Published online Jun 26, 2019. doi: 10.4330/wjc.v11.i6.159
Peer-review started: February 26, 2019
First decision: April 15, 2019
Revised: April 29, 2019
Accepted: June 12, 2019
Article in press: June 13, 2019
Published online: June 26, 2019
Processing time: 123 Days and 17.8 Hours
Cardiovascular diseases are the number one cause of morbidity and mortality in the United States and worldwide. The induction of the endoplasmic reticulum (ER) stress, a result of a disruption in the ER homeostasis, was found to be highly associated with cardiovascular diseases such as hypertension, diabetes, ischemic heart diseases and heart failure. This review will discuss the latest literature on the different aspects of the involvement of the ER stress in cardiovascular complications and the potential of targeting the ER stress pathways as a new therapeutic approach for cardiovascular complications.
Core tip: The central mechanisms involved in heart failure, a public health crisis, remain unknown. Current therapies, in addition to their strong side effects, neither halt nor reverse heart complications. The endoplasmic reticulum (ER) stress has been shown to be involved in cardiovascular diseases. Here we analyzed the role and mechanism of the ER stress in heart failure.
- Citation: Belmadani S, Matrougui K. Broken heart: A matter of the endoplasmic reticulum stress bad management? World J Cardiol 2019; 11(6): 159-170
- URL: https://www.wjgnet.com/1949-8462/full/v11/i6/159.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i6.159
The endoplasmic reticulum (ER), one of the largest organelles in the eukaryotic cells was described for the first time in 1945 by Porter et al[1]. The ER is responsible for protein synthesis, and folding of most secreted and membrane protein, which represent approximately 35% of all protein[2].The ER is also the site of protein translocation, calcium homeostasis, lipid, and steroid biosynthesis[3]. Effective ER function relies on various quality control factors such as molecular chaperones, protein oxidoreductases, and enzymes involved in glycosylation, sulfation and proteolysis[4]. This highly organized machinery requires an optimal ER environment. Various factors such as myocardial ischemia, diabetes, hypertension, and heart failure can disrupt this environment provoking the accumulation of misfolded proteins[4]. When the ER homeostasis is altered by the accumulation of unfolded/misfolded protein; signaling pathways are activated triggering an adaptive response known as the unfolded protein response (UPR). The primary goal of the UPR is to restore the protein balance by suppressing protein translation, increased clearance of unfolded or misfolded proteins and promoting cell survival. Unfortunately, if the ER homeostasis is not restored, the cell dysfunction and death signaling pathways is launched. The UPR re-establishes homeostasis through three distinct branches that are initiated by the ER-resident protein folding sensors, inositol-requiring protein-1 (IRE1α), activating transcription factor-6 (ATF6) or protein kinase RNA-like ER kinase (PERK). Each branch uses a unique mechanism to activate transcription factors and up-regulate UPR target genes. These three ER-transmembrane proteins serve both as sensors for the ER stress and effectors for the response to the ER stress induction. Under basal conditions, the ER-resident transmembrane proteins ATF6, IRE1, and PERK are maintained in an inactive state via their binding to the ER chaperone glucose-regulated protein (GRP78)[5]. Under stress conditions where misfolded proteins are increased in the ER, GRP78 binds misfolded protein and releases from the ER stress sensors, leading to their activation.
IRE1 is the most ancient ER transmembrane protein containing an ER-luminal sensor domain recognizing unfolded peptides, kinase and endoribonuclease (RNase) domain on its cytosolic portion[6]. IRE1 has two isoforms, IRE1α and IRE1β[6]. IRE1α is ubiquitously expressed whereas IRE1β is only expressed in the gut[6]. In the absence of the ER stress, GRP78 binds to the luminal domain of IRE1α. Under stress situations, IRE1 is activated by homodimerization after release from GRP78 and auto-phosphorylation leading to the activation of the kinase and the endoribonuclease activity of IRE1. Active IRE1α splices a transcription factor X-box-binding-protein-1 (XBP1) mRNA to spliced XBP1 (XBP1s). XBP1s is a potent transcription factor for a variety of genes involved in retrograde transport of proteins from the ER to cytosol and in ER-induced protein degradation[7]. Moreover, IRE1 degrades mRNAs via regulated IRE1-dependent mRNA decay (RIDD) mechanism to reinstate homeostasis in the ER.
ATF6, a 670 amino acids type II transmembrane protein with a bZIP transcription factor motif. At resting conditions ATF6 is localized at the ER through its interaction with GRP78. Following the stress, unfolded/misfolded proteins accumulation enhance the release of the ATF6 and its translocation to the Golgi apparatus where the luminal and transmembrane domains are cleaved by site-1 and site-2 proteases (S1P and S2P) via regulated intra-membrane proteolysis. This results in an active ATF6 capable of interacting with regulatory sequences called ER stress response elements and regulating the expression of chaperones, X box-binding protein 1 (XBP1) toward restoring protein folding and cellular homeostasis[8,9].
Like IRE1, PERK has a protein kinase activity, and after its dissociation from GRP78, PERK dimerizes and autophosphorylates. Active PERK phosphorylates the eukaryotic initiation factor 2α (eIF2α), which blocks unfolded protein translation promoting cell survival and also activates the transcription of the ATF4 to decrease Unfolded protein level in the ER via the activation of various UPR genes[10]. If the adaptive mechanisms do not sufficiently recover the ER homeostasis, the UPR can switch from a pro-adaptive to a pro-apoptotic role[11].
Numerous studies have linked the disruption of the ER homeostasis to the pathophysi-ology of many diseases including heart diseases. However, the specific role of the ER stress signaling in the heart is yet to be defined and whether ER stress signaling is detrimental or protective in the heart is still a challenging question that needs to be answered[4,12]. In cardiomyocyte, Bcl2 proteins family was shown to induce apoptosis via calcium signaling during ER stress induction[13]. In line with this study, prolonged ER stress triggered cardiomyocyte apoptosis and oxidation of CaMKII, a redox-sensitive enzyme, which was rescued by antioxidant or CamKII inhibitors treatments[14]. Furthermore, it has been shown that the oxidation of CaMKII may lead to cardiac dysfunction and apoptosis[15]. In this context, Roe et al[14] showed that CaMKII oxidation mediates ER stress-induced cardiac dysfunction and apoptosis and could be used as a potential target in cardiac diseases triggered by the ER stress. GRP78 has been found to increase in patients with heart failure suggesting the implication of the UPR activation in heart failure[16]. Patients with heart failure display a structural and architecture alteration of the ER as well as dys-regulation of the ER proteins involved in the UPR response[17]. In fact, spliced XBP1s, GRP78, ATF4, and CHOP were all induced in failing human heart[16-20]. Using the transverse aortic constriction (TAC) mouse model to induce heart failure, Okada et al[20] showed that the ER stress was induced in both hypertrophic and failing heart. Remarkably, the ER stress-CHOP and apoptosis were only seen in failing heart but not in hypertrophic heart indicating the differential effect of the ER stress pathology-dependent. Moreover, the ER stress-CHOP deficient mice develop less cardiac hypertrophy, fibrosis, and cardiac dysfunction compared to wild mice. Our recent study showed that the inhibition of the ER stress protected the heart against myocardial infarction induced by ischemia-reperfusion injury[21]. Together these studies suggest that the ER stress could be involved in the development of myocardial infarction, cardiac hypertrophy, and the transition from hypertrophy to heart failure[22,23].
Recently, PERK was shown to protect the heart from pressure overload-induced heart failure[24]. Cardiomyocyte-specific disruption of PERK did not affect the cardiac structure or function under normal conditions but exacerbates the development of heart failure in response to TAC[25]. The hearts of PERK knockout mice showed a dramatic reduction in Serca2α expression and an increase in apoptosis and UPR genes expression (GRP78, GRP94, CHOP) in response to TAC. These results suggest the importance of PERK in the maintenance of intracellular calcium homeostasis, control of the ER stress level and cell survival[25].
The activation of the UPR has been shown in ischemic heart diseases[26,27]. PERK activation was also observed in ischemic hearts, and its overexpression seems to promote cell survival while its down regulation is detrimental to cells[28,29].
XBP1s and GRP78 were increased in the ischemic heart of patients and animal models[27,30]. XBP1s seem to be cardio-protective in mice after ischemia-reperfusion injury[31,32]. Moreover, the ER stress-CHOP, PUMA and Tribbles3 downstream effect-ors of PERK play a significant role in cell death induced by the ER stress after myocardial ischemia-reperfusion injury[33].
ATF6, the third ER stress member that is activated during myocardial I/R injury. Using genetic and pharmacological approaches, Glembotski’s group and others showed that ATF6 protects the heart against myocardial I/R injury probably through the induction of the ERAD machinery leading to the degradation of misfolded proteins in the ER[34-37]. Recently, it has been found that thrombospondin-4 protects the heart by promoting the adaptive response of the ER stress through the activation of the ER stress ATF6[38,39]. The authors showed that ATF6 location and activity could be determined via its interaction with thrombospondin-4. These results suggest the benefit of enhancing the adaptive response mediated by ATF6 as a potential therapy to target ischemic heart diseases.
In recent years, various studies found links between the ER stress pathways and infla-mmation[40]. Ischemic heart disease, a significant cause of death is recognized as an inflammatory disease involving infiltration of monocytes and macrophages. Recently, cardiac-specific expression of monocyte chemoattractant protein-1 (MCP-1) in mice causes heart failure, which was correlated with the activation of a cluster of the ER stress-related genes[41]. It has been shown that the production of the pro-inflammatory cytokine such IFNγ, TNF-α, MCP-1, and IL-8 required the activation of the IRE1 and XBP1[42,43]. IRE1 has also been linked to inflammation mediated by NFκB cascade via its binding to TRAF2[44,45]. Moreover, ATF 6 can also trigger NFκB mediated inflammation through AKT phosphorylation[46].
When the UPR fails to reestablish the ER homeostasis, the detrimental apoptotic signaling pathway is activated. Up to date, it is still a mystery how cells chose between the adaptive/survival pathway vs the detrimental /death once the UPR machinery is triggered. Under sustained ER stress induction, IRE1α triggers apoptosis via the activation of JNK and p38 through TRAF2 and ASK1 mechanism[47,48].
Cardiac myocyte lacking ASK1 were resistant to apoptosis induced by the hydrogen peroxide[49]. Cardiac overexpression of ASK1 showed an increase in cardiac apoptosis in a mice model of TAC while ASK1 deficient mice were protected from heart failure[50]. In a rat model of I/R injury, the inhibition of ASK1 was able to reduce apoptosis and myocardial infarct size[51]. The p38 activates the ER stress CHOP and both p38 and JNK can activate Bax to initiate apoptosis. IRE1 is also known to activate caspase12 leading to apoptosis[52,53]. Moreover, the RNase activity of IRE1 known as RIDD may promote cell death via the degradation of mRNAs involved in protein survival[54]. It is worth noting that IRE1 exerts two opposing functions: death and survival depending on the conformational of the protein and the intensity of the stress mild vs high. Under mild conditions of the ER stress, IRE1 helps to relieve the stress by splicing XBP-1. Under high-prolonged ER stress, IRE1 triggers apoptosis via the interaction with TRAF2 and ASK1[54]. Erhardt’s group recently described that the ER stress requires the proapoptotic Bcl-2 family protein (Puma) to promote apoptosis in cardiac myocytes[55]. Puma is critical for cell death related to I/R[56]. Thus, the overexpression of PUMA in cardiac myocytes contributes to apoptosis induced by the ER stress while deletion protects the heart from I/R injury[57]. These results suggest inhibition of Puma activity may be used to treat cardiac infarcts or prevent heart failure by blocking ER stress-induced apoptosis[56,58]. Additionally, evidence suggests that the ER stress-CHOP plays a pivotal role in mitochondria-dependent apoptosis in the heart with pressure overload[22]. It is clear that the ER stress induction is a mechanism that leads to apoptosis and therefore tissue damage.
Autophagy or “self-eating” is a highly conserved cell-recycling program for the clea-rance of damaged proteins and organelles. Autophagy has been reported in many cells type of the cardiovascular system and been classified into microautophagy, macroautophagy, and chaperone-mediated autophagy. Autophagy is necessary for the preservation of normal cardiac function. However, deficient or excessive cardiac autophagy is considered as a maladaptive response. Moreover, autophagy is regarded as “double edge sword” for its different role in the cardiovascular system.
Recently, the ER stress emerges as an important inducer of autophagy and a link between the ER stress, autophagy and cardiac function have been proposed[59,60]. Although, abundant data showed that cross talks exist between the ER stress and autophagy, the molecular mechanism is yet to be determined[61]. Zhang et al[62] recently showed that mitochondrial aldehyde dehydrogenase (ALDH2) was able to alleviate ER stress-induced cardiomyopathy via autophagy reduction. Reticulon, a membrane-associated protein localized at the ER has been shown to be involved in the induction of autophagy leading to the ER stress induction demonstrating the relationship between autophagy, reticulum and the ER stress[63]. Furthermore, the activation of the IRE1 induces autophagy via its interaction with TRAF2 and the activation of JNK leading to the regulation of Beclin-1 expression. Moreover, advance glycation products (AGEs) were able to trigger autophagy in cardiac myocytes probably via the ER stress signaling. In fact, crosstalk between advanced AGEs and ER stress signaling could mediate the induction of autophagy by AGES[64,65]. In a mouse model of sepsis, Cardiac-specific overexpression of the antioxidant metallothionein (MT) was able to rescue cardiac contractility dysfunction probably via ER stress and oxidative stress modulation[66]. In a swine model of hypertension, the progression of LVH has been shown to involve an early activation of the ER stress followed by an increase in autophagy leading to apoptosis[67]. SIRT1, a member of the sirtuins family, histone/protein deacetylases known to be crucially involved in signaling related to cell death/survival and has been found to be activated in the heart to promote cell adaptation and survival under stress[68]. Recently Lemaire’s group reported that in cardiac cells, Sirt1 was able to modulate the induction of autophagy in response to the ER stress induction suggesting the possibility of tuning the adaptive autophagy in cardiac pathologies related to ER stress[69].
In summary, the ER stress and autophagy play an important role in the patho-genesis of cardiac complications. Although, ample studies established the interplay and the interaction between the ER stress and autophagy, and their role in the progression of heart diseases, the molecular mechanism remained unknown. Who are the players, how can we better tune the ER stress and autophagy? It is evident now that the ER stress and autophagy are influencing each other. Therefore, a good understanding of the interconnection between these two important physiological processes, especially under pathological conditions will be of great importance and may shed light on developing new therapeutic strategies to rescue the cardiovascular system.
MicroRNAs (miRNAs or miRs) are a class of conserved small, 20-23 nucleotide, single-stranded, non-coding RNAs that post-transcriptionally regulate gene expression[70]. They were first described in the 1993 and had been linked to various cellular stress such oxidative stress, inflammation, and the ER stress in the setting of cardiovascular complications[71]. The miRNAs are increasingly recognized as a master regulator of the ER stress and an important player in the UPR response, which manage the UPR balance between survival and cell death during the ER stress-induction. In fact, several miRNAs have been demonstrated to be regulated by the ER stress and to regulate the ER stress by optimizing the levels of key proteins involved in the UPR. For instance PERK pathway induces the expression of miR-30c-2*, which represses XBP1s synthesis at the translational level[72]. Although miR-30c-2* level increases after the ER stress induction along with the XBP1s level, miR-30c-2* was still capable of affecting the XBP1 level in the course of the UPR[72]. In cardiac myocytes and using a Tamoxifen-inducible ATF6 in the heart of transgenic mice, activated ATF6 regulates the expression of 13 miRNAs[73]. The miRNA-455, one of the miRNAs down regulated by ATF6, negatively regulates calreticulin (a calcium chaperone protein) involved in the folding of nascent polypeptides[73]. Therefore, the ER stress ATF6 down regulates miRNA-455, which up-regulates calreticulin, a cardio-protective gene. While the ER stress ATF6 regulates the expression of miRNAs, it was also a target of miR-702[74]. Together, the two studies showed the existence of interplay between miRNAs and the pro-adaptive activity of the UPR in the heart. Another class of miRNAs linked to the ER stress includes member of miRNA-30 family. The miRNA30 is one of the most abundant miRNAs expressed in the myocardium and has been shown to be down-regulated in heart failure and hypertension in both vascular smooth muscle cells and cardiac neonate cells. Under ER stress conditions, miRNA-30 was down-regulated while GRP78 was up-regulated. Moreover, GRP78 up-regulation seems to modulate miRNA-30 expression through the inhibition of the C/EBP trans-activity by CHOP in the myocardium[75]. Interestingly, Knockdown of miRNA-30 in cardiac cells triggered ER stress and identified the ER stress ATF6/CHOP and caspase-12 as indirect targets of this miRNAs. While the transfection of miR-30 was able to abolish the ER stress suggesting that miRNA30 plays a role in the regulation of cell death and miRNA30 replacement could be considered as an approach for targeting the ER stress and the related pathological diseases[76]. Recent studies indicated that miRNA214 is a negative regulator of angiogenesis in the retina and heart[19,77]. XBP1 was found to be a direct target of miR214 in endothelial cells. The blockade of the endogenous miRNA214 expression regulated cardiac function and cardiac angiogenesis. Interestingly, cardiac overexpression of miRNA-214 in mice had no morphological changes suggesting that miRNA214 regulates cardiac and vascular angiogenesis only when XBP-1 is dys-regulated[78]. This study highlighted another scenario of ‘’cross talk’’ between miRNAs and the ER stress components in the cardiovascular system. Independently of XBP-1, a recent study proposed a new role of the ER stress sensor IRE1α in the modulation of miRNA-200 and miRNA-466 and the improvement of bone marrow derived progenitor cells (BMPC) function via its endonuclease activity in diabetes[79]. This study outlined the importance of the ER stress IRE1α as a crucial modulator of the fate/function of BMPCs during angio-genesis and tissue repair via the modulation of miRNA expression levels and may be therefore involved in another ischemic setting such ischemic heart diseases and heart failure. Further studies are needed to determine the mechanism that inhibits IRE1α activity in diabetic BMPCs and the potential of expanding these findings to other cardiovascular complications, such as the heart failure[80]. The ER stress ATF4, a downstream effector of the ER stress PERK, has been linked to miR-663 in endothelial cells[81]. The inhibition of miRNA663 during the ER stress induction leads to a decrease in the ER stress ATF4 expression as well as its target gene, the VEGF. Moreover, miRNA708 was the first ER stress-induced miRNA discovered[82].
MicroRNAs and the ER stress interaction is a very young research area. More work is required to unravel the array of microRNA targets and determine their function in the ER stress-induced death/survival. Moreover, it is essential to recognize that the results obtained so far showing the interaction/link/correlation/regulation of the ER stress by miRNAs or vice versa represent a promising avenue for cardiovascular diseases. As mentioned above, one of the significant challenges of the ER stress response in pathological situations is the fact that it is difficult to distinguish between the protective pathways and the detrimental pathways once the UPR response is triggered. Differentiating between the detrimental pathways and the adaptive pathways of the ER stress players via its miRNAs target will advance the field tremendously and opens new opportunities for novel therapeutic strategies targeting ER stress via miRNAs in cardiovascular diseases.
ER stress has been involved in numerous cardiovascular diseases such as diabetes, hypertension, myocardial infarction and heart failure. Therefore, targeting the ER stress in cardiovascular disease via the activation of the adaptive pathway of the UPR or the inhibition of the detrimental pro-apoptotic pathways of the UPR will be a beneficial therapy for cardiovascular diseases.
Chemical chaperones, small molecules that work similarly to the endogenous molecular chaperone machinery to stabilize misfolded proteins, facilitate their proper folding and reduce the ER stress. Among the chemical chaperones that have been extensively used in various diseases related to the ER stress, Tauroursodeoxycholic (TUDCA) and 4-phenylbutyric acid (PBA).
TUDCA is a non-toxic hydrophilic bile acid that functions as a chemical chaperone and has been extensively used in colitis, pulmonary fibrosis, biliary cirrhosis, and recently in patients with obesity and insulin resistant[83-85]. In animal models, TUDCA has been shown to protect the heart against myocardial dysfunction in obesity, and reduce apoptosis in a mouse model of myocardial infarction[86]. Under pressure overload, TUDCA was shown to attenuate cardiac remodeling through down-regulation of the GRP78 and GRP94 and the regulation of the ER stress PERK phosphorylation and eIF2α[87]. Moreover, in a mouse model of heart failure induced by calreticulin overexpression, the inhibition of the UPR using TUDCA decreased cardiac fibrosis, which was mediated through the inhibition of the ER stress IRE1 activation and XBP1 splicing[88]. Together, these results highlight the cardioprotective effect of TUDCA treatment and the therapeutic potential of using TUDCA in the management of cardiac complications[66].
PBA, a low-molecular-weight aromatic fatty acid, has a chaperone-like activity and has been shown to attenuate cardiac hypertrophy, fibrosis, and apoptosis in a pressure overload animal model[89]. In isolated rat hearts subjected to I/R injury, 4-PBA was revealed to be a potent cardioprotective agent via: (1) The reduction in the I/R injury-induced myocardial dysfunction and cell apoptosis; (2) The delay of the onset of the ER stress via the regulation of Grp78 expression, PERK phosphorylation; and (3) The inhibition of oxidative stress[90]. In a cell and a clinically relevant dog model for atrial fibrillation, the blockade of the ER stress by PBA inhibits the induction of the autophagy and suppresses cardiomyocytes remodeling suggesting the potential of using PBA to protect the heart against clinical atrial fibrillation[91]. Furthermore, PBA and TUDCA were also able to reduce the cardio-toxicity effect of doxorubicin (a chemotherapeutic agent commonly used in cancer). Moreover, PBA and TUDCA reduced cardiomyocyte apoptosis and alleviated cardiac dysfunction in a mouse model of cardiomyopathy induced by doxorubicin[92]. Considering that TUDCA and PBA are FDA-approved chemical chaperones and already used clinically for the treatment of some diseases, it will be exciting and safer to test TUDCA and PBA in patients with cardiovascular complications related to the ER disturbance. Future basic and clinical studies are critically needed to determine: (1) The right doses required to obtain the cardioprotective effect; and (2) To delineate the mechanism of how chemical chaperones promote the protein folding.
Statin therapy has been shown to be beneficial for heart failure treatment[93]. Interestingly, a recent study showed that in a mouse model of pressure overload and this effect was associated with a reduction in the ER stress[94-97]. The results suggest that the reduction in the ER stress might be a novel mechanism for the beneficial effect of statin for heart failure[98]. Moreover, in a rat model of heart failure, the modulation of the ER stress markers such as Caspase12, the ER stress-CHOP, and GRP78 was proposed as a mechanism by which Atorvastatin (another statin drug) protects the heart against heart failure[99]. Interestingly, the administration of Atorvastatin improved left ventricular ejection fraction and attenuated left ventricular remodeling in patients with heart failure[100]. These results could be clinically relevant for the treatment and the prevention of heart failure.
Apelin recently discovered as an endogenous ligand for the G protein-coupled receptor APJ and has been shown to be a beneficial therapy for patients with heart failure[101-103]. Apelin seems to have a positive effect on peripheral and coronary vasodila-tation, cardiac output, and cardiac function[104,105]. The cardioprotective effect of apelin could be mediated through the inhibition of the ER stress dependent apoptosis[106]. Furthermore, in a mouse model of obesity-induced cardiac compli-cations, exogenous administration of apelin attenuates myocardial contractile dysfunctions and cardiac hypertrophy through the inhibition of the ER stress and the restoration of autophagy[107]. On the other hand, apelin 13 (the main subtype of apelin in the human heart) induced cardiomyocytes hypertrophy through autophagy and the ER stress mechanisms[108]. From these studies, the benefits vs the detrimental role of apelin in cardiac complication seem to depend on the conditions basic vs stress and could be explained by the UPR status of the ER stress adaptive vs detrimental UPR response.
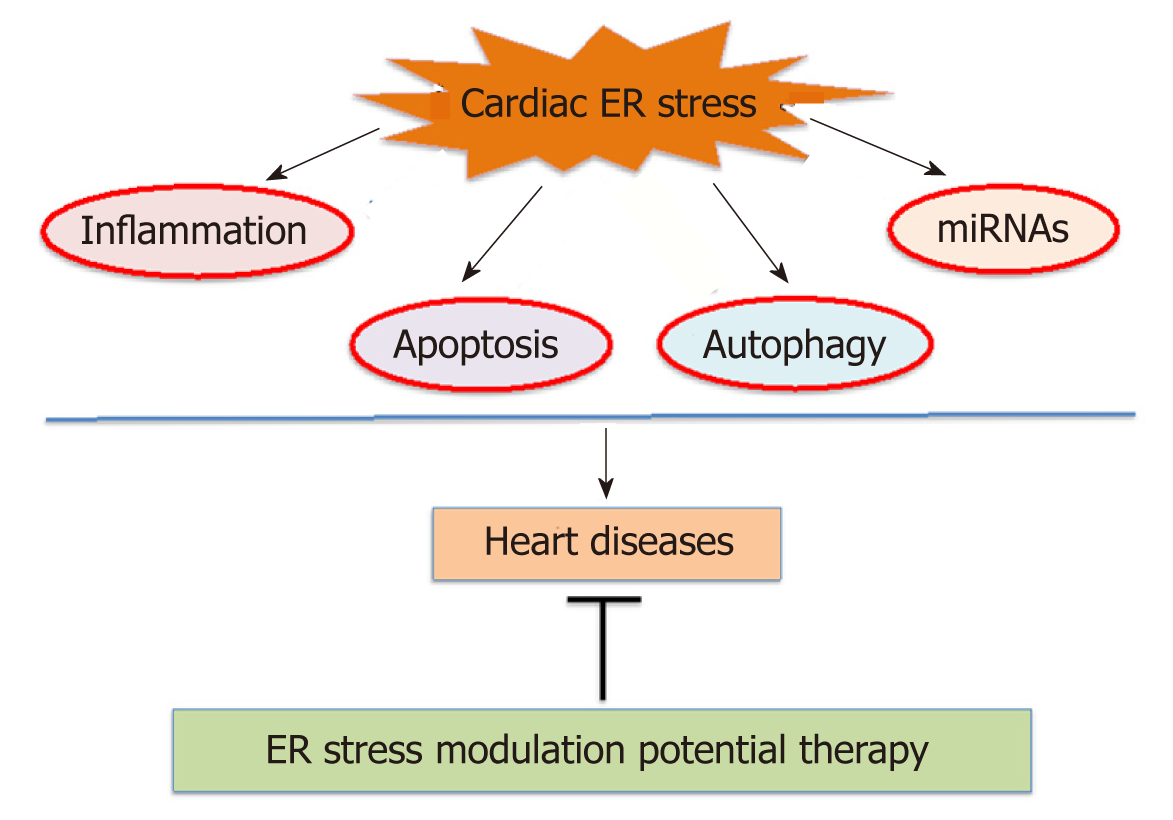
Adenosine monophosphate-activated kinase (AMPK) recognized as an intracellular energy and stress sensor that function to maintain intracellular homeostasis during stress conditions. Dysregulation of AMPK has been reported in humans and animal models of metabolic syndrome[109-111]. The 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and metformin, antidiabetic drugs activate AMPK, reduce the ER stress and slow the progression of heart failure[112]. Additionally, AICAR activates nuclear factor-E2-related factor (Nrf2) through AMPK independent pathways, which helps combat oxidative damage. Increased expression of Nrf2 reduces cardiac hypertrophy, myocardial infarct, and the progression of heart failure. However, AMPK and Nrf2 pathways show convergence as well[113]. Therapies that activate AMPK and Nrf2, as well as the UPR and apoptotic pathways, hold promise in the treatment cardiac complications. Moreover, therapeutic efforts aimed at oxidative stress also reduce the ER stress. Thus, the ER stress appears to be, a key player in cardiovascular complications and a large number of drugs seemed to protect the heart against failure involved the ER stress modulation. Targeting the ER stress pathways hold a great feature for patients with cardiac complications. As the prevalence of heart diseases raises yearly worldwide, it becomes significant to understand the relationship between heart failure and the ER stress. There is still much to understand about the contribution of the ER stress in heart complications (Figure 1).
Significant attention was given to the ER stress in the recent years from “bench to bed” due to its involvement in numerous cardiovascular diseases such as diabetes, hypertension, myocardial infarction, and heart failure. Although many studies have characterized signaling pathways of the ER stress and the UPR in general and particularly in the cardiac field, many questions remained to be addressed. How can we tame the ER stress and what is the best way to control it? How can we balance “too much or too little” of the ER stress to promote survival and inhibit apoptosis in cardiac pathology? How can we integrate conventional therapies (AMP kinase drugs, ACE inhibitors, autophagy (activators/inhibitors) with the UPR target against cardiovascular diseases? How can we use MicroRNAs and gene therapy to regulate the ER stress toward a better and safe future therapy? Can the chemical chaperone be “the ER stress therapy” by excellence against cardiac complications? Only the future will tell us.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Petix NR, Ueda H S-Editor: Ji FF L-Editor: A E-Editor: Xing YX
| 1. | Porter KR, Claude A, Fullam EF. A study of tissue culture cells by electron microscopy: Methods and preliminary observations. J Exp Med. 1945;81:233-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Palade GE. The endoplasmic reticulum. J Biophys Biochem Cytol. 1956;2:85-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 377] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | van der Kant R, Neefjes J. Small regulators, major consequences - Ca²⁺ and cholesterol at the endosome-ER interface. J Cell Sci. 2014;127:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res. 2007;101:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 6. | Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708-5717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 632] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 338] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2260] [Cited by in RCA: 2366] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 9. | Jin JK, Blackwood EA, Azizi K, Thuerauf DJ, Fahem AG, Hofmann C, Kaufman RJ, Doroudgar S, Glembotski CC. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ Res. 2017;120:862-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 10. | Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2430] [Cited by in RCA: 2559] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 11. | Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2167] [Cited by in RCA: 2102] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 12. | Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1840] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 13. | Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, Brown JH, Molkentin JD, Kranias EG, Dorn GW 2nd. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Roe ND, Ren J. Oxidative activation of Ca(2+)/calmodulin-activated kinase II mediates ER stress-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2013;304:H828-H839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 895] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 16. | Dally S, Monceau V, Corvazier E, Bredoux R, Raies A, Bobe R, del Monte F, Enouf J. Compartmentalized expression of three novel sarco/endoplasmic reticulum Ca2+ATPase 3 isoforms including the switch to ER stress, SERCA3f, in non-failing and failing human heart. Cell Calcium. 2009;45:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Ortega A, Roselló-Lletí E, Tarazón E, Molina-Navarro MM, Martínez-Dolz L, González-Juanatey JR, Lago F, Montoro-Mateos JD, Salvador A, Rivera M, Portolés M. Endoplasmic reticulum stress induces different molecular structural alterations in human dilated and ischemic cardiomyopathy. PLoS One. 2014;9:e107635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Duan Q, Ni L, Wang P, Chen C, Yang L, Ma B, Gong W, Cai Z, Zou MH, Wang DW. Deregulation of XBP1 expression contributes to myocardial vascular endothelial growth factor-A expression and angiogenesis during cardiac hypertrophy in vivo. Aging Cell. 2016;15:625-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Duan Q, Yang L, Gong W, Chaugai S, Wang F, Chen C, Wang P, Zou MH, Wang DW. MicroRNA-214 Is Upregulated in Heart Failure Patients and Suppresses XBP1-Mediated Endothelial Cells Angiogenesis. J Cell Physiol. 2015;230:1964-1973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Mali V, Haddox S, Hornersmith C, Matrougui K, Belmadani S. Essential role for EGFR tyrosine kinase and ER stress in myocardial infarction in type 2 diabetes. Pflugers Arch. 2018;470:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 23. | Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 805] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 24. | Liu X, Kwak D, Lu Z, Xu X, Fassett J, Wang H, Wei Y, Cavener DR, Hu X, Hall J, Bache RJ, Chen Y. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension. 2014;64:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Lu Z, Xu X, Fassett J, Kwak D, Liu X, Hu X, Wang H, Guo H, Xu D, Yan S, McFalls EO, Lu F, Bache RJ, Chen Y. Loss of the eukaryotic initiation factor 2α kinase general control nonderepressible 2 protects mice from pressure overload-induced congestive heart failure without affecting ventricular hypertrophy. Hypertension. 2014;63:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Glembotski CC. The role of the unfolded protein response in the heart. J Mol Cell Cardiol. 2008;44:453-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Sawada T, Minamino T, Fu HY, Asai M, Okuda K, Isomura T, Yamazaki S, Asano Y, Okada K, Tsukamoto O, Sanada S, Asanuma H, Asakura M, Takashima S, Kitakaze M, Komuro I. X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1280-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Szegezdi E, Duffy A, O'Mahoney ME, Logue SE, Mylotte LA, O'brien T, Samali A. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349:1406-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 30. | Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PPA, Ferdous A, Gillette TG, Scherer PE, Hill JA. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156:1179-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 329] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 32. | Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabò R, Di Lisa F, Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17:923-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Wang S, Binder P, Fang Q, Wang Z, Xiao W, Liu W, Wang X. Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br J Pharmacol. 2018;175:1293-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 34. | Martindale JJ. Protecting the Myocardium from Ischemia and Reperfusion Injury Via Inducible Activation of ATF6 Or Constitutive Expression of MKK62006: ProQuest. Available from: URL: https://www.proquest.com/APAC-CN/. |
| 35. | Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735-29745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Belmont PJ, Chen WJ, San Pedro MN, Thuerauf DJ, Gellings Lowe N, Gude N, Hilton B, Wolkowicz R, Sussman MA, Glembotski CC. Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene Derlin-3 in the ischemic heart. Circ Res. 2010;106:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Toko H, Takahashi H, Kayama Y, Okada S, Minamino T, Terasaki F, Kitaura Y, Komuro I. ATF6 is important under both pathological and physiological states in the heart. J Mol Cell Cardiol. 2010;49:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H, Prywes R, Lorenz JN, Mori K, Lawler J, Robbins J, Molkentin JD. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149:1257-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 39. | Doroudgar S, Glembotski CC. ATF6 [corrected] and thrombospondin 4: the dynamic duo of the adaptive endoplasmic reticulum stress response. Circ Res. 2013;112:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33-40. [PubMed] [DOI] [Full Text] |
| 41. | Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol. 2006;291:H1411-H1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324-2330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 44. | Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 602] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 45. | Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 47. | Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1088] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 48. | Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2324] [Article Influence: 93.0] [Reference Citation Analysis (1)] |
| 49. | He X, Liu Y, Sharma V, Dirksen RT, Waugh R, Sheu SS, Min W. ASK1 associates with troponin T and induces troponin T phosphorylation and contractile dysfunction in cardiomyocytes. Am J Pathol. 2003;163:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A. 2003;100:15883-15888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Gerczuk PZ, Breckenridge DG, Liles JT, Budas GR, Shryock JC, Belardinelli L, Kloner RA, Dai W. An apoptosis signal-regulating kinase 1 inhibitor reduces cardiomyocyte apoptosis and infarct size in a rat ischemia-reperfusion model. J Cardiovasc Pharmacol. 2012;60:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2547] [Cited by in RCA: 2611] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 53. | Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 54. | Logue SE, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 55. | Toth A, Jeffers JR, Nickson P, Min JY, Morgan JP, Zambetti GP, Erhardt P. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52-H60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Nickson P, Toth A, Erhardt P. PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovasc Res. 2007;73:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Mandl A, Huong Pham L, Toth K, Zambetti G, Erhardt P. Puma deletion delays cardiac dysfunction in murine heart failure models through attenuation of apoptosis. Circulation. 2011;124:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Mandl A. Puma Deletion Delays Cardiac Dysfunction in Mouse Heart Failure Models Through Attenuation of Apoptosis. 2014; Available from: URL: http://aok.pte.hu/hu/egyseg/almenu/1670/docs/phd/file/dolgozatok/2014/Mandl_Adel_PhD_dolgozat.pdf. |
| 59. | Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, Mollereau B. ER stress inhibits neuronal death by promoting autophagy. Autophagy. 2012;8:915-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 60. | Wang S, Chen X, Nair S, Sun D, Wang X, Ren J. Deletion of protein tyrosine phosphatase 1B obliterates endoplasmic reticulum stress-induced myocardial dysfunction through regulation of autophagy. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3060-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 62. | Zhang Y, Wang C, Zhou J, Sun A, Hueckstaedt LK, Ge J, Ren J. Complex inhibition of autophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1919-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Zhang C, Syed TW, Liu R, Yu J. Role of Endoplasmic Reticulum Stress, Autophagy, and Inflammation in Cardiovascular Disease. Front Cardiovasc Med. 2017;4:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 64. | Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, He X, Wang N. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol. 2014;13:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 65. | Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012;97:2231-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 66. | Ceylan-Isik AF, Sreejayan N, Ren J. Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol. 2011;50:107-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 67. | Zhang X, Gibson ME, Li ZL, Zhu XY, Jordan KL, Lerman A, Lerman LO. Autophagy Portends the Level of Cardiac Hypertrophy in Experimental Hypertensive Swine Model. Am J Hypertens. 2016;29:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Tanno M, Kuno A, Horio Y, Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic Res Cardiol. 2012;107:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 69. | Guilbert A, Prola A, Ventura-Clapier V, Lemaire C. P79 Sirt1 modulates endoplasmic reticulum stress-induced autophagy in heart. Cardiovasc Res. 2014;103:S13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14460] [Cited by in RCA: 16089] [Article Influence: 1005.6] [Reference Citation Analysis (2)] |
| 71. | Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8672] [Cited by in RCA: 8878] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 72. | Byrd AE, Aragon IV, Brewer JW. MicroRNA-30c-2* limits expression of proadaptive factor XBP1 in the unfolded protein response. J Cell Biol. 2012;196:689-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Belmont PJ, Chen WJ, Thuerauf DJ, Glembotski CC. Regulation of microRNA expression in the heart by the ATF6 branch of the ER stress response. J Mol Cell Cardiol. 2012;52:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Zhang WG, Chen L, Dong Q, He J, Zhao HD, Li FL, Li H. Mmu-miR-702 functions as an anti-apoptotic mirtron by mediating ATF6 inhibition in mice. Gene. 2013;531:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Tang Q, Len Q, Liu Z, Wang W. Overexpression of miR-22 attenuates oxidative stress injury in diabetic cardiomyopathy via Sirt 1. Cardiovasc Ther. 2018;36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 76. | Chen M, Ma G, Yue Y, Wei Y, Li Q, Tong Z, Zhang L, Miao G, Zhang J. Downregulation of the miR-30 family microRNAs contributes to endoplasmic reticulum stress in cardiac muscle and vascular smooth muscle cells. Int J Cardiol. 2014;173:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | van Mil A, Grundmann S, Goumans MJ, Lei Z, Oerlemans MI, Jaksani S, Doevendans PA, Sluijter JP. MicroRNA-214 inhibits angiogenesis by targeting Quaking and reducing angiogenic growth factor release. Cardiovasc Res. 2012;93:655-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255-18260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1183] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 79. | Wang JM, Qiu Y, Yang ZQ, Li L, Zhang K. Inositol-Requiring Enzyme 1 Facilitates Diabetic Wound Healing Through Modulating MicroRNAs. Diabetes. 2017;66:177-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 80. | Belmadani S, Matrougui K. The Unraveling Truth About IRE1 and MicroRNAs in Diabetes. Diabetes. 2017;66:23-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Afonyushkin T, Oskolkova OV, Bochkov VN. Permissive role of miR-663 in induction of VEGF and activation of the ATF4 branch of unfolded protein response in endothelial cells by oxidized phospholipids. Atherosclerosis. 2012;225:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Behrman S, Acosta-Alvear D, Walter P. A CHOP-regulated microRNA controls rhodopsin expression. J Cell Biol. 2011;192:919-927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 683] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 84. | Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1994] [Cited by in RCA: 1959] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 85. | Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab. 2010;12 Suppl 2:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 86. | Rivard AL, Steer CJ, Kren BT, Rodrigues CM, Castro RE, Bianco RW, Low WC. Administration of tauroursodeoxycholic acid (TUDCA) reduces apoptosis following myocardial infarction in rat. Am J Chin Med. 2007;35:279-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Rani S, Sreenivasaiah PK, Kim JO, Lee MY, Kang WS, Kim YS, Ahn Y, Park WJ, Cho C, Kim DH. Tauroursodeoxycholic acid (TUDCA) attenuates pressure overload-induced cardiac remodeling by reducing endoplasmic reticulum stress. PLoS One. 2017;12:e0176071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 88. | Groenendyk J, Lee D, Jung J, Dyck JR, Lopaschuk GD, Agellon LB, Michalak M. Inhibition of the Unfolded Protein Response Mechanism Prevents Cardiac Fibrosis. PLoS One. 2016;11:e0159682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 89. | Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim DH. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun. 2012;421:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 90. | Jian L, Lu Y, Lu S, Lu C. Chemical Chaperone 4-Phenylbutyric Acid Reduces Cardiac Ischemia/Reperfusion Injury by Alleviating Endoplasmic Reticulum Stress and Oxidative Stress. Med Sci Monit. 2016;22:5218-5227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Wiersma M, Meijering RAM, Qi XY, Zhang D, Liu T, Hoogstra-Berends F, Sibon OCM, Henning RH, Nattel S, Brundel BJJM. Endoplasmic Reticulum Stress Is Associated With Autophagy and Cardiomyocyte Remodeling in Experimental and Human Atrial Fibrillation. J Am Heart Assoc. 2017;6:pii: e006458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 92. | Fu HY, Sanada S, Matsuzaki T, Liao Y, Okuda K, Yamato M, Tsuchida S, Araki R, Asano Y, Asanuma H, Asakura M, French BA, Sakata Y, Kitakaze M, Minamino T. Chemical Endoplasmic Reticulum Chaperone Alleviates Doxorubicin-Induced Cardiac Dysfunction. Circ Res. 2016;118:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 93. | Boklage SH, Malangone-Monaco E, Lopez-Gonzalez L, Ding Y, Henriques C, Elassal J. Statin Utilization Patterns and Outcomes for Patients with Acute Coronary Syndrome During and Following Inpatient Admissions. Cardiovasc Drugs Ther. 2018;32:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Zhao H, Liao Y, Minamino T, Asano Y, Asakura M, Kim J, Asanuma H, Takashima S, Hori M, Kitakaze M. Inhibition of cardiac remodeling by pravastatin is associated with amelioration of endoplasmic reticulum stress. Hypertens Res. 2008;31:1977-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Xu Z, Okamoto H, Akino M, Onozuka H, Matsui Y, Tsutsui H. Pravastatin attenuates left ventricular remodeling and diastolic dysfunction in angiotensin II-induced hypertensive mice. J Cardiovasc Pharmacol. 2008;51:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Han ZH, Wu XS, Zhang XX, Hu R, Zhao H, Wang CM, Ren XJ, Jiang TY, Zhang WD, Chen F. [The primary observation on the effect of pravastatin to non-ischemic heart failure]. Zhonghua Xinxueguanbing Zazhi. 2007;35:603-606. [PubMed] |
| 97. | Nakaya R, Uzui H, Shimizu H, Nakano A, Mitsuke Y, Yamazaki T, Ueda T, Lee JD. Pravastatin suppresses the increase in matrix metalloproteinase-2 levels after acute myocardial infarction. Int J Cardiol. 2005;105:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Liao Y, Takashima S, Zhao H, Asano Y, Shintani Y, Minamino T, Kim J, Fujita M, Hori M, Kitakaze M. Control of plasma glucose with alpha-glucosidase inhibitor attenuates oxidative stress and slows the progression of heart failure in mice. Cardiovasc Res. 2006;70:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Song XJ, Yang CY, Liu B, Wei Q, Korkor MT, Liu JY, Yang P. Atorvastatin inhibits myocardial cell apoptosis in a rat model with post-myocardial infarction heart failure by downregulating ER stress response. Int J Med Sci. 2011;8:564-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 100. | Sola S, Mir MQ, Lerakis S, Tandon N, Khan BV. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol. 2006;47:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 101. | Xu J, Chen L, Jiang Z, Li L. Biological functions of Elabela, a novel endogenous ligand of APJ receptor. J Cell Physiol. 2018;233:6472-6482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 102. | Luo X, Liu J, Zhou H, Chen L. Apelin/APJ system: A critical regulator of vascular smooth muscle cell. J Cell Physiol. 2018;233:5180-5188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Huang Z, Wu L, Chen L. Apelin/APJ system: A novel potential therapy target for kidney disease. J Cell Physiol. 2018;233:3892-3900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 104. | Gao LR, Xu RY, Zhang NK, Chen Y, Wang ZG, Zhu ZM, Fei YX, Cao Y, Xu HT, Yang Y. Increased apelin following bone marrow mononuclear cell transplantation contributes to the improvement of cardiac function in patients with severe heart failure. Cell Transplant. 2009;18:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan AD, Newby DE. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121:1818-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 106. | Zhang Z, Yu B, Tao GZ. Apelin protects against cardiomyocyte apoptosis induced by glucose deprivation. Chin Med J (Engl). 2009;122:2360-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 107. | Ceylan-Isik AF, Kandadi MR, Xu X, Hua Y, Chicco AJ, Ren J, Nair S. Apelin administration ameliorates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol. 2013;63:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 108. | Xie F, Wu D, Huang SF, Cao JG, Li HN, He L, Liu MQ, Li LF, Chen LX. The endoplasmic reticulum stress-autophagy pathway is involved in apelin-13-induced cardiomyocyte hypertrophy in vitro. Acta Pharmacol Sin. 2017;38:1589-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Hall DT, Griss T, Ma JF, Sanchez BJ, Sadek J, Tremblay AMK, Mubaid S, Omer A, Ford RJ, Bedard N, Pause A, Wing SS, Di Marco S, Steinberg GR, Jones RG, Gallouzi IE. The AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), but not metformin, prevents inflammation-associated cachectic muscle wasting. EMBO Mol Med. 2018;10:pii: e8307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 110. | Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des. 2014;20:5507-5509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 504] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 111. | Viollet B, Guigas B, Leclerc J, Hébrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf). 2009;196:81-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 385] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 112. | Malick R, Belmadani S. Endoplasmic Reticulum Stress and Heart Complication in Diabetes. J Diabetes Metab. 2015;6:12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, Sun X. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |