Published online May 26, 2019. doi: 10.4330/wjc.v11.i5.149
Peer-review started: February 11, 2019
First decision: April 16, 2019
Revised: April 19, 2019
Accepted: May 14, 2019
Article in press: May 14, 2019
Published online: May 26, 2019
Processing time: 112 Days and 17.6 Hours
The prevalence of atrial fibrillation (AF) is on the rise in the aging population with congenital heart disease (CHD). A few case series have described the feasibility and early outcomes associated with radiofrequency catheter ablation of AF centered on electrically isolating pulmonary veins (PVs) in patients with CHD. In contrast, cryoballoon ablation has not previously been studied in this patient population despite its theoretical advantages, which include a favorable safety profile and shorter procedural time.
To assess the safety and feasibility of cryoballoon ablation for AF in an initial cohort of patients with CHD.
The study population consisted of consecutive patients with CHD who underwent cryoballoon ablation for AF at the Montreal Heart Institute between December 2012 and June 2017. Procedural complications, acute success, and 1-year freedom from recurrent AF after a single procedure with or without antiarrhythmic drugs were assessed. Procedures were performed under conscious sedation. Left atrial access was obtained via a single transseptal puncture or through an existing atrial septal defect (ASD). Cryoballoon occlusion was assessed by distal injection of 50% diluted contrast into the PV. At least one 240-s cryothermal application was performed when complete PV occlusion was obtained. Following ablation, patients were routinely followed at outpatient visits at 1, 3, 6, and 12 mo, and annually thereafter.
Ten patients with a median age of 57.9 (interquartile range 48.2-61.7 years) and 60% female, met the inclusion criteria and were followed for 2.8 (interquartile range 1.4-4.5) years. Two patients had moderately complex CHD (sinus venosus ASD with partial anomalous pulmonary venous return; aortic coarctation with a persistent left superior vena cava), with the remainder having simple defects. AF was paroxysmal in 8 (80.0%) and persistent in 2 (20.0%) patients. The PV anatomy was normal in 6 (60.0%) patients. Four had left common PV (n = 3) and/or 3 right PV (n = 2). Electrical pulmonary vein isolation (PVI) was acutely successful in all patients. One patient had transient phrenic nerve palsy that recovered during the intervention. No major complications occurred. One year after a single ablation procedure, 6 (60%) patients remained AF-free. One patient with recurrent AF had recovered PV conduction and underwent a second PVI procedure. A second patient underwent ablation of an extra-pulmonary vein trigger for AF.
Cryoballoon ablation for AF is feasible and safe in patients with simple and moderate forms of CHD, with an excellent acute success rate and modest 1-year freedom from recurrent AF.
Core tip: A few studies have described radiofrequency ablation for atrial fibrillation (AF) in patients with congenital heart disease (CHD). Herein, we report the first case series of cryoballoon ablation for the treatment of AF in patients with CHD. Ten patients with CHD, median age 57.9 years, underwent cryoballoon ablation and were followed for a median of 2.8 years. Pulmonary vein isolation was acutely successful in all patients. No major complications occurred. One year after a single procedure, 6 (60%) patients remained AF-free. In conclusion, cryoballoon ablation is feasible and appears to be safe, with an excellent acute success rate and modest 1-year freedom from recurrent AF.
- Citation: Abadir S, Waldmann V, Dyrda K, Laredo M, Mondésert B, Dubuc M, Khairy P. Feasibility and safety of cryoballoon ablation for the treatment of atrial fibrillation in patients with congenital heart disease. World J Cardiol 2019; 11(5): 149-158
- URL: https://www.wjgnet.com/1949-8462/full/v11/i5/149.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i5.149
Patients with congenital heart disease (CHD) tend to live longer, but they are subject to numerous late complications of which arrhythmias are prominent[1]. Atrial arrhythmias are the leading cause of morbidity and hospitalizations, with an estimated prevalence of 50% by the age of 65 years[2,3]. Although intra-atrial reentrant tachycardia (IART) is the most pervasive arrhythmia in patients with CHD, the prevalence of atrial fibrillation (AF) is on the rise in the aging population. Indeed, AF has already surpassed IART as the most common presenting arrhythmia in patients with CHD aged over 50 years[4].
A few case series have described the feasibility and early outcomes associated with radiofrequency catheter ablation of AF centered on electrically isolating pulmonary veins (PVs) in patients with CHD. In the largest series of 57 patients, single procedure arrhythmia-free survival rates on or off antiarrhythmic drugs were 63% at 1 year and 22% at 5 years[5]. Cryoballoon ablation for paroxysmal AF is generally considered non-inferior to radiofrequency ablation in patients with normal hearts or acquired heart disease[6]. However, cryoballoon ablation has not previously been assessed in patients with CHD. The theoretical advantages include the favorable safety profile and shorter procedural time, which could be of value when targeting multiple substrates, as is often the case in patients with CHD[6,7]. Therefore, we assessed our early experience with cryoballoon ablation in patients with CHD.
The study population consisted of all consecutive patients with CHD who underwent cryoballoon ablation for AF at the Montreal Heart Institute between December 2012 and June 2017. Eligible patients were identified through the institutional adult CHD catheter ablation database and the tailored informatics system, CONGENERATE, which contains comprehensive diagnostic and procedural codes for patients followed at the Montreal Heart Institute Adult Congenital Center. All patients had symptomatic and drug refractory paroxysmal or early persistent AF (< 1 year duration), documented by a surface electrocardiogram (ECG). Written informed consent for procedures was obtained in all patients. The study was approved by the local institutional review bo-ard.
Patients were anticoagulated a minimum of 4 wk prior to the intervention. For those on vitamin K antagonists, the anticoagulant was continued with a targeted international normalized ratio of 2 to 3. Direct oral anticoagulants were interrupted 24 h prior to the procedure. All patients underwent pre-procedural transesophageal echocardiography to rule-out thrombus, in addition to cardiac computed tomography (CT) or magnetic resonance imaging (MRI) to assess PV anatomy and exclude PV stenosis.
Procedures were performed under conscious sedation, with boluses of remifentanil for analgesia and a continuous infusion of propofol. A diagnostic 6-French deflectable decapolar catheter was positioned in the coronary sinus and a 9-French 9-MHz intracardiac echocardiography (ICE) catheter placed in the right atrium. Left atrial access was obtained via a single transseptal puncture or through an existing atrial septal defect (ASD) under ICE and fluoroscopic guidance. In the setting of an ASD closure device, ICE was used to identify areas of the native septum considered suitable for the transseptal puncture. Intravenous heparin was administered as a combination of boluses and an infusion to achieve and maintain an activated clotting time (ACT) > 300 s after transseptal access. The standard transseptal sheath was exchanged for a 15-French FlexCath (Medtronic CryoCath LP, Montreal, Canada) steerable sheath, through which a first- or second-generation 23- or 28-mm cryoballoon was advanced to the left atrium.
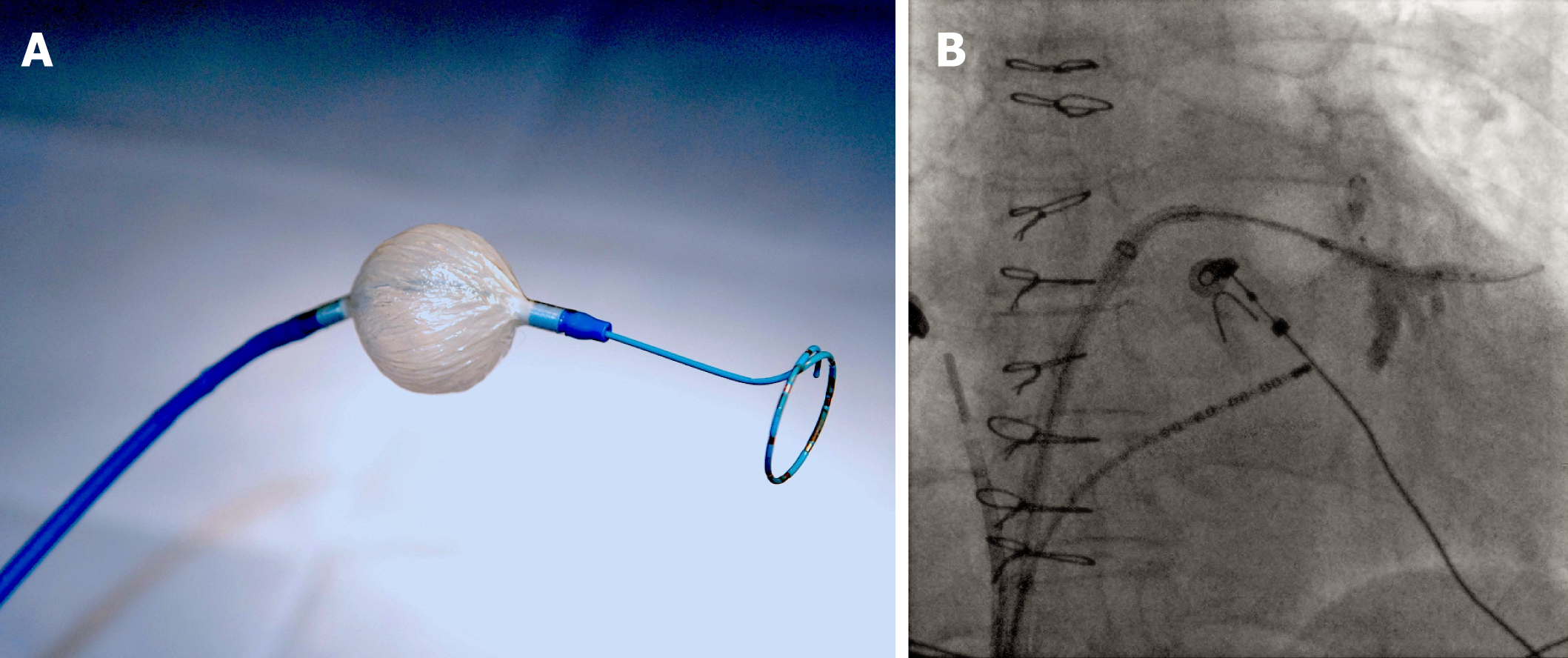
The size of the cryoballoon was selected according to the PV diameters determined by CT, MRI, or PV angiography, with a preference for the larger 28-mm size (Figure 1A). PV potentials were recorded by a circular mapping catheter (Achieve, Medtronic, Minneapolis, MN, USA) introduced in the central lumen of the cryoballoon catheter. The Achieve catheter was advanced distally into the PV to optimize support during cryoballoon positioning. The cryoballoon was inflated within the left atrium under fluoroscopic guidance and advanced to the PV ostium. The Achieve catheter was then withdrawn proximally to record PV potentials. Cryoballoon occlusion was assessed by distal injection of 50% diluted contrast into the PV. At least one standard 240-s cryothermal application was delivered when complete PV occlusion was obtained. Additional lesions were not systematically applied in the absence of a clinical reason to do so, such as delayed pulmonary vein isolation (PVI) or relatively warm ablation temperatures[8,9].
During cryoballoon ablation of right-sided PVs, diaphragmatic excursion was monitored by abdominal palpation while pacing the right phrenic nerve with the decapolar catheter positioned at the superior vena cava cranial to the right superior PV. In addition, diaphragmatic electromyographic monitoring of the compound motor action potential was systematically performed using a technique described previously[10,11]. The procedural endpoint was PVI, as assessed by entrance and exit block. No extra-PV substrate was systematically targeted, although additional arrhythmias were also treated.
Oral anticoagulation was restarted the evening following the intervention, typically 6 h post-procedure, and continued for a minimum of 6 mo. Patients were discharged home within 24 h. All patients were treated with proton-pump inhibitors for 4 wk. The decision to pursue antiarrhythmic therapy post-procedure was at the physician’s discretion based on clinical elements. Patients were routinely followed at outpatient visits with ECG recordings at 1, 3, 6, and 12 mo, and then annually. Regular telephone interviews were also performed and medical consultations were promptly scheduled in the event of symptoms suggestive of arrhythmia. For patients with recurrent symptoms not captured by ECGs, 24-h Holter and/or event recorder monitoring was performed. Recurrence was defined as any episode of AF lasting more than 30 s after a 3-mo blanking period. The primary endpoint was 1-year freedom from recurrent AF after a single procedure, with or without antiarrhythmic drugs.
Continuous variables are presented as median and interquartile range (IQR; 25th-75th percentile) and categorical variables as frequencies and percentages. Recurrence-free survival was plotted using the Kaplan-Meier product limit method. Complete data were available for all patients. Considering the descriptive nature of the study, inferential statistics were not performed. Statistical analysis was conducted using R software, version 3.3.2 (R Project for Statistical Computing, Vienna, Austria).
Ten patients with a median age of 57.9 (IQR 48.2-61.7) years and 60% female, met the inclusion criteria and underwent cryoballoon ablation for AF. Baseline clinical characteristics are summarized in Table 1. Eight patients had simple forms of CHD [i.e., ASD (n = 6), ASD associated with ventricular septal defect (VSD; n = 1), and quadricuspid aortic valve with aortic stenosis (n = 1)]. Two patients had moderately complex CHD [i.e., sinus venosus ASD with partial anomalous pulmonary venous return (n = 1; Figure 1B), and aortic coarctation with a persistent left superior vena cava (n = 1)]. Three patients with ASDs had percutaneous device closure 3 to 6 months after the AF ablation procedure. In the remaining 7 patients, cryoballoon ablation was performed a median of 15.5 (IQR 8.2-30.3) years after the repair of CHD (Table 2).
| n = 10 | |
| Age, yr | 57.9 (48.2-61.7) |
| Female gender, n (%) | 6 (60.0) |
| Type of congenital heart disease, n (%) | |
| Simple | 8 (80.0) |
| Atrial septal defect | 6 (60.0) |
| Atrial and ventricular septal defects | 1 (10.0) |
| Quadricuspid aortic valve with aortic stenosis | 1 (10.0) |
| Moderate | 2 (20.0) |
| Sinus venosus atrial septal defect with PAPVR | 1 (10.0) |
| Aortic coarctation with persistent left superior vena cava | 1 (10.0) |
| Age at repair, yr | 44.3 (12.9-54.7) |
| Hypertension, n (%) | 5 (50.0) |
| Dyslipidemia, n (%) | 3 (30.0) |
| Diabetes mellitus, n (%) | 1 (10.0) |
| Body mass index > 30 kg/m2, n (%) | 2 (20.0) |
| Current smoker, n (%) | 1 (10.0) |
| Coronary artery disease, n (%) | 3 (30.0) |
| Symptoms/signs associated with atrial fibrillation, n (%) | |
| Palpitations | 10 (100.0) |
| Dyspnea | 8 (80.0) |
| Congestive heart failure | 2 (20.0) |
| Prior hospitalization for atrial fibrillation, n (%) | 7 (70.0) |
| Left ventricular ejection fraction, % | 60 (55-60) |
| Left atrial volume, mL/m2 | 34.5 (27.3-44.0) |
| Pattern of atrial fibrillation, n (%) | |
| Paroxysmal | 8 (80.0) |
| Persistent | 2 (20.0) |
| Time from diagnosis of atrial fibrillation to procedure, yr | 4.6 (0.9-10.3) |
| Number of antiarrhythmic drugs tried | 2 (2-3) |
| Pharmacological therapy, n (%) | |
| Antiarrhythmic drug | 10 (100.0) |
| Beta-blockers | 7 (70.0) |
| Amiodarone | 3 (30.0) |
| Sotalol | 2 (20.0) |
| Flecainide | 2 (20.0) |
| Propafenone | 1 (10.0) |
| Dofetilide | 1 (10.0) |
| Dronedarone | 1 (10.0) |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 4 (40.0) |
| Anticoagulant | 8 (80.0) |
| Diuretic | 2 (20.0) |
| Patient # | Age(yr) | Sex | CHD | Type of repair | Age at repair(yr) | Age at first AF(yr) | AF pattern | Number AADs | LA volume (mL/m2) |
| 1 | 46.4 | F | ASD + VSD | Surgical patch | 1.5 | 38.5 | Paroxysmal | 5 | 27 |
| 2 | 55.8 | F | ASD | Percutaneous device | 55.7 | 55.3 | Paroxysmal | 5 | 52 |
| 3 | 60.0 | F | SVASD + PAPVR | Surgical patch | 44.3 | 46.3 | Paroxysmal | 2 | 26 |
| 4 | 69.2 | F | ASD | Percutaneous device | 53.7 | 67.8 | Paroxysmal | 2 | 45 |
| 5 | 69.5 | F | ASD | Surgical patch | 24.3 | 68.6 | Paroxysmal | 2 | 39 |
| 6 | 62.3 | F | ASD | None | N/A | 61.4 | Paroxysmal | 2 | 23 |
| 7 | 15.4 | M | AoCo + LSVC | Surgical AoCo repair | 0.0 | 14.4 | Paroxysml | 3 | 30 |
| 8 | 59.9 | M | Quadricuspid AS | Aortic valvuloplasty | 59.0 | 37.9 | Persistent | 3 | 45 |
| 9 | 38.8 | M | ASD | None | N/A | 28.5 | Paroxysmal | 2 | 28 |
| 10 | 53.4 | M | ASD | None | N/A | 53.0 | Persistent | 2 | 41 |
All patients experienced palpitations during AF episodes. In addition, 8 (80.0%) patients reported dyspnea, with 2 (20.0%) having associated congestive heart failure. Seven (70.0%) patients had unplanned hospitalizations for AF, and 6 (60.0%) had electrical cardioversions. The AF pattern was paroxysmal in 8 (80.0%) and early persistent in 2 (20.0%). Patients were referred for AF ablation a median of 4.6 (IQR 0.9-10.3) years after the initial diagnosis of AF and had received a median of 2 (IQR 2-3) antiarrhythmic drugs. All patients had preserved left ventricular ejection fractions (when in sinus rhythm), with a median indexed left atrial volume of 34.5 (IQR 27.3-44.0) mL/m2, as shown by echocardiography. IART and/or focal atrial tachycardia (FAT) were also documented in 5 (50.0%) patients, with one having had a prior catheter ablation procedure (for a cavotricuspid isthmus-dependent IART, lateral right atrial IART, and inferoseptal non-automatic FAT).
The PV anatomy was normal in 6 (60.0%) patients. Two patients had a left common PV (LCPV), 1 had a LCPV with 3 right PVs, and 1 had 3 right PVs. In all patients, left atrial access was obtained through a portion of the native or surgically repaired atrial septum. A single 28-mm cryoballoon was used in 6 (60.0%) and a single 23-mm cryoballoon in 3 (30.0%) patients. In one patient, both a 28- and 23-mm cryoballoon was used. The main procedural characteristics are summarized in Table 3. PVI was achieved in all patients. Transient phrenic nerve palsy occurred in one patient, requiring prompt termination of the cryoballoon application. Diaphragmatic excursion fully recovered during the intervention. No major complications occurred.
| n = 10 | |
| Access to the left atrium, n (%) | |
| Across an atrial septal defect | 3 (30.0) |
| Trans-septal puncture across the native septum | 5 (50.0) |
| Trans-septal puncture across a surgical patch | 2 (20.0) |
| Trans-septal puncture across a percutaneous closure device | 0 (0.0) |
| Cryoballoon size, n (%) | |
| 23 mm | 4 (40.0) |
| 28 mm | 7 (70.0) |
| Total cryoablation time, s | |
| Left superior pulmonary vein | 374 (252-475) |
| Left inferior pulmonary vein | 480 (240-480) |
| Left common pulmonary vein | 480 (480-700) |
| Right superior pulmonary vein | 360 (261-453) |
| Right inferior pulmonary vein | 315 (247-450) |
| Number of applications | |
| Left superior pulmonary vein | 2 (1.5-2.0) |
| Left inferior pulmonary vein | 1 (1.0-2.0) |
| Left common pulmonary vein | 2 (2.0-3.5) |
| Right superior pulmonary vein | 2 (1.25-2.75) |
| Right inferior pulmonary vein | 2 (1.0-2.0) |
| Minimal temperature reached, oC | |
| Left superior pulmonary vein | -49 (-49, -51) |
| Left inferior pulmonary vein | -45 (-41, -52) |
| Left common pulmonary vein | -48 (-46, -54) |
| Right superior pulmonary vein | -45 (-40, -51) |
| Right inferior pulmonary vein | -45 (-39, -54) |
| Total procedural time, min | 183.0 (152.5, 224.0) |
| Total fluoroscopy time, min | 33.5 (27.5-43.0) |
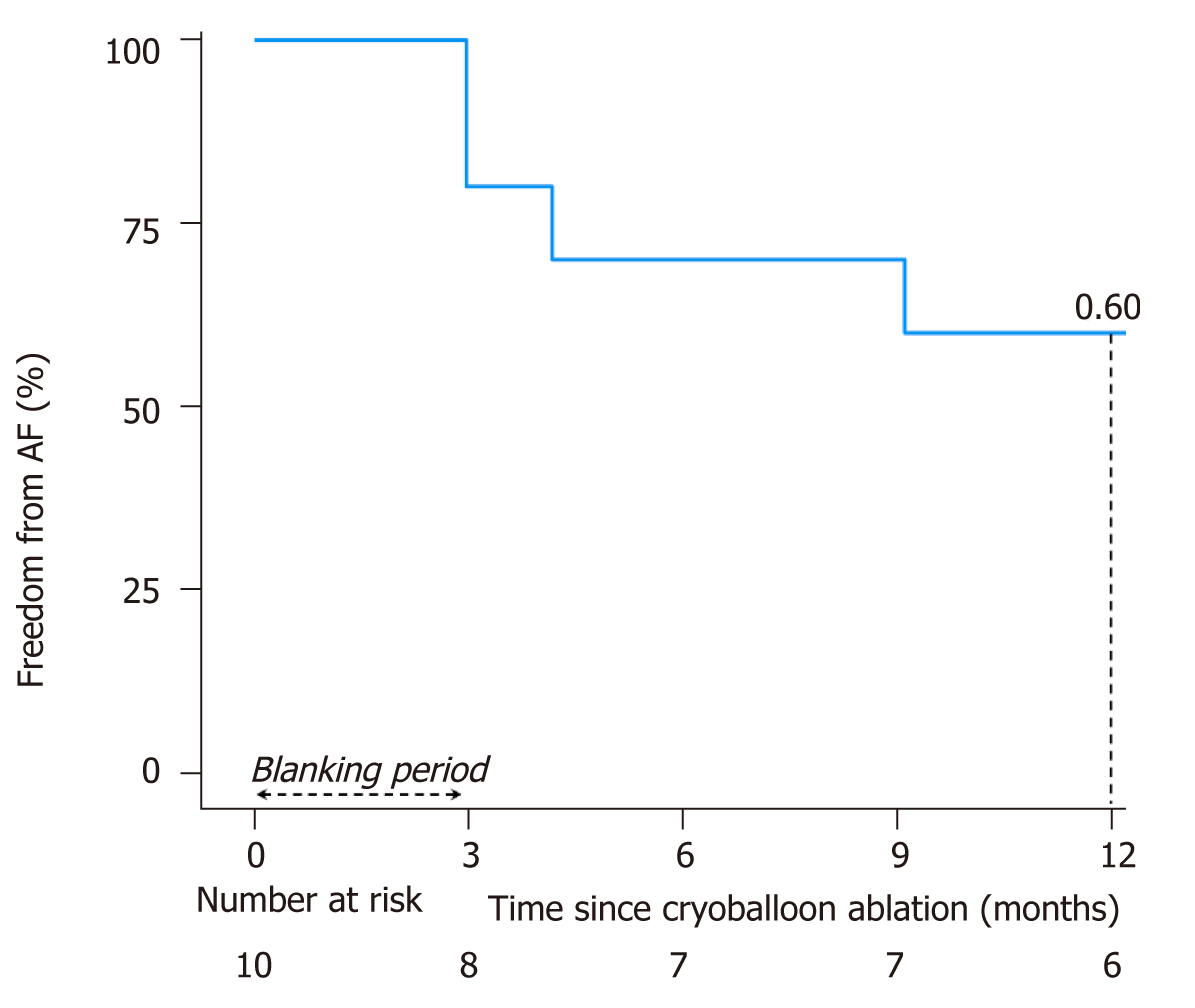
Patients were followed for a median of 2.8 (IQR 1.4 to 4.5) years after ablation. One year after a single procedure, 6 (60%) patients remained AF-free, including 4 (66.7%) without antiarrhythmic agents. Propafenone was continued in 2 patients. Freedom from AF is plotted in Figure 2. Two of the four patients with recurrent AF had a subsequent catheter ablation procedure. One had recovered PV conduction and underwent antral PVI with radiofrequency energy. In the other patient, AF was triggered by a scar-based IART circuit that was ablated with radiofrequency energy, along with the cavotricuspid isthmus.
This study is the first to report the feasibility and safety of cryoballoon ablation for AF in patients with CHD. The main findings were as follows: The procedures were acutely successful in all patients, no major complications occurred, and the 1-year single-procedure success rate was modest and within the range reported for radiofrequency catheter ablation.
With AF poised to become the next arrhythmia epidemic after IART in patients with CHD, a better understanding of the underlying mechanisms and management are key challenges in the coming years. Atrial arrhythmias are notoriously difficult to control with antiarrhythmic drugs in patients with CHD. Moreover, in those with fragile physiologies, these arrhythmias can result in rapid hemodynamic deteri-oration, heart failure, and sudden death. The disappointing results with long-term medical therapy have contributed to the growing preference for non-pharmacological options[12,13]. However, although numerous studies have focused on IART or FAT in CHD, few have reported the acute and long-term outcomes of AF ablation and none have used the cryoballoon.
The largest report on radiofrequency catheter ablation of AF included 57 patients of whom 35 (61.4%) had mild, 10 (17.5%) had moderate, and 12 (21.1%) had severe forms of CHD[5]. If PVI failed to restore sinus rhythm, additional linear lesions were performed and complex fractionated atrial electrograms were targeted. Consistent with our results, the one-year arrhythmia-free survival rate after a single procedure was 63%. The rate then declined to 22% at 5 years. In another series of 36 patients with CHD and AF, the majority of whom had atrial (61%) or ventricular (17%) septal defects, antral PVI was performed[14]. Additional ablation sites in some patients included the superior vena cava junction, left atrial septum and posterior wall, coronary sinus ostium, and crista terminalis. After a single procedure, freedom from recurrent AF in the absence of antiarrhythmic drugs was 42% at 300 d and 27% at 4 years. These rates were not significantly different to age-matched controls without CHD. Two series of 39 and 45 patients with AF and ASD closure devices reported successful transseptal access in 90%-98%, with 76-77% having freedom from recurrent arrhythmias with or without antiarrhythmic drugs at 12 to 14 mo[15]. For more complex forms of CHD, the literature is largely limited to case reports of AF ablation[16,17].
The small sample size in our early case series precludes definitive conclusions as to whether the observed 1-year arrhythmia free survival rate of 60% is significantly lower than the 75%-80% rate reported with cryoballoon ablation in patients without CHD[6,18-20]. However, anatomical, mechanistic, and technical aspects can potentially contribute to lower success rates in patients with CHD. First, access to the left atrium can lead to an unusual course of the ablation catheter by virtue of abnormal septal anatomy, traversing an existing ASD, or puncturing at an unconventional site. The resulting lack of support can render it more difficult to achieve complete cryoballoon PV occlusion. The high number of applications in each PV and relatively lengthy fluoroscopy times reflect these technical challenges. Second, although it is well documented that most triggers of paroxysmal AF arise from PVs in patients with structurally normal hearts, this is not necessarily the case for those with CHD[21]. Anatomical differences, surgical scarring, hemodynamic sequelae, and/or hypoxic stress can contribute to a higher prevalence of extra-PV triggers, as observed in one of our patients[22]. Focal non-PV drivers for AF have been described in a few patients with CHD[23]. These drivers were characterized by circumscribed areas exhibiting continuous electrical activity coexisting with parts of the atrium activated in a regular manner. The application of radiofrequency energy at these sites terminated AF. These extra-PV substrates cannot be targeted by the cryoballoon. Third, AF ablation outcomes are more favorable in patients with a short history of AF and no extensive atrial remodeling[24]. In contrast, considering the challenges discussed, CHD patients are typically referred later in their disease course, often after failing several antiarrhythmic drugs[14]. A higher perceived level of difficulty combined with uncertain outcomes may discourage operators from considering AF ablation at an earlier stage. Delayed referral can theoretically impact results due to a higher degree of atrial structural and electrical remodeling changes. Lastly, chronic volume and pressure loads in patients with CHD result in thickening of atrial walls that can hinder the creation of durable circumferential PV ablation lesions[25].
In our study, a substantial proportion of patients had factors classically associated with AF in the general population. Hypertension, dyslipidemia, smoking, higher body mass index, and coronary artery disease were also recently associated with AF in a multicenter study of patients with heterogeneous forms of CHD[4]. This observation paves the way for future studies as to whether education and preventive risk factor management can significantly impact the AF burden in patients with CHD. Furthermore, it highlights the epidemiological changes associated with the aging CHD population and the potential interplay between CHD and acquired comorbidities.
In addition to AF, half the patients in our study had coexisting atrial arrhythmias, mainly IART, and benefitted from catheter ablation of these other substrates. The co-occurrence of AF and other atrial arrhythmias is well described in patients with CHD[4]. Considering the propensity to develop various forms of arrhythmias, catheter ablation procedures in adults with CHD should generally be considered palliative[12]. There is evidence to suggest that even if arrhythmias are not entirely eliminated, clinical outcome is improved[26].
This single-center retrospective study represents the first assessment of cryoballoon ablation for AF in CHD and demonstrates the feasibility and safety of this technique. The study is underpowered to determine the factors associated with recurrent arrhythmias. Although ECGs were systematically performed at regular follow-up intervals, continuous monitoring was symptom-based such that asymptomatic self-terminating episodes of AF may have escaped detection. The population was limited to patients with simple or moderate forms of CHD; therefore, the results should not be extrapolated to those with complex CHD. No direct comparisons of AF ablation using radiofre-quency energy in patients with CHD, or cryoballoon ablation in controls without CHD were carried out.
In conclusion, cryoballoon ablation for AF is feasible and appears to have an acceptable safety profile in patients with simple and moderate forms of CHD. In this initial experience, the acute success rate for PVI was high, with a modest 1-year event-free survival rate after a single procedure. Recurrences may be due to non-PV triggers. Further studies are required to provide mechanistic insights regarding triggers and substrates for AF in the various forms of CHD, and to compare cryo-balloon ablation with radiofrequency catheter ablation.
The prevalence of atrial fibrillation (AF) is on the rise in the growing aging population with congenital heart disease (CHD). Although a few case series have described the feasibility and early outcomes associated with radiofrequency catheter ablation of AF, cryoballoon ablation has not previously been studied in this patient population.
The theoretical advantages of cryoballoon ablation include its favorable safety profile and shorter procedural time, which could be valuable when targeting multiple arrhythmias during a single intervention, as is often the case in patients with CHD.
We sought to assess the feasibility, safety, and recurrence-free survival in our initial experience with cryoballoon ablation for AF in patients with CHD.
A single-center cohort study was conducted which enrolled consecutive patients with CHD and cryoballoon ablation for AF was carried out at the Montreal Heart Institute between December 2012 and June 2017. Procedural complications, acute success, and 1-year freedom from recurrent AF after a single procedure with or without antiarrhythmic drugs were assessed.
Ten patients with CHD and a median age of 57.9 years underwent cryoballoon ablation and were followed for a median of 2.8 years. Pulmonary vein isolation was acutely successful in all patients. No major complications occurred. One year after a single procedure, 6 (60%) patients remained AF-free.
Cryoballoon ablation for AF is feasible and appears to have an acceptable safety profile in patients with CHD. In our initial experience, the acute success rate for PVI was high, with a modest 1-year event-free survival rate after a single procedure.
Further studies are required to provide mechanistic insights regarding triggers and substrates for AF in the various forms of CHD, and to compare cryoballoon ablation with radio-frequency catheter ablation.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pastromas S, Vermeersch P S-Editor: Ji FF L-Editor: A E-Editor: Wang J
| 1. | Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 647] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 2. | Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Yang H, Kuijpers JM, de Groot JR, Konings TC, van Dijk A, Sieswerda GT, Post MC, Mulder BJM, Bouma BJ. Impact of atrial arrhythmias on outcome in adults with congenital heart disease. Int J Cardiol. 2017;248:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Labombarda F, Hamilton R, Shohoudi A, Aboulhosn J, Broberg CS, Chaix MA, Cohen S, Cook S, Dore A, Fernandes SM, Fournier A, Kay J, Macle L, Mondésert B, Mongeon FP, Opotowsky AR, Proietti A, Rivard L, Ting J, Thibault B, Zaidi A, Khairy P; AARCC. Increasing Prevalence of Atrial Fibrillation and Permanent Atrial Arrhythmias in Congenital Heart Disease. J Am Coll Cardiol. 2017;70:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Sohns C, Nürnberg JH, Hebe J, Duckeck W, Ventura R, Konietschke F, Cao C, Siebels J, Volkmer M. Catheter Ablation for Atrial Fibrillation in Adults With Congenital Heart Disease: Lessons Learned From More Than 10 Years Following a Sequential Ablation Approach. JACC Clin Electrophysiol. 2018;4:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque JP, Tondo C; FIRE AND ICE Investigators. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2016;374:2235-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1434] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 7. | Andrade JG, Khairy P, Dubuc M. Catheter cryoablation: biology and clinical uses. Circ Arrhythm Electrophysiol. 2013;6:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Dorwarth U, Schmidt M, Wankerl M, Krieg J, Straube F, Hoffmann E. Pulmonary vein electrophysiology during cryoballoon ablation as a predictor for procedural success. J Interv Card Electrophysiol. 2011;32:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Fürnkranz A, Köster I, Chun KR, Metzner A, Mathew S, Konstantinidou M, Ouyang F, Kuck KH. Cryoballoon temperature predicts acute pulmonary vein isolation. Heart Rhythm. 2011;8:821-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Franceschi F, Dubuc M, Guerra PG, Delisle S, Romeo P, Landry E, Koutbi L, Rivard L, Macle L, Thibault B, Talajic M, Roy D, Khairy P. Diaphragmatic electromyography during cryoballoon ablation: a novel concept in the prevention of phrenic nerve palsy. Heart Rhythm. 2011;8:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Franceschi F, Dubuc M, Guerra PG, Khairy P. Phrenic nerve monitoring with diaphragmatic electromyography during cryoballoon ablation for atrial fibrillation: the first human application. Heart Rhythm. 2011;8:1068-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, Deal BJ, Dearani JA, Groot Nd, Dubin AM, Harris L, Janousek J, Kanter RJ, Karpawich PP, Perry JC, Seslar SP, Shah MJ, Silka MJ, Triedman JK, Walsh EP, Warnes CA. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11:e102-e165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 13. | Hernández-Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, Chessa M, Combes N, Dagres N, Diller G, Ernst S, Giamberti A, Hebe J, Janousek J, Kriebel T, Moltedo J, Moreno J, Peinado R, Pison L, Rosenthal E, Skinner JR, Zeppenfeld K; ESC Scientific Document Group. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace. 2018;20:1719-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 14. | Philip F, Muhammad KI, Agarwal S, Natale A, Krasuski RA. Pulmonary vein isolation for the treatment of drug-refractory atrial fibrillation in adults with congenital heart disease. Congenit Heart Dis. 2012;7:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Santangeli P, Di Biase L, Burkhardt JD, Horton R, Sanchez J, Bailey S, Zagrodzky JD, Lakkireddy D, Bai R, Mohanty P, Beheiry S, Hongo R, Natale A. Transseptal access and atrial fibrillation ablation guided by intracardiac echocardiography in patients with atrial septal closure devices. Heart Rhythm. 2011;8:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Frankel DS, Shah MJ, Aziz PF, Hutchinson MD. Catheter ablation of atrial fibrillation in transposition of the great arteries treated with mustard atrial baffle. Circ Arrhythm Electrophysiol. 2012;5:e41-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kirubakaran S, Rajani R, Linton N, Kiesewetter C, Anderson D, O'Neill M. Catheter ablation for persistent atrial fibrillation in a patient with previous repair of total anomalous pulmonary venous connection. Circ Arrhythm Electrophysiol. 2013;6:e54-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN; STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 672] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 19. | Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, Hörmann P, Schmidt K, Horn N, Brinkmeier-Theofanopoulou M, Kunzmann K, Riexinger T, Schymik G, Merkel M, Schmitt C. Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients With Paroxysmal Atrial Fibrillation: The Prospective, Randomized, Controlled, Noninferiority FreezeAF Study. Circulation. 2015;132:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 20. | Aryana A, Singh SM, Kowalski M, Pujara DK, Cohen AI, Singh SK, Aleong RG, Banker RS, Fuenzalida CE, Prager NA, Bowers MR, D'Avila A, O'Neill PG. Acute and Long-Term Outcomes of Catheter Ablation of Atrial Fibrillation Using the Second-Generation Cryoballoon versus Open-Irrigated Radiofrequency: A Multicenter Experience. J Cardiovasc Electrophysiol. 2015;26:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5432] [Cited by in RCA: 5390] [Article Influence: 199.6] [Reference Citation Analysis (0)] |
| 22. | Chang HY, Lo LW, Lin YJ, Chang SL, Hu YF, Li CH, Chao TF, Chung FP, Ha TL, Singhal R, Chong E, Yin WH, Tsao HM, Hsieh MH, Chen SA. Long-term outcome of catheter ablation in patients with atrial fibrillation originating from nonpulmonary vein ectopy. J Cardiovasc Electrophysiol. 2013;24:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | de Groot NM, Zeppenfeld K, Wijffels MC, Chan WK, Blom NA, Van der Wall EE, Schalij MJ. Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery: role of circumscribed areas with heterogeneous conduction. Heart Rhythm. 2006;3:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1446] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 25. | Sherwin ED, Triedman JK, Walsh EP. Update on interventional electrophysiology in congenital heart disease: evolving solutions for complex hearts. Circ Arrhythm Electrophysiol. 2013;6:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Triedman JK, Alexander ME, Love BA, Collins KK, Berul CI, Bevilacqua LM, Walsh EP. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |