiguat and placebo groups (12.9% vs 13.9%, P = 0.83), indicating that the primary outcome was driven by the lower rate of HF-related hospitalization in the vericiguat group (38.3% vs 42.4%, P = 0.02)[2]. By contrast, our pooled analysis of vericiguat in HFrEF population showed no inter-group difference in both the incidence of the primary composite (P = 0.34), cardiovascular mortality (P = 0.29), and total HF-related hospitalizations (P = 0.34). Nonetheless, mirroring our pooled results, the VICTORIA trial showed no significant difference in the incidence of all-cause mortality between vericiguat and the placebo groups (20.3% vs 21.2%, P = 0.38). This underscores that the lower hospitalization rate in the vericiguat arm did not translate into clinical survival benefits. Together, these findings call for caution while interpreting the findings of the VICTORIA trial.
The present meta-analysis sought to address the overall discrepancies by systematically adjusting the definition of primary composite outcome and by excluding patients on the suboptimal dose of sGC stimulators. By design, our study prevents the influence of both known and unknown confounding factors due to the inclusion of high-quality studies. Our study showed no clinical benefits of sGC stimulator in terms of reducing mortality or HF-related hospitalization when the overall outcomes were stratified by type of HF and regimen of trial medication. These findings contrast with the most contemporary VICTORIA trial, which showed a decrease in the incidence of HF-related total hospitalizations and death from cardiovascular causes. Also unique was a demonstration of the consistent ineffectiveness of vericiguat and riociguat to reduce all-cause mortality across all included trials. Moreover, the calculation of the net clinical benefit may serve to inform clinical decision making, suggesting that sGC stimulators offer no benefits and could potentially be harmful.
Our study is constrained by the limitations of the included studies. Patient-level data were missing to measure the impact of non-compliance on overall clinical outcomes. Long term follow-up data was lacking in more than half of the included studies, limiting our ability to calculate their predictive effects. Some studies focused on non-clinical primary outcomes (pro-BNP, PAP) neglecting a significant amount of clinical complications such as myocardial infarction and mortality, reducing the precision of estimated complications. Due to the paucity of long-term follow-up data, it is unclear if these results could be extrapolated to patients with HFpEF. The ongoing DYNAMIC study might shed more light on the efficacy of sGC in patients with HFpEF[15].
It can also be argued that the assessment of the efficacy and safety of the sGC stimulators is a bivariate exercise, and summarizing it in a unidimensional variable (net clinical benefit) could be misleading. For example, a large number of relatively minor episodes of hypotension versus a small improvement in HF-related hospitalization rate may lead to a negative calculated Net Clinical Benefit (NCB). Still, given the vast disparity between the impact on the quality of life between an episode of hypotension versus HF-related hospitalization, it may falsely undervalue the benefit of therapy. Therefore, the NCB value should preferably be interpreted in the context of the nature of both adverse and beneficial events, without committing to value judgment. That being said, in our case, we found no significant beneficial effect of sGC stimulators and a higher incidence of adverse effects rendering this a moot point.
Vericiguat and riociguat offer no additional benefits to current guideline-based medical therapy in terms of reducing the incidence of hospitalization or mortality in patients with HFpEF or HFrEF. Further larger-scale studies are needed to validate these findings.
Despite treatment with traditional pharmacologic management, patients with heart failure (HF) have a dismal prognosis, with approximately 1 million HF-related hospitalizations (HHF) occurring annually, accounting for over 6.5 million hospital days and $37.2 billion each year.
This has led researchers to study the efficacy of alternate drugs in preventing HF exacerbations, which include soluble guanylate cyclase (sGC) stimulators vericiguat and riociguat. Multiple clinical trials have attempted to explore the utility of vericiguat and riociguat in patients with HF. However, there a lack of large scale studies to determine the true merits of sGC stimulators in patients with HF.
Therefore, we performed this meta-analysis to determine the efficacy and safety of sGC stimulators in HF patients.
The MEDLINE (PubMed, Ovid), Embase, Clinicaltrials.org and Cochrane databases were queried with various combinations of medical subject headings (MeSH) to identify relevant articles. All randomized control trials (RCT) until March 31, 2020, comparing the safety and efficacy of sGC in HF were evaluated for inclusion. The primary efficacy endpoint was a composite of the first hospitalization for HF and death from cardiovascular causes. The secondary efficacy endpoints were the components of the primary outcome, total HF-related hospitalizations, cardiovascular and all-cause mortality. The statistical analysis was performed using the Cochran–Mantel–Haenszel test on a random-effect model to calculate relative risk (RR) for the dichotomous outcomes of RCTs. The overall quality of the included RCTs was high.
Six RCTs comprising 5604 patients (2801 sGC stimulator and 2803 placebo) were included. The primary endpoint (a composite of cardiovascular mortality and first HF-related hospitalization) was reduced in patients receiving sGC stimulators compared to placebo [RR 0.92, 95% confidence interval (CI): 0.85-0.99, P = 0.02]. The incidence of total HF-related hospitalizations were also lower in sGC group (RR 0.91, 95%CI: 0.86-0.96, P = 0.0009), however, sGC stimulators had no impact on all-cause and cardiovascular mortality (RR 0.96, 95%CI: 0.86-1.07, P = 0.45) and (RR 0.94, 95%CI: 0.83-1.06, P = 0.29), respectively. The overall safety endpoints (composite of hypotension and syncope) were also identical between the two groups (RR 1.50, 95%CI: 0.93-2.42, P = 0.10). For the primary composite endpoint, the number needed to treat was 35, the number needed to harm was -44 and the overall net clinical benefit was -9.
Data published in literature revealed no additional benefits to guideline-based medical therapy in reducing incidence of HF hospitalization and mortality with sGC stimulator use. Large scale studies are required to determine the efficacy of sGC stimulators in patients with HF.
As it is unclear whether sGC stimulators have any additional benefit in improving the prognosis of HF patients due to a lack of substantial research, large scale studies are needed to determine their efficacy in reducing HF related hospitalization rates.
We sincerely thank Dr. Smith D, and Dr. Eisenstaedt R for providing research opportunities and resources in the institute.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM S-Editor: Yan JP L-Editor: A P-Editor: Li JH
| 1. | Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4163] [Cited by in RCA: 4809] [Article Influence: 687.0] [Reference Citation Analysis (1)] |
| 2. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1841] [Article Influence: 167.4] [Reference Citation Analysis (1)] |
| 3. | Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM; VICTORIA Study Group. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2020;382:1883-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 835] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 4. | Mogensen UM, Gong J, Jhund PS, Shen L, Køber L, Desai AS, Lefkowitz MP, Packer M, Rouleau JL, Solomon SD, Claggett BL, Swedberg K, Zile MR, Mueller-Velten G, McMurray JJV. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2018;20:760-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4078] [Cited by in RCA: 4740] [Article Influence: 430.9] [Reference Citation Analysis (0)] |
| 6. | Erdmann E, Semigran MJ, Nieminen MS, Gheorghiade M, Agrawal R, Mitrovic V, Mebazaa A. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur Heart J. 2013;34:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Follmann M, Ackerstaff J, Redlich G, Wunder F, Lang D, Kern A, Fey P, Griebenow N, Kroh W, Becker-Pelster EM, Kretschmer A, Geiss V, Li V, Straub A, Mittendorf J, Jautelat R, Schirok H, Schlemmer KH, Lustig K, Gerisch M, Knorr A, Tinel H, Mondritzki T, Trübel H, Sandner P, Stasch JP. Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure. J Med Chem. 2017;60:5146-5161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise AV, Roessig L, Semigran MJ; Left Ventricular Systolic Dysfunction Associated With Pulmonary Hypertension Riociguat Trial (LEPHT) Study Group. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 460] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 10. | Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 11. | Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Müller K, Roessig L, Pieske B; SOCRATES-REDUCED Investigators and Coordinators. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015;314:2251-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 12. | Rosenkranz S, Ghofrani HA, Beghetti M, Ivy D, Frey R, Fritsch A, Weimann G, Saleh S, Apitz C. Riociguat for pulmonary arterial hypertension associated with congenital heart disease. Heart. 2015;101:1792-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CSP, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 14. | Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3796] [Cited by in RCA: 4932] [Article Influence: 352.3] [Reference Citation Analysis (0)] |
| 15. | Mascherbauer J, Grünig E, Halank M, Hohenforst-Schmidt W, Kammerlander AA, Pretsch I, Steringer-Mascherbauer R, Ulrich S, Lang IM, Wargenau M, Frey R, Bonderman D. Evaluation of the pharmacoDYNAMIC effects of riociguat in subjects with pulmonary hypertension and heart failure with preserved ejection fraction : Study protocol for a randomized controlled trial. Wien Klin Wochenschr. 2016;128:882-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |