Published online Oct 26, 2020. doi: 10.4330/wjc.v12.i10.484
Peer-review started: March 19, 2020
First decision: July 5, 2020
Revised: July 19, 2020
Accepted: August 15, 2020
Article in press: August 15, 2020
Published online: October 26, 2020
Processing time: 218 Days and 4.9 Hours
There is risk of stenosis and thrombosis of the superior vena cava after upper extremity central catheter replacement. This complication is more serious among patients with single ventricle physiology, as it might preclude them from undergoing further life-sustaining palliative surgery.
To describe complications associated with the use of upper extremity percutaneous intravenous central catheters (PICCs) in children with single ventricle physiology.
A single institution retrospective review of univentricular patients who underwent superior cavopulmonary anastomoses as their stage 2 palliation procedure from January 2014 until December 2018 and had upper body PICCs placed at any point prior to this procedure. Clinical data including ultrasonography, cardiac catheterization, echocardiogram reports and patient notes were used to determine the presence of thrombus or stenosis of the upper extremity and cervical vessels. Data regarding the presence and duration of upper extremity PICCs and upper extremity central venous catheter (CVC), and use of anticoagulation were recorded.
Seventy-six patients underwent superior cavopulmonary anastomoses, of which 56 (73%) had an upper extremity PICC at some point prior to this procedure. Median duration of PICC usage was 24 d (25%, 75%: 12, 39). Seventeen patients (30%) with PICCs also had internal jugular or subclavian central venous catheters (CVCs) in place at some point prior to their superior cavopulmonary anastomoses, median duration 10 d (25%, 75%: 8, 14). Thrombus was detected in association with 2 of the 56 PICCs (4%) and 3 of the 17 CVCs (18%). All five patients were placed on therapeutic dose of low molecular weight heparin at the time of thrombus detection and subsequent cardiac catheterization demonstrated resolution in three of the five patients. No patients developed clinically significant venous stenosis.
Use of upper extremity PICCs in patients with single ventricle physiology prior to super cavopulmonary anastomosis is associated with a low rate of catheter-associated thrombosis.
Core Tip: There is a wide variation in practice in terms of the preferred central venous access site and catheter-type for patients who undergo surgery for single ventricle heart disease. Thrombosis is a serious concern in patients with single ventricle physiology. This study aims to describe the use of upper body percutaneously inserted central catheters in patients with single ventricle physiology prior to their superior cavopulmonary anastomosis procedure at our institution. Our study shows that upper body percutaneous intravenous central catheters are associated with a low rate of clinically significant catheter-related thrombosis.
- Citation: Kaipa S, Mastropietro CW, Bhai H, Lutfi R, Friedman ML, Yabrodi M. Upper body peripherally inserted central catheter in pediatric single ventricle patients. World J Cardiol 2020; 12(10): 484-491
- URL: https://www.wjgnet.com/1949-8462/full/v12/i10/484.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i10.484
Children with critical congenital heart disease (CHD) often require a central venous catheter (CVC) in the medication administration, fluid resuscitation, nutrition and hemodynamic monitoring prior to surgical repair and for post-operative care management. A subset of children with CHD can require central venous access for a longer period of time[1,2]. The use of central venous catheters (CVCs) or percutaneously inserted central catheters (PICCs) for prolonged periods can result in complications including venous stenosis and thrombosis, especially in neonates[3,4]. Central venous thrombosis is a cause of considerable morbidity and mortality in children who undergo surgery for CHD[5,6]. Patients who undergo surgery for CHD are at high risk of developing thrombosis due to a hypercoagulable state often related to cardiopulmonary bypass and blood product transfusions[1,7,8].
There are several options for CVCs location including umbilical in neonates, femoral, internal jugular, or subclavian vein, transthoracic intracardiac catheters, and PICCs[9]. Considerable variation exists between centers in the most commonly used catheters and sites, and no formal recommendations have been published to guide current practice[9-11].
Infants with single ventricle physiology need to have patent superior vena cava and upper-extremity veins to ensure adequate passive pulmonary blood flow[12]. Stenosis or thrombosis of these central veins can delay surgery and, in some patients, preclude further palliation surgeries. Due to these concerns about obstruction of upper extremity veins many institutions discourage the use of upper extremity CVCs or PICCs in this patient population. However, data on the rate of venous thrombosis in children with single ventricle physiology in whupper extremity CVCs are placed are limited[8,13,14]. In our practice, the use of upper extremity PICCs in this patient population is common.
We aim to describe our use of upper extremity PICCs in children with single ventricle physiology who have undergone superior cavopulmonary anastomosis as their stage two palliation surgery.
This study was approved by the Institutional Review Board at our institution. This is a retrospective cohort study of pediatric patients undergoing cardiac surgery at our institution over a 5-year period–January 2014 to December 2018. Patients who underwent superior cavopulmonary anastomoses (Hemi-Fontan or bidirectional Glenn procedure) were identified from our institutional pediatric cardiac surgical database and data was extracted from chart reviews of the electronic medical records. Patients who required cervical extracorporeal membrane oxygenation support at any point prior to their superior cavopulmonary anastomosis procedure and patients who did not undergo a first stage palliation procedure were excluded from the study.
All patients at our institution undergo diagnostic cardiac catheterization prior their superior cavopulmonary anastomosis for surgical planning purposes. Routine screening for the presence of CVC- or PICC-associated thromboses however is not performed, studies are ordered based on the discretion of the primary care team. All patients had a diagnostic cardiac catheterization to assess hemodynamics prior to the second stage pallation surgery.
Location and type of central venous access is not protocolized. For most patients, an umbilical venous catheter is placed in neonates with CHD at birth. If a patient continues to need stable intravenous access beyond 48 h of life, a PICC is placed in the intervention radiology suite and the umbilical venous catheter removed. Intraoperatively, most patients receive a double lumen intra-cardiac, right atrial catheter is placed. For some patients, an internal jugular or subclavian CVC is placed as an additional venous access site. The ongoing need for a central venous access is assessed daily during the multidisciplinary morning rounds. For patients who are deemed to require central venous access for a prolonged period to time postoperatively, which includes most of the patients with single ventricle physiology who undergo stage 1 palliation procedures, a PICC is placed and right atrial catheters and other CVCs are removed. A heparin infusion (2 U/mL) is administered as a carrier fluid for all central venous access lumens while they are in place.
Beginning in January 2016, central venous catheter-related thrombus prophylaxis protocol in our cardiac intensive care unit was implemented. For patients who were deemed to require their central venous access for 72 h or more, low molecular-weight heparin (LMWH) is started within 48 h post-operatively or within 12 h after the placement of central venous access for non-surgical patients. The dose is adjusted by a designated clinical pharmacist based on Anti-Xa activity (goal range: 0.25-0.49 IU/mL). Prophylactic LMWH protocol is delayed in for significant post-operative bleeding, concern for high risk of bleeding, use of another agent for therapeutic anticoagulation, compromised renal function (creatinine clearance < 30 mL/min/1.73 m2), or concern for heparin-induced thrombocytopenia. LMWH is initiated at 0.75 mg/kg/dose every 12 h for patients who were less than 2 mo old and 0.5 mg/kg/dose for patients who were greater than 2 mo old.
Patient characteristics including demographic data, diagnoses, and first stage palliation procedures were collected. Data regarding type and duration of the central venous access, number of catheters, and use of anticoagulation after the cardiac surgery were also collected. We also collected information regarding central venous access utilized during any hospital admissions during the inter-stage period between stage 1 palliation procedures and superior cavopulmonary anastomosis. Outcome data regarding thrombosis was collected from vascular ultrasound, computerized tomography, fluoroscopy, echocardiogram and cardiac catheterization studies obtained prior to superior cavopulmonary anastomosis.
Data are presented using descriptive statistics. Categorical data are presented as frequency with percentage and continuous variables are presented as median with (25%, 75%) unless otherwise noted. Chi square test was used to compare the rate of complications between the group of patients with PICCs and CVCs.
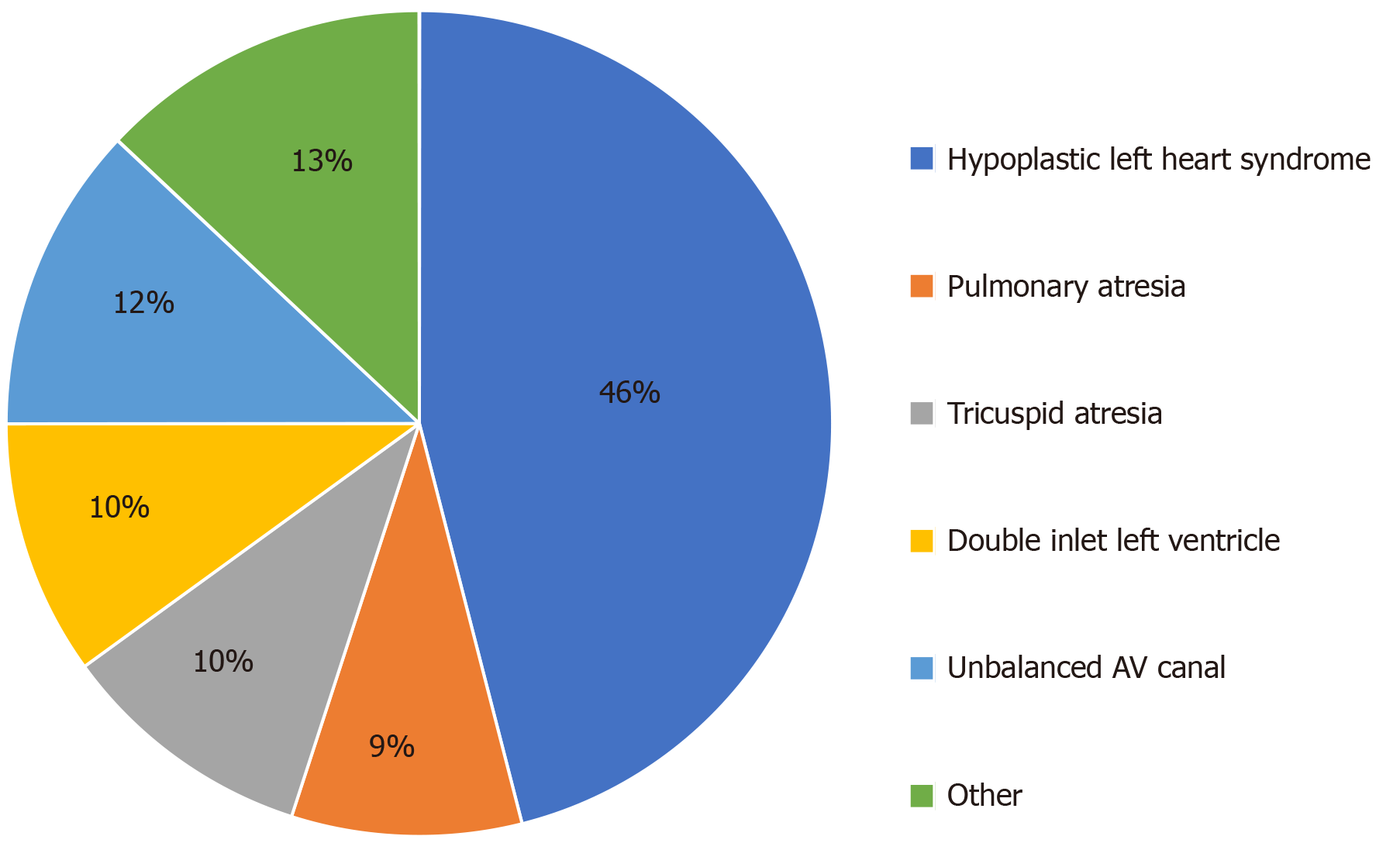
We reviewed 76 patients who underwent cardiac catheterization prior undergoing superior cavopulmonary anastomoses as their stage 2 palliation procedure (hemi-Fontan or bidirectional Glenn). Patient characteristics are shown in (Table 1). The median age at first stage palliation surgery was 9 d (25%, 75%: 6, 15). Thirty (39%) patients were female and 35 (46%) patients had hypoplastic left heart syndrome. The distribution of the primary cardiac defects is shown in (Figure 1).
| Variable | Patients |
| Birth weight in kgs (mean) | 3.13 |
| Age at first palliation surgery in days (IQR) | 9 (6, 15) |
| Female sex, n (%) | 30 (39) |
| Non-hispanic white, n (%) | 48 (64) |
| Prematurity, n (%) | 10 (15) |
| HLHS, n (%) | 46% |
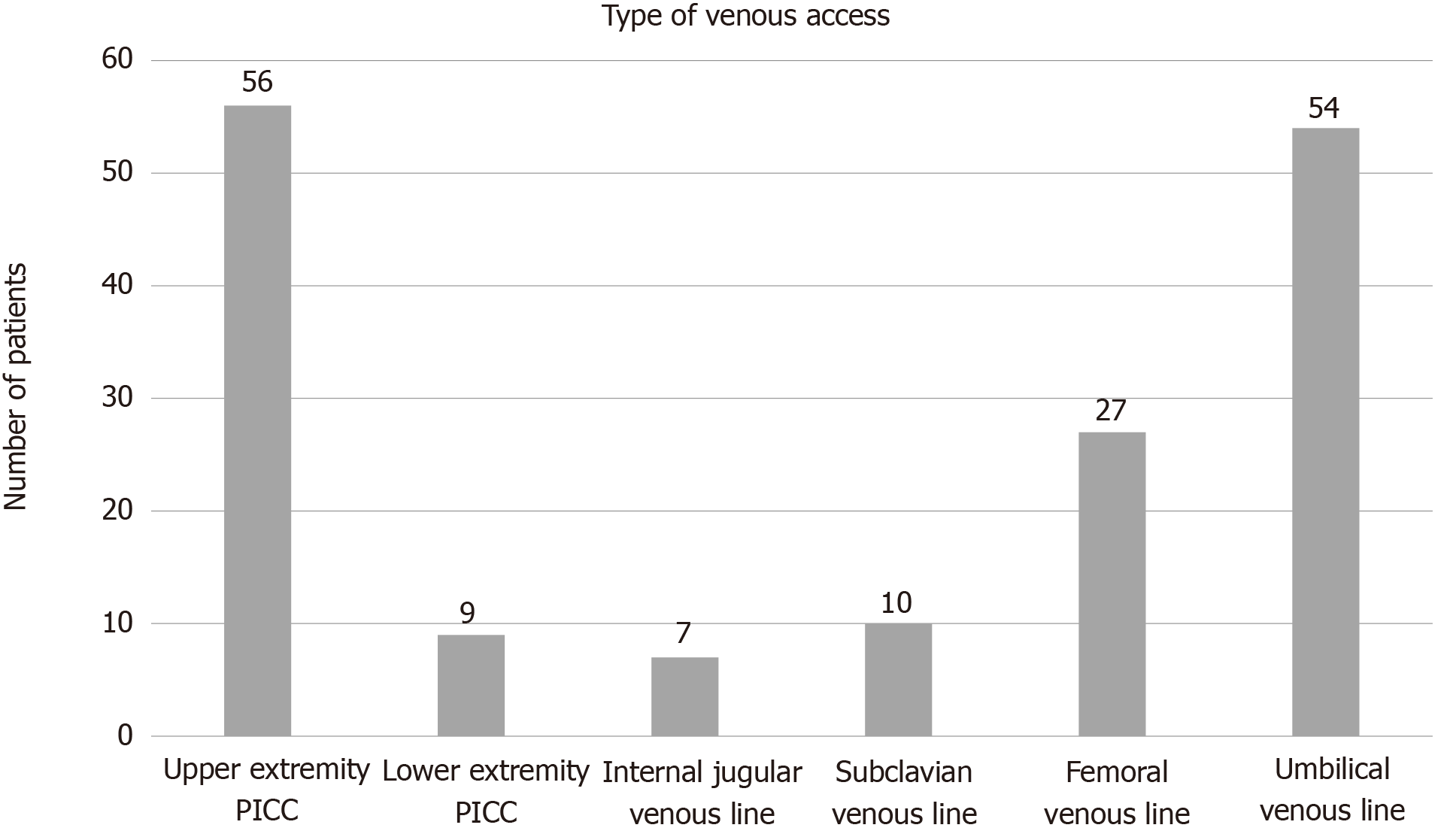
All patients had at least one CVC or PICC placed prior to their second stage procedure. The types of CVCs and PICCs used are summarized in (Figure 2). Fifty-six patients (73%) had an upper extremity PICC at some point prior to their superior cavopulmonary anastomosis with a median duration of 24 d (25%, 75%: 12, 39). Seventeen patients who had PICCs also had an upper extremity CVC (internal jugular or subclavian vein) in place at some point prior to superior cavopulmonary anastomosis with a median duration 10 d (25%, 75%: 8, 14). No patient had an upper extremity PICC and CVC in place simultaneously. Sixty-eight patients (89%) received aspirin and 68 patients (89%) received LMWH prophylaxis while PICCs or CVCs were in place.
Venous thrombus was identified in 5 patients (7%), all of which were seen on upper extremity vascular ultrasound. Three patients had hypoplastic left heart syndrome and underwent the Norwood operation, one patient had tricuspid atresia and underwent pulmonary artery band placement, and one patient had double outlet right ventricle and underwent pulmonary artery band placement. No thrombi were detected via other radiologic studies or cardiac catheterization. In total, upper extremity ultrasounds were obtained in 11 (14%) patients due to suspicion for thrombus. Thrombus was detected in association with 2 of the 56 PICCs (43.6%) and 3 of the 17 CVCs (18%) and the incidence of thrombosis was significantly different between the PICCs vs CVCs (P < 0.04). Thrombosis was identified at a median of 5 d (Range: 4-18 after their first stage palliation surgery and a median of 9 d (Range: 7-16) after placement of PICC or CVC. All 5 patients were switched from prophylactic LMWH dose to therapeutic LMWH after identification of thrombosis and subsequent cardiac catheterization demonstrated thrombus resolution in 3 of 5 patients. The presence of thrombosis in two patients delayed their surgery and, in one patient, precluded the patient from having the second stage surgery. These two patients had PICCs and chorionic villi sampling prior the cardiac catheterization. None of the other 71 patients were found to have thrombosis on cardiac catheterization. Characteristics of the patients with upper extremity thrombus are shown in (Table 2).
| Diagnosis | First stage operation | Age at surgery (in days) | Age at diagnosis thrombus (in days) | Type of venous access | Anti-coagulation prior to thrombus | Resolution of thrombus |
| DORV | PA band | 6 | 10 | Subclavian | No | Yes |
| HLHS | Hybrid | 20 | 25 | Subclavian | Yes | Yes |
| HLHS | Norwood | 14 | 18 | UE PICC | No | Yes |
| Tricuspid atresia | PA band | 9 | 27 | UE PICC | No | No |
| HLHS | Norwood | 9 | 18 | Subclavian | Yes | No |
At our institute, we utilize upper extremity PICCs in most of our single ventricle physiology patients. This cohort demonstrates that the practice of using upper body PICC lines has a low rate of thrombosis among single ventricle physiology patients. There is a large range of reported incidence of venous thrombosis after cardiac surgery in the literature. A prior study from our institute identified a rate of thrombosis of 6% among all patients who underwent cardiac surgery which similar is to this cohort[1]. Manlhiot et al[2], reported thrombosis in 11% of pediatric patients with CHD[2]. In another case series with 89 umbilical venous catheters and femoral central venous catheters, the incidence of the thrombosis among single ventricle patient was high as 42%[15]. In a third study, Miller et al[4] reported no cases of thrombosis in 156 right internal jugular vein catheters in patients who underwent Glenn or Fontan operations[4].
Upper body venous thrombosis is a major concern for single ventricle patients because it could preclude them from further palliative operations in the future. It is important to weigh the risks versus the benefits when deciding the location and type of central venous catheter. In general, pediatric patients will require a deep sedation or mechanical ventilation during central venous catheter replacement. In our institute, PICCs are placed in interventional radiology suite, if a patient’s condition allows for a transfer. Patients receive local anesthetic and minimum sedation for placement with a high success rate.
Obtaining venous access for pediatric patients who undergo congenital heart surgery can be challenging and might take a significant amount of time after induction of anesthesia or require surgical replacement in some cases. As a result, having a PICCs replaced prior to the surgery will facilitate the preoperative process and save operating room time along. Upper extremity venous access will be at the head of the operation table for easy access by the pediatric cardiac anesthesia team. Moreover, most of the single ventricle patients will require several diagnostic or interventional cardiac catheterization over the course of their life, upper extremity PICCs will decrease the number of the femoral venous access attempts.
There is limited data comparing safety and complication rate of PICCs over CVCs in children. Most of the studies have focused on complications with PICCs in neonates and children with leukemia[8,16]. There are few studies published that compare the rates of infection and thrombosis in the hospitalized pediatric patients with PICCs versus CVCs. Noonan et al[17] recently published their experience of the 2709 venous catheters, 126 PICCs and 1583 CVCs. They reported that the rates of central line-associated blood steam catheters (CLABSIs) and venous thromboembolism were higher among patients with PICCs[17]. Data measuring the different complications between PICCs and CVCs among patients with congenital heart disease is very sparse. In this cohort, we did not report the incidence of the CLABSIs, as we have a very low CLABSI rate at our institute with over 800 CLABSI free days during the timeframe of this study.
The majority of patients (60%) who developed thrombosis had upper body CVCs at some point in addition to the PICCs. There was resolution of thrombosis on subsequent cardiac catheterization prior to the second stage palliative after treating them with low-molecular-weight heparin in 60% (3/5) of patients with thrombosis. There were two patients who had PICCs without additional CVC who developed non-occlusive thrombosis, which resolved prior to the cardiac catheterization. There was no one excluded from the second stage from the group who had only PICCs due to the present of the thrombosis. Another interesting finding from our cohort was that patients who were diagnosed with catheter-related thrombosis developed it in close proximity to the first stage palliation surgery. This suggests that the immediate post-operative period is prothrombotic and early anticoagulation, once the risk of bleeding is reasonable, may be beneficial.
This is a retrospective study at the single center. We do not perform routine upper body ultrasound for thrombosis detection. Imaging for thrombosis identification was directed by symptoms prior second stage cardiac catheterization which might underestimate the rate. Additionally, patients had to survive until pre-stage II diagnostic cardiac catheterization in order to be assessed for the outcomes. The majority of our patients were receiving anti-coagulation which might contribute to this low rate of thrombosis. There is no controlled evaluation for other important clinical indicators like anticoagulation practices or rate of CLABSIs
In this retrospective study of children with single ventricle physiology, the placement of percutaneously inserted central catheter in the upper extremity is a reliable way to achieve long-lasting central venous access and associated with a low risk of clinically significant stenosis or thrombosis.
There is risk of stenosis and thrombosis of the superior vena cava after upper extremity central catheter replacement. This complication is more serious among patients with single ventricle physiology, as it might preclude them from undergoing further life-sustaining palliative surgery. Data on the rate of venous thrombosis in children with single ventricle physiology with upper extremity central venous catheters are limited. Also, there is a wide variation in practice regarding the choice of central access in this population across the centers.
To study the risk of using upper body percutaneously inserted central catheter (PICC) in single ventricle patients. The results of this study could be used to develop a multicenter study to determine the risk and benefit of using this type and location of the catheter in this population.
To describe the incidence of thrombosis associated with the use of PICCs in patients with single ventricle physiology.
We retrospectively reviewed the charts of patients with single ventricle physiology who underwent second stage palliation surgery. Data regarding the type and duration of central venous access were collected in addition to the data regarding thrombosis or stenosis.
We reviewed a total of seventy-six patients underwent superior cavopulmonary anastomoses, of which 56 (73%) had an upper extremity PICC at some point prior to this procedure. Median duration of PICC usage was 24 d (25%, 75%: 12, 39). Seventeen patients (30%) with PICCs also had internal jugular or subclavian central venous catheters (CVCs) in place at some point prior to their superior cavopulmonary anastomoses with a median duration of 10 days (25%, 75%: 8, 14). Thrombus was detected in association with 2 of the 56 PICCs (4%) and 3 of the 17 CVCs (18%) and the incidence of thrombosis was significantly different between the PICCs vs CVCs (P < 0.04). All five patients were placed on therapeutic dose of low molecular weight heparin at the time of thrombus detection and subsequent cardiac catheterization demonstrated resolution in three of the five patients. No patients developed clinically significant venous stenosis.
The placement of PICC in the upper extremity in children with single ventricle physiology was associated with low risk of clinically significant stenosis or thrombosis and provide a reliable way to have long-lasting central venous access.
Further research and multicenter studies specifically looking at the incidence of complications with upper body PICCs in single ventricle patients are warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dai HL S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Murphy LD, Benneyworth BD, Moser EAS, Hege KM, Valentine KM, Mastropietro CW. Analysis of Patient Characteristics and Risk Factors for Thrombosis After Surgery for Congenital Heart Disease. Pediatr Crit Care Med. 2018;19:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Manlhiot C, Menjak IB, Brandão LR, Gruenwald CE, Schwartz SM, Sivarajan VB, Yoon H, Maratta R, Carew CL, McMullen JA, Clarizia NA, Holtby HM, Williams S, Caldarone CA, Van Arsdell GS, Chan AK, McCrindle BW. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation. 2011;124:1511-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Williamson J. Prevention and early recognition of complications of central venous catheterization. Am Heart J. 1976;92:667-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Miller JW, Vu DN, Chai PJ, Kreutzer JH, John JB, Vener DF, Jacobs JP. Upper body central venous catheters in pediatric cardiac surgery. Paediatr Anaesth. 2013;23:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Stefanescu Schmidt AC, Armstrong A, Kennedy KF, Nykanen D, Aboulhosn J, Bhatt AB. Prediction of adverse events after catheter-based procedures in adolescents and adults with congenital heart disease in the IMPACT registry. Eur Heart J. 2017;38:2070-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Guzzetta NA. Thrombosis in Neonates and Infants After Cardiac Surgery-Another Piece of the Puzzle. J Cardiothorac Vasc Anesth. 2017;31:1949-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Stein ML, Quinonez LG, DiNardo JA, Brown ML. Complications of Transthoracic Intracardiac and Central Venous Lines in Neonates Undergoing Cardiac Surgery. Pediatr Cardiol. 2019;40:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Noailly Charny PA, Bleyzac N, Ohannessian R, Aubert E, Bertrand Y, Renard C. Increased Risk of Thrombosis Associated with Peripherally Inserted Central Catheters Compared with Conventional Central Venous Catheters in Children with Leukemia. J Pediatr. 2018;198:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, Blitt C, Caplan RA, Connis RT, Domino KB, Fleisher LA, Grant S, Mark JB, Morray JP, Nickinovich DG, Tung A. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116:539-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 343] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 10. | Mitto P, Barankay A, Späth P, Richter JA. Central venous catheterization in infants and children with congenital heart diseases--experiences with 400 consecutive punctures. J Cardiothorac Anesth. 1989;3:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Petäjä J, Lundström U, Sairanen H, Marttinen E, Griffin JH. Central venous thrombosis after cardiac operations in children. J Thorac Cardiovasc Surg. 1996;112:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Yabrodi M, Mastropietro CW. Hypoplastic left heart syndrome: from comfort care to long-term survival. Pediatr Res. 2017;81:142-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Atchison CM, Amankwah E, Wilhelm J, Arlikar S, Branchford BR, Stock A, Streiff M, Takemoto C, Ayala I, Everett A, Stapleton G, Jacobs ML, Jacobs JP, Goldenberg NA. Risk factors for hospital-associated venous thromboembolism in critically ill children following cardiothoracic surgery or therapeutic cardiac catheterisation. Cardiol Young. 2018;28:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Silvey M, Hall M, Bilynsky E, Carpenter SL. Increasing rates of thrombosis in children with congenital heart disease undergoing cardiac surgery. Thromb Res. 2018;162:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Aiyagari R, Song JY, Donohue JE, Yu S, Gaies MG. Central venous catheter-associated complications in infants with single ventricle: comparison of umbilical and femoral venous access routes. Pediatr Crit Care Med. 2012;13:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Janes M, Kalyn A, Pinelli J, Paes B. A randomized trial comparing peripherally inserted central venous catheters and peripheral intravenous catheters in infants with very low birth weight. J Pediatr Surg. 2000;35:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Noonan PJ, Hanson SJ, Simpson PM, Dasgupta M, Petersen TL. Comparison of Complication Rates of Central Venous Catheters Versus Peripherally Inserted Central Venous Catheters in Pediatric Patients. Pediatr Crit Care Med. 2018;19:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |