Published online Nov 26, 2019. doi: 10.4330/wjc.v11.i11.266
Peer-review started: April 23, 2019
First decision: August 2, 2019
Revised: September 8, 2019
Accepted: October 6, 2019
Article in press: October 6, 2019
Published online: November 26, 2019
Processing time: 215 Days and 12.4 Hours
Transthyretin amyloid (TTR) cardiomyopathy is a disease of insidious onset, which is often accompanied by debilitating neurological and/or cardiac complications. The true prevalence is not fully known due to its elusive presentation, being often under-recognized and usually diagnosed only late in its natural history and in older patients. Because of this, effective treatment options are usually precluded by multiple comorbidities and frailty associated with such patients. Therefore, high clinical suspicion with earlier and better detection of this disease is needed. In this review, the novel applications of multimodality imaging in the diagnostic pathway of TTR cardiomyopathy are explored. These include the complimentary roles of transthoracic echocardiography, cardiac magnetic resonance, nuclear scintigraphy and positron emission tomography in quantifying cardiac dysfunction, diagnosis and risk stratification. Recent advances in novel therapeutic options for TTR have further enhanced the importance of a timely and accurate diagnosis of this disease.
Core tip: Non-invasive diagnosis of transthyretin amyloid (TTR) cardiomyopathy is improving with significant developments in multiple imaging modalities available to date. A greater appreciation of the various strengths and limitations of these imaging modalities is vital, as is high clinical suspicion and timely investigation for the disease, which remains insidious and elusive. This is of particular relevance in light of emerging novel effective therapeutic options. This focused review aims to highlight the role of multimodality imaging in the diagnosis and risk stratification of patients with TTR cardiomyopathy.
- Citation: Traynor BP, Shamsi A, Voon V. Multi-modality imaging in transthyretin amyloid cardiomyopathy. World J Cardiol 2019; 11(11): 266-276
- URL: https://www.wjgnet.com/1949-8462/full/v11/i11/266.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i11.266
Transthyretin amyloid (TTR) cardiomyopathy is a disease characterized by extracellular accumulation of abnormal amyloid protein fibrils due to autosomal dominant hereditary mutation transmission or from a wild type (acquired) form, previously referred to as senile amyloidosis. Transthyretin is a protein primarily synthesized in the liver and can dissociate, subsequently aggregating to produce amyloid. Distinctively, TTR cardiomyopathy lies in one part of the spectrum of amyloid cardiomyopathy compared to primary systemic amyloidosis or light-chain amyloid (AL) cardiomyopathy, often due to plasma cell dyscrasia.
However, amyloid cardiomyopathy, particularly the TTR subtype, is often under-diagnosed, as patients are often asymptomatic or present with nonspecific symptoms early in the trajectory of the disease. Although certain electrocardiographic markers (i.e., low voltage QRS) may suggest the presence of amyloid cardiomyopathy, these markers are not specific, particularly for TTR[1]. Left ventricular (LV) hypertrophy criteria on electrocardiography has only been observed in 25% of TTR cardiomyopathy[2]. While biomarkers such as natriuretic peptides and troponin may be elevated in TTR cardiomyopathy, inferring worse prognosis, their utility in diagnosis of the disease is limited[3,4]. The diagnostic yield is further challenged by the utility of the gold standard of endomyocardial biopsy, which may be limited by sampling errors in early disease and false positive/negative rates of approximately 10%[5].
Therefore, the true prevalence of TTR cardiomyopathy is not fully known as it is usually diagnosed late in its natural history when the disease is well established. Previous reports using imaging and histological evidence have estimated TTR cardiomyopathy prevalence to be between 0.36% to 25% in different cohorts of elderly patients, including those with aortic stenosis and heart failure with preserved ejection fraction. These have been associated with worse outcomes[6-12]. With that, these observations support the need for higher clinical suspicion and earlier screening and diagnosis of TTR cardiomyopathy with non-invasive imaging modalities.
Indeed, the timely detection of TTR cardiomyopathy may allow earlier implementation of disease-modifying therapy, improving survival. Conventionally, orthotopic liver and/or heart transplantation has been offered to these patients as possible curative treatments, as the misfolded TTR protein is synthesized in the liver[13]. Advanced age at liver transplantation and duration of disease have been associated with increased mortality[13]. Patients are also more likely to be suitable surgical candidates at earlier stages of the disease. Furthermore, recent studies have demonstrated beneficial outcomes in patients with TTR treated with novel medical therapies[14,15]. Published data from the ATTR-ACT trial has shown significant reductions in all-cause mortality in TTR-diagnosed patients treated with Tafamidis, a novel agent with TTR stabilizing properties, along with improvements in cardiovascular-related hospitalizations and quality of life measurements[14]. The authors of this study speculate that treatment with this agent early in the disease course will convey greater benefit, similar to its effect in TTR familial amyloid neuropathy[16]. In a subpopulation of the APOLLO study, the RNA inhibitor, Patisiran, has shown statistically significant improvements in certain exploratory endpoints measuring cardiac function, including natriuretic peptide levels, LV wall thickness and global longitudinal strain[15]. These therapeutic options offer promising solutions and support the need for a timely diagnosis. Otherwise, TTR cardiomyopathy is commonly associated with long-term debilitating neurological and cardiac complications such as arrhythmias and heart failure[17].
With that, this focused review aims to highlight the role of multimodality imaging in the diagnosis and risk stratification of patients with TTR cardiomyopathy.
Echocardiography is the primary initial imaging modality performed in the investigation of amyloid cardiomyopathy when clinically suspected. While it is a widely available and inexpensive imaging modality, its ability to differentiate between amyloid cardiomyopathy subtypes is limited and when amyloid cardiomyopathy is suspected based on echocardiography, further investigations are necessary to confirm TTR cardiomyopathy.
Increased LV wall thickness, particularly in the absence of high electrocardiographic voltages, and diastolic dysfunction are among the common early echocardiographic features seen which can raise suspicion of amyloid cardiomyopathy, although the differentials for such features are wide[18,19]. In the later stages of the disease, a restrictive filling pattern and biatrial dilatation may be accompanied by pleural and/or pericardial effusions[19-21]. Although not highly specific, LV wall thickness tends to increase to a greater degree in TTR compared to AL cardiomyopathy[18].
Using myocardial strain analysis, the presence of relative apical sparing of longitudinal strain is very characteristic of amyloid cardiomyopathy and has been demonstrated as a reproducible method of accurately differentiating amyloid cardiomyopathy from other causes of LV hypertrophy. In a study comparing 55 patients with amyloid cardiomyopathy to 30 patients with LV hypertrophy due to due to either hypertrophic cardiomyopathy or aortic stenosis, the presence of relative apical longitudinal strain was 93% sensitive and 82% specific in identifying amyloid cardiomyopathy[22].
This apical sparing pattern of global circumferential strain is usually observed unless severe diastolic dysfunction is present[23]. Furthermore, this imaging technique may better aid the identification of amyloid cardiomyopathy in challenging patient subgroups with mild LV wall thickening and preserved ejection fraction[24]. Despite that, there is limited data on echocardiographic features specific to TTR cardiomyopathy. In a study of biopsy-proven TTR patients using speckle-tracking echocardiography, acquired TTR was characterized by lower LV ejection fraction, as well as lower basal and mid LV radial strain compared to inherited TTR[25].
In addition, only few echocardiographic markers have demonstrated prognostic value specific to TTR cardiomyopathy. Among these, impairment of left atrial function, using conventional and strain-derived speckle-tracking parameters, has been demonstrated in amyloid cardiomyopathy and closely correlates to LV deformation. Acquired TTR was associated with worse left atrial function when compared to inherited TTR or AL[26]. In terms of strain imaging, 4-chamber longitudinal strain was significantly associated with major adverse cardiovascular events in amyloid cardiomyopathy, superior to traditional parameters[27]. Relative apical sparing pattern of global longitudinal strain may indicate worse prognosis, particularly when combined with low LV ejection fraction[28]. In assessing the right ventricle, TAPSE can independently predict major adverse events in amyloid cardiomyopathy patients[29]. Right ventricular dilatation has also been associated with more severe cases of amyloid cardiomyopathy and infers very poor prognosis[30].
In 1975, 99mTc -methylene diphosphonate accumulation in amyloid cardiomyopathy was reported for the first time[31]. Since then, multiple bone scintigraphy tracers have been tested, although their cellular binding mechanisms are not fully known. Several of these tracers have been predominantly utilized and are described below. Scintigraphy tracer uptake in TTR cardiomyopathy has been suggested as possibly due to the increased number of small microcalcifications seen in the myocardium in TTR[32]. The presence of cardiac tracer uptake confirms amyloid cardiomyopathy but has not been able to exclusively differentiate TTR cardiomyopathy from other subtypes. In addition, the absence of tracer uptake does not rule out amyloid cardiomyopathy.
The authors of a large study of 1217 patients that underwent radionuclide scintigraphy, either 99mTechnetium-3,3-disphono-1,2-propanodicarboxylic acid (99mTc-DPD), 99mTechnetium pyrophosphate (99mTc-PYP) or 99mTc-hydroxymehtylene diphosphonate (99mTc-HMDP) proposed a non-invasive diagnostic criteria for TTR cardiomyopathy[33]. TTR was suggested by a score of 2 or 3 with the use of the Perugini visual score of myocardial radiotracer enhancement (Table 1). Grade 2 or 3 enhancement was shown to be 90% sensitive and 97% specific for TTR cardiomyopathy using this scoring system. Furthermore, when grade 2 or 3 uptake is combined with absence of monoclonal proteins in serum or urine testing, the diagnostic accuracy improves further. A specificity and positive predictive value of 100% has been demonstrated in this regard. This was consistent among all three of the radiotracers used in the study.
| Score | Cardiac uptake and bone uptake |
| Score 0 | Absent cardiac uptake and normal bone uptake |
| Score 1 | Mild cardiac uptake |
| Score 2 | Moderate cardiac uptake accompanied by attenuated bone uptake |
| Score 3 | Strong cardiac uptake with mild/absent bone uptake |
Interestingly, while absence of abnormal cardiac uptake of radionucleotide tracer confers a prognostic benefit, Perugini grade stratification at diagnosis has yet to show prognostic significance in TTR cardiomyopathy[34]. These observations are further supported by a study of a large cohort of patients undergoing scintigraphy for non-cardiac reasons. Of 12521 patients included, myocardial tracer uptake was demonstrated in 0.36%[6].
Despite the added value of nuclear scintigraphy in the diagnostic pathway of amyloid cardiomyopathy, there remains low penetrance and high variability in its utilization[35], thus indicating a greater need for standardization in technique between centres.
99mTc-DPD scintigraphy is a highly sensitive technique for imaging TTR cardiomyopathy. In a study utilizing 99mTc-DPD scintigraphy, all 158 patients with TTR and clinical cardiac involvement demonstrated cardiac tracer uptake[36]. In the diagnosis of TTR cardiomyopathy, a study comparing 15 patients with TTR cardiomyopathy to 10 patients with AL-related cardiomyopathy revealed both sensitivity and specificity of 100% in identifying the TTR cohort using 99mTc-DPD scintigraphy[37]. Another more recent study comparing a larger group of 45 patients with TTR cardiomyopathy to 34 with AL cardiomyopathy and 15 controls again showed high levels of accuracy with positive and negative predictive values of 88% and 100% using a visual score of ≥ 2[38]. 99mTc -DPD use as a modality in diagnosing and differentiating TTR from AL cardiomyopathy has also been supported by a study of a small Australian cohort of 13 TTR patients, all showing diagnostic tracer uptake, while 25% of patients with AL-related cardiac involvement showed uptake[39].
99mTc-DPD has been observed to distribute predominantly in the cardiac septal and basal segments and lowest uptake is found in the apical and apico-antero-lateral segments[40].
Furthermore, reasonable intermodality agreement 99mTc-DPD has been shown with cardiac magnetic resonance (CMR) in the identification of TTR cardiomyopathy. Significantly improved estimation of cardiac involvement was seen using 99mTc –DPD scintigraphy when compared to late gadolinium enhancement (LGE) on CMR in a study of 18 patients diagnosed with TTR. These consecutively diagnosed patients had a mean age of 50 years, 56% were female and 56% were asymptomatic[41]. Interestingly, amyloid fibril composition has been shown to affect the result of 99mTc-DPD scintigraphy. Among 55 biopsy-proven TTR patients, all of those with type A fibrils, and none of those with type B, showed tracer uptake. Type B fibrils were associated with early-onset V30M mutation and in patients carrying the Y114C mutation in inherited TTR, whereas type A was noted in all other mutations currently examined as well as in acquired TTR cardiomyopathy[42].
99mTc-PYP is currently the most commonly used form of nuclear scintigraphy. There is growing evidence behind its use of as a cardiac tracer in TTR. In a large multicenter study of 171 patients with CA, 121 due to TTR, 99mTc-PYP showed 91% sensitivity and 92% specificity in diagnosing TTR cardiomyopathy[43]. Another study demonstrated the ability of 99mTc -PYP cardiac imaging to distinguish AL from TTR cardiomyopathy with a sensitivity of 97% and specificity of 100% when heart-to-contralateral ratio of > 1.5 was used[44]. Furthermore, 99mTc-PYP scintigraphy showed reduced uptake in the apical segments of the LV in TTR. This correlates with apical sparing of longitudinal strain seen on echocardiography[45].
In addition, there may be potential to diagnose early TTR cardiomyopathy. An observational study of carriers of inherited TTR mutations included 12 asymptomatic carriers with normal echocardiographic and biochemical parameters. Cardiac 99mTc-PYP uptake was abnormal by visual scoring, comparing cardiac to bone tracer uptake, in 84%. Grade 2 or 3 tracer avidity, indicating TTR deposition, was seen in 58%[46]. However, serial 99mTc-PYP scanning has not been shown to track disease progression accurately, as demonstrated in a small study, which showed no significant change in tracer uptake after 18 mo despite obvious clinical progression of disease[47].
Radiolabelled amyloid ligands have previously been developed to investigate for amyloid deposits in the brain in Alzheimer’s disease. These tracers have also shown some utility in amyloid cardiomyopathy. Its concomitant use with nuclear scintigraphy aids in confirming localization of tracer uptake in heart. A systematic review of six studies involving the use of positron emission tomography (PET) in amyloid cardiomyopathy, including 98 patients, demonstrated a pooled sensitivity of 95% and specificity of 98% in differentiating amyloid cardiomyopathy from controls[48]. Although the individual studies have been small, due to high levels of accuracy, the use of PET and scintigraphy may potentially aid in screening early phases of TTR cardiomyopathy where structural disease may not be apparent on echocardiography or CMR[49]. This requires further exploration. Evidence of PET studies utilizing various cardiac tracers are described below.
11C-Pittsburgh compound B, a radiotracer commonly used in the investigation of Alzheimer’s disease, has the ability to identify amyloid cardiomyopathy due to both type A and type B amyloid fibrils. While this method does not distinguish between TTR and AL, it may help identify certain patients with type B amyloid fibril disease, predominantly V30M mutation-associated TTR cardiomyopathy where 99mTc-DPD scintigraphy has shown a lack of tracer uptake. However, the mechanism of this is not fully known[42,50]. In addition, the utility of this compound is limited by its very short half-life and difficult production.
18F-florbetaben PET has been shown to help identify patients with amyloid cardiomyopathy, due to TTR or AL. Percentage 18F-florbetaben retention was shown to predict myocardial dysfunction in amyloid cardiomyopathy[51]. In another study of 14 patients, 9 with AL or TTR cardiomyopathy and 5 controls, 18F-florbetapir uptake was seen in all patients with amyloid cardiomyopathy and none of the controls[49]. An autopsy study of 20 patients with autopsy-documented amyloid cardiomyopathy, either due to AL or TTR, and 10 controls, showed binding of 18F-florbetapir, a similar tracer to 18F-florbetaben, in myocardial sections in all amyloid cardiomyopathy patients and in none of the controls[52].
18F-fluorine sodium fluoride is a PET tracer that has been shown, in a small study, to differentiate biopsy-proven TTR from AL cardiomyopathy and controls. Tracer uptake was shown to be present in all of the TTR cardiomyopathy patients and none of either the AL-related patients or controls[53]. This radioisotope was also able to quantify the degree and regional distribution of tracer uptake. However, another report of two patients with TTR cardiomyopathy did not show any uptake of this tracer[54]. The authors hypothesized that specific TTR mutation may influence radioisotope uptake. Therefore, while 18F-fluorine sodium fluoride shows promise as a TTR-specific investigative and disease-monitoring tool, it requires further investigation in larger studies.
Currently, there is limited evidence regarding the utility of computed tomography (CT) in diagnosing TTR cardiomyopathy. Myocardial iodine concentration and ratio were increased in amyloid cardiomyopathy and can accurately distinguish amyloid cardiomyopathy from non-amyloid hypertrophic cardiomyopathy and healthy controls with an AUC of 0.99. At a threshold of 0.65, iodine ratio demonstrated a sensitivity of 100% and a specificity of 92% in diagnosing amyloid cardiomyopathy[55]. Myocardial extracellular volume measured using CT has been shown to accurately track laboratory and echocardiographic markers of amyloid cardiomyopathy severity and correlate with bone scintigraphy quantification of amyloid burden[56]. Furthermore, determining the myocardial extracellular volume previously required blood sampling to measure haematocrit level. However, recently, a methodology of calculating the extracellular volume, using a calculation involving the attenuation of blood, has eliminated the need for blood sampling from this process. This improves the feasibility of using CT as a potentially useful imaging modality in amyloid cardiomyopathy[57].
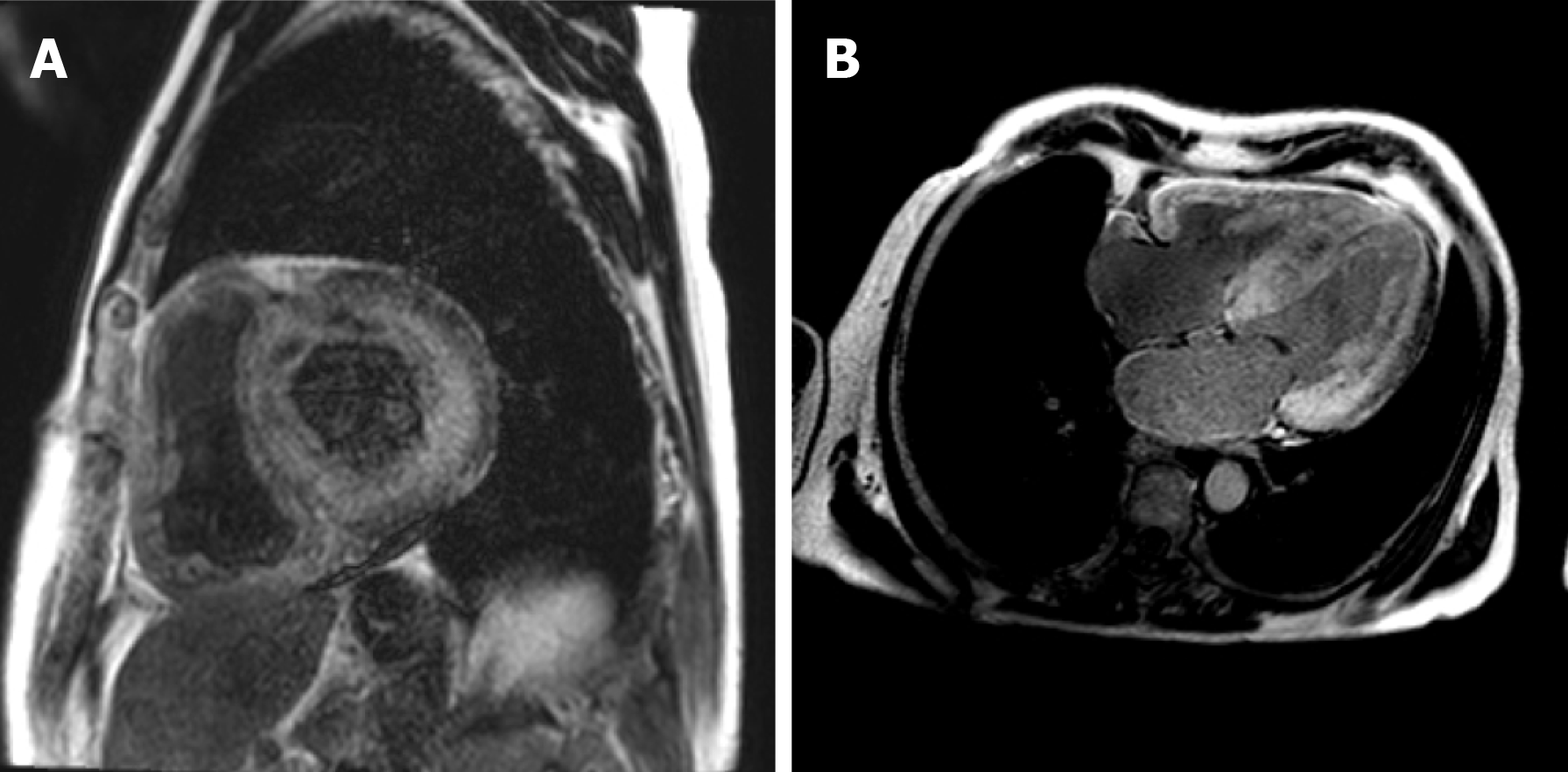
Cardiac magnetic resonance (CMR) is a useful imaging modality in the diagnosis of amyloid cardiomyopathy. Its utility in assessing abnormal myocardial interstitium was described in 2005[58]. Characteristic features seen in amyloid cardiomyopathy were described as a subendocardial tram-line pattern on LGE imaging which can progress to transmural enhancement in later stages of the disease[59] (Figure 1). Alongside LGE, conventional sequences and non-contrast techniques including native T1 mapping can help diagnose amyloid cardiomyopathy and quantify amyloid burden, although caution should be applied in the setting of ectopic beats, which is not uncommonly associated with amyloid cardiomyopathy, but may result in overlapping blood pool and subsequent false positive diffuse elevation of T1 levels. Cardiac involvement in patients with inherited TTR can be seen in patients without clinical cardiac signs or increased LV wall thickness on CMR, suggesting a potential role in detecting pre-clinical amyloid cardiomyopathy in certain at-risk patients[60].
LGE on CMR has been shown to be of high diagnostic value in amyloid cardiomyopathy and has achieved a diagnostic sensitivity of 85% and specificity of 92% in a meta-analysis of five studies[61]. Transmural pattern of LGE has been shown to be more associated with TTR than AL, although the classically described circumferential subendocardial or transmural LGE is not seen in most patients with amyloid cardiomyopathy. Other findings which are more suggestive of TTR include greater intraventricular septal wall thickness and right ventricular LGE[62]. These investigators also proposed a scoring system, derived from CMR with LGE, which differentiates TTR from AL with 87% sensitivity and 96% specificity[62].
Furthermore, the results of a study by Fontana and colleagues suggested that phase sensitive inversion recovery should replace conventional magnitude inversion recovery for LGE determination in the setting of amyloid cardiomyopathy. Phase sensitive inversion recovery helps to remove the potential confounder of incorrect inversion recovery time selection in diffuse infiltrative disease[63]. Higher proportion of left atrial LGE has been shown to have a strong association with amyloid cardiomyopathy and may help in differentiating amyloid cardiomyopathy from other cardiomyopathies. A sensitivity of 76% and specificity of 94% has been shown where left atrial LGE is > 33%, with significant reduction in left atrial emptying function[64].
However, LGE has some limitations in the investigation of amyloid cardiomyopathy. LGE does not enable assessment of diffuse changes in interstitial space secondary to amyloid deposition or quantitative assessment of expanded interstitium. This is due to inversion time adjustment to the least-enhancing myocardial region. As a result, absence of LGE does not confirm normal myocardium in amyloid cardiomyopathy[65]. Another limitation associated with gadolinium enhancement is the risk of nephropathy. Caution is warranted due to the high prevalence of renal impairment in patients with amyloid cardiomyopathy.
T1 native mapping using non-contrast MRI has shown high levels of diagnostic accuracy in detecting AL cardiomyopathy. In a study of 53 AL amyloidosis patients, 28 patients with confirmed AL cardiomyopathy were compared to 36 healthy controls and 17 patients with aortic stenosis. Accuracy of 92% was seen using a non-contrast T1 cut-off of 1020 ms[66]. Compared to TTR cardiomyopathy, T1 elevations are higher in AL cardiomyopathy but similar diagnostic and disease-tracking performance has been shown in TTR. In TTR, T1 also correlates with left atrial area and with PR interval and QRS duration on electrocardiogram[67]. Quantification methods of myocardial T1, such as weighted mean shortened modified look-locker inversion recovery sequence T1 values have been shown to be significantly higher in amyloid cardiomyopathy when compared to healthy controls[68]. T1 mapping allows detection of diffuse myocardial disease and quantitative assessments, which are limited in LGE imaging[65].
T1 mapping can accurately identify patients with LGE-confirmed cardiac involvement in TTR and correlates well with the degree of amyloid deposition[69]. As a result, it can help improve detection rates of amyloid cardiomyopathy when used in combination with LGE sequences. It is also a particularly useful tool when contrast is contraindicated due to renal impairment and when LGE artefacts occur due to poor breath-holding and arrhythmias; diagnostic problems commonly seen in these patients.
Myocardial extracellular volume is another cardiac mapping technique using CMR that is a validated indicator of myocardial fibrosis[70]. It involves T1 mapping acquisitions before and after T1-shortening contrast injection. Both T1 mapping and extracellular volume have recently been shown to perform well as diagnostic techniques in differentiating TTR from other causes of hypertrophic cardiomyopathy[71]. While not a specific feature of amyloid cardiomyopathy, it has been identified as a potential disease-marker to track therapeutic response in the reduction of hepatic amyloid burden following the use of anti-serum amyloid P component antibody in systemic amyloidosis[72].
Extracellular volume correlates with amyloid burden and has been shown to be an independent prognostic factor for survival in TTR cardiomyopathy patients[73]. Furthermore, extracellular volume has been suggested as a more robust marker in TTR cardiomyopathy when compared to T1 mapping as it has shown independent prediction of mortality, where T1 mapping has not[71]. In this regard, T1 mapping and extracellular volume are divergent when comparing TTR to AL cardiomyopathy. Extracellular volume is higher in TTR, reflecting proportionally more amyloid deposition. In contrast, native T1 levels, reflecting both interstitial and cellular changes, are lower in TTR[74]. However, these differing myocardial observations are poorly understood.
CMR-measured longitudinal strain can demonstrate the relative apical sparing and base-to-apex gradient in longitudinal strain, with significantly reduced global longitudinal strain, which is characteristic of amyloid cardiomyopathy[75]. Strain analysis using CMR can help diagnose LGE-positive amyloid cardiomyopathy patients while avoiding the need for contrast medium. Peak circumferential strain level and variability may be more sensitive when compared to LGE imaging in detecting early cardiac involvement in amyloid cardiomyopathy[76]. Basal segments strain parameters can accurately identify cardiac involvement in patients with amyloidosis[77].
Operator-independent heart deformation analysis using CMR has been shown to accurately reproduce radial and circumferential regional myocardial motion patterns, which correlate with feature-tracking indices in amyloid cardiomyopathy[78].
Reduced T2 ratio, comparing the T2 signal intensity of myocardium to skeletal muscle, has shown some utility in amyloid cardiomyopathy diagnosis and can predict mortality[79]. Myocardial oedema, as assessed by T2 mapping, is elevated in both TTR and AL cardiomyopathy, although to a higher degree in AL[80].
The use of multi-modality imaging in the diagnosis and management of suspected TTR cardiomyopathy is becoming increasingly accurate and necessary. In light of recent evidence for disease-specific therapeutic agents, high clinical suspicion coupled with earlier utilization of non-invasive imaging modalities are essential for diagnosing this insidious and elusive disease.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular
Country of origin: Ireland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bamoshmoosh M, Iacoviello M S-Editor: Dou Y L-Editor: A E-Editor: Liu MY
| 1. | Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 512] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 2. | Dungu J, Sattianayagam PT, Whelan CJ, Gibbs SD, Pinney JH, Banypersad SM, Rowczenio D, Gilbertson JA, Lachmann HJ, Wechalekar A, Gillmore JD, Hawkins PN, Anderson LJ. The electrocardiographic features associated with cardiac amyloidosis of variant transthyretin isoleucine 122 type in Afro-Caribbean patients. Am Heart J. 2012;164:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 516] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 4. | Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem. 2009;55:2098-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Ardehali H, Qasim A, Cappola T, Howard D, Hruban R, Hare JM, Baughman KL, Kasper EK. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J. 2004;147:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Longhi S, Guidalotti PL, Quarta CC, Gagliardi C, Milandri A, Lorenzini M, Potena L, Leone O, Bartolomei I, Pastorelli F, Salvi F, Rapezzi C. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovasc Imaging. 2014;7:531-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, George I, Kodali S, Leon MB, Hahn R, Bokhari S, Maurer MS. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 531] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 8. | Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, Roberts N, Hutt DF, Rowczenio DM, Whelan CJ, Ashworth MA, Gillmore JD, Hawkins PN, Moon JC. Occult Transthyretin Cardiac Amyloid in Severe Calcific Aortic Stenosis: Prevalence and Prognosis in Patients Undergoing Surgical Aortic Valve Replacement. Circ Cardiovasc Imaging. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 9. | Damy T, Costes B, Hagège AA, Donal E, Eicher JC, Slama M, Guellich A, Rappeneau S, Gueffet JP, Logeart D, Planté-Bordeneuve V, Bouvaist H, Huttin O, Mulak G, Dubois-Randé JL, Goossens M, Canoui-Poitrine F, Buxbaum JN. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J. 2016;37:1826-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | González-López E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 829] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 11. | Bennani Smires Y, Victor G, Ribes D, Berry M, Cognet T, Méjean S, Huart A, Roussel M, Petermann A, Roncalli J, Carrié D, Rousseau H, Berry I, Chauveau D, Galinier M, Lairez O. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging. 2016;32:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru-Enari S, Paetau A, Tienari PJ, Myllykangas L. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 414] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 13. | Carvalho A, Rocha A, Lobato L. Liver transplantation in transthyretin amyloidosis: issues and challenges. Liver Transpl. 2015;21:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C; ATTR-ACT Study Investigators. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1823] [Article Influence: 260.4] [Reference Citation Analysis (0)] |
| 15. | Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Planté-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA, Suhr OB. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1528] [Cited by in RCA: 2042] [Article Influence: 291.7] [Reference Citation Analysis (0)] |
| 16. | Keohane D, Schwartz J, Gundapaneni B, Stewart M, Amass L. Tafamidis delays disease progression in patients with early stage transthyretin familial amyloid polyneuropathy: additional supportive analyses from the pivotal trial. Amyloid. 2017;24:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Castaño A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20:163-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Falk RH, Quarta CC, Dorbala S. How to image cardiac amyloidosis. Circ Cardiovasc Imaging. 2014;7:552-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Klein AL, Hatle LK, Taliercio CP, Taylor CL, Kyle RA, Bailey KR, Seward JB, Tajik AJ. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;16:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 119] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Siqueira-Filho AG, Cunha CL, Tajik AJ, Seward JB, Schattenberg TT, Giuliani ER. M-mode and two-dimensional echocardiographic features in cardiac amyloidosis. Circulation. 1981;63:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 227] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 644] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 23. | Lo Q, Haluska B, Chia EM, Lin MW, Richards D, Marwick T, Thomas L. Alterations in regional myocardial deformation assessed by strain imaging in cardiac amyloidosis. Echocardiography. 2016;33:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Pagourelias ED, Mirea O, Duchenne J, Van Cleemput J, Delforge M, Bogaert J, Kuznetsova T, Voigt JU. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: A Head-to-Head Comparison of Deformation and Nondeformation Parameters. Circ Cardiovasc Imaging. 2017;10:e005588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 25. | Minamisawa M, Koyama J, Sekijima Y, Ikeda S, Kozuka A, Ebisawa S, Miura T, Motoki H, Okada A, Izawa A, Ikeda U. Comparison of the standard and speckle tracking echocardiographic features of wild-type and mutated transthyretin cardiac amyloidoses. Eur Heart J Cardiovasc Imaging. 2016;17:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Nochioka K, Quarta CC, Claggett B, Roca GQ, Rapezzi C, Falk RH, Solomon SD. Left atrial structure and function in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2017;18:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Kado Y, Obokata M, Nagata Y, Ishizu T, Addetia K, Aonuma K, Kurabayashi M, Lang RM, Takeuchi M, Otsuji Y. Cumulative Burden of Myocardial Dysfunction in Cardiac Amyloidosis Assessed Using Four-Chamber Cardiac Strain. J Am Soc Echocardiogr. 2016;29:1092-1099.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Senapati A, Sperry BW, Grodin JL, Kusunose K, Thavendiranathan P, Jaber W, Collier P, Hanna M, Popovic ZB, Phelan D. Prognostic implication of relative regional strain ratio in cardiac amyloidosis. Heart. 2016;102:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Bodez D, Ternacle J, Guellich A, Galat A, Lim P, Radu C, Guendouz S, Bergoend E, Couetil JP, Hittinger L, Dubois-Randé JL, Plante-Bordeneuve V, Deux JF, Mohty D, Damy T. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid. 2016;23:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Patel AR, Dubrey SW, Mendes LA, Skinner M, Cupples A, Falk RH, Davidoff R. Right ventricular dilation in primary amyloidosis: an independent predictor of survival. Am J Cardiol. 1997;80:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | VanAntwerp JD, O'Mara RE, Pitt MJ, Walsh S. Technetium-99m-diphosphonate accumulation in amyloid. J Nucl Med. 1975;16:238-240. [PubMed] |
| 32. | Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol. 2016;25:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation. 2016;133:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1406] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 34. | Hutt DF, Fontana M, Burniston M, Quigley AM, Petrie A, Ross JC, Page J, Martinez-Naharro A, Wechalekar AD, Lachmann HJ, Quarta CC, Rezk T, Mahmood S, Sachchithanantham S, Youngstein T, Whelan CJ, Lane T, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur Heart J Cardiovasc Imaging. 2017;18:1344-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 35. | Harb SC, Haq M, Flood K, Guerrieri A, Passerell W, Jaber WA, Miller EJ. National patterns in imaging utilization for diagnosis of cardiac amyloidosis: A focus on Tc99m-pyrophosphate scintigraphy. J Nucl Cardiol. 2017;24:1094-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Hutt DF, Quigley AM, Page J, Hall ML, Burniston M, Gopaul D, Lane T, Whelan CJ, Lachmann HJ, Gillmore JD, Hawkins PN, Wechalekar AD. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging. 2014;15:1289-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 37. | Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi-Reggiani L, Fallani F, Branzi A, Rapezzi C. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99m Tc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 658] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 38. | Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, Ferlini A, Salvi F, Gallo P, Gagliardi C, Branzi A. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Moore PT, Burrage MK, Mackenzie E, Law WP, Korczyk D, Mollee P. The Utility of 99mTc-DPD Scintigraphy in the Diagnosis of Cardiac Amyloidosis: An Australian Experience. Heart Lung Circ. 2017;26:1183-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Abulizi M, Cottereau AS, Guellich A, Vandeventer S, Galat A, Van Der Gucht A, Plante-Bordeneuve V, Dubois-Randé JL, Bodez D, Rosso J, Damy T, Itti E. Early-phase myocardial uptake intensity of 99mTc-HMDP vs 99mTc-DPD in patients with hereditary transthyretin-related cardiac amyloidosis. J Nucl Cardiol. 2018;25:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Minutoli F, Di Bella G, Mazzeo A, Donato R, Russo M, Scribano E, Baldari S. Comparison between (99m)Tc-diphosphonate imaging and MRI with late gadolinium enhancement in evaluating cardiac involvement in patients with transthyretin familial amyloid polyneuropathy. AJR Am J Roentgenol. 2013;200:W256-W265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Pilebro B, Suhr OB, Näslund U, Westermark P, Lindqvist P, Sundström T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci. 2016;121:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 43. | Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, Pozniakoff T, Ruberg FL, Miller EJ, Berk JL, Dispenzieri A, Grogan M, Johnson G, Bokhari S, Maurer MS. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis. JAMA Cardiol. 2016;1:880-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 44. | Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 470] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 45. | Sperry BW, Vranian MN, Tower-Rader A, Hachamovitch R, Hanna M, Brunken R, Phelan D, Cerqueira MD, Jaber WA. Regional Variation in Technetium Pyrophosphate Uptake in Transthyretin Cardiac Amyloidosis and Impact on Mortality. JACC Cardiovasc Imaging. 2018;11:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 46. | Haq M, Pawar S, Berk JL, Miller EJ, Ruberg FL. Can 99mTc-Pyrophosphate Aid in Early Detection of Cardiac Involvement in Asymptomatic Variant TTR Amyloidosis? JACC Cardiovasc Imaging. 2017;10:713-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Castaño A, DeLuca A, Weinberg R, Pozniakoff T, Blaner WS, Pirmohamed A, Bettencourt B, Gollob J, Karsten V, Vest JA, Chiuzan C, Maurer MS, Bokhari S. Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol. 2016;23:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 48. | Kim YJ, Ha S, Kim YI. Cardiac amyloidosis imaging with amyloid positron emission tomography: A systematic review and meta-analysis. J Nucl Cardiol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 49. | Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR, Di Carli MF, Moore SC, Falk RH. Imaging cardiac amyloidosis: a pilot study using ¹⁸F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41:1652-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 50. | Pilebro B, Arvidsson S, Lindqvist P, Sundström T, Westermark P, Antoni G, Suhr O, Sörensen J. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR). J Nucl Cardiol. 2018;25:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 51. | Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac Amyloid Imaging with 18F-Florbetaben PET: A Pilot Study. J Nucl Med. 2016;57:1733-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 52. | Park MA, Padera RF, Belanger A, Dubey S, Hwang DH, Veeranna V, Falk RH, Di Carli MF, Dorbala S. 18F-Florbetapir Binds Specifically to Myocardial Light Chain and Transthyretin Amyloid Deposits: Autoradiography Study. Circ Cardiovasc Imaging. 2015;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Morgenstern R, Yeh R, Castano A, Maurer MS, Bokhari S. 18Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis. J Nucl Cardiol. 2018;25:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Gagliardi C, Tabacchi E, Bonfiglioli R, Diodato S, Nanni C, Guidalotti P, Lorenzini M, Lodi F, Milandri A, Rapezzi C, Fanti S. Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? J Nucl Cardiol. 2017;24:746-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Chevance V, Damy T, Tacher V, Legou F, Ridouani F, Luciani A, Kobeiter H, Rahmouni A, Deux JF. Myocardial iodine concentration measurement using dual-energy computed tomography for the diagnosis of cardiac amyloidosis: a pilot study. Eur Radiol. 2018;28:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Treibel TA, Bandula S, Fontana M, White SK, Gilbertson JA, Herrey AS, Gillmore JD, Punwani S, Hawkins PN, Taylor SA, Moon JC. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr. 2015;9:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 57. | Treibel TA, Fontana M, Steeden JA, Nasis A, Yeung J, White SK, Sivarajan S, Punwani S, Pugliese F, Taylor SA, Moon JC, Bandula S. Automatic quantification of the myocardial extracellular volume by cardiac computed tomography: Synthetic ECV by CCT. J Cardiovasc Comput Tomogr. 2017;11:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 59. | Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 1151] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 60. | Deux JF, Damy T, Rahmouni A, Mayer J, Planté-Bordeneuve V. Noninvasive detection of cardiac involvement in patients with hereditary transthyretin associated amyloidosis using cardiac magnetic resonance imaging: a prospective study. Amyloid. 2014;21:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Zhao L, Tian Z, Fang Q. Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 62. | Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, Lachmann HJ, Wechalekar A, Gillmore JD, Whelan CJ, Hawkins PN, Anderson LJ. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 63. | Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, Maestrini V, Barcella W, Rosmini S, Bulluck H, Sayed RH, Patel K, Mamhood S, Bucciarelli-Ducci C, Whelan CJ, Herrey AS, Lachmann HJ, Wechalekar AD, Manisty CH, Schelbert EB, Kellman P, Gillmore JD, Hawkins PN, Moon JC. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation. 2015;132:1570-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 64. | Kwong RY, Heydari B, Abbasi S, Steel K, Al-Mallah M, Wu H, Falk RH. Characterization of Cardiac Amyloidosis by Atrial Late Gadolinium Enhancement Using Contrast-Enhanced Cardiac Magnetic Resonance Imaging and Correlation With Left Atrial Conduit and Contractile Function. Am J Cardiol. 2015;116:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Hashimura H, Kimura F, Ishibashi-Ueda H, Morita Y, Higashi M, Nakano S, Iguchi A, Uotani K, Sugimura K, Naito H. Radiologic-Pathologic Correlation of Primary and Secondary Cardiomyopathies: MR Imaging and Histopathologic Findings in Hearts from Autopsy and Transplantation. Radiographics. 2017;37:719-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN, Neubauer S, Moon JC. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 500] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 67. | Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, Pica S, Castelletti S, Piechnik SK, Robson MD, Gilbertson JA, Rowczenio D, Hutt DF, Lachmann HJ, Wechalekar AD, Whelan CJ, Gillmore JD, Hawkins PN, Moon JC. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 68. | van den Boomen M, Slart RHJA, Hulleman EV, Dierckx RAJO, Velthuis BK, van der Harst P, Sosnovik DE, Borra RJH, Prakken NHJ. Native T1 reference values for nonischemic cardiomyopathies and populations with increased cardiovascular risk: A systematic review and meta-analysis. J Magn Reson Imaging. 2018;47:891-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Oda S, Utsunomiya D, Morita K, Nakaura T, Yuki H, Kidoh M, Hirata K, Taguchi N, Tsuda N, Shiraishi S, Namimoto T, Hirakawa K, Takashio S, Izumiya Y, Yamamuro M, Hokimoto S, Tsujita K, Ueda M, Yamashita T, Ando Y, Yamashita Y. Cardiovascular magnetic resonance myocardial T1 mapping to detect and quantify cardiac involvement in familial amyloid polyneuropathy. Eur Radiol. 2017;27:4631-4638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJ, Schmitt M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 312] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 71. | Martinez-Naharro A, Kotecha T, Norrington K, Boldrini M, Rezk T, Quarta C, Treibel TA, Whelan CJ, Knight DS, Kellman P, Ruberg FL, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc Imaging. 2019;12:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 72. | Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, Fontana M, Moon JC, Pinzani M, Gillmore JD, Hawkins PN, Pepys MB. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015;373:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 73. | Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, Kotecha T, Francis R, Hutt DF, Rezk T, Rosmini S, Quarta CC, Whelan CJ, Kellman P, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol. 2017;70:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 74. | Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado D, White SK, Bulluck H, Herrey AS, Hawkins PN, Moon J. AL and ATTR cardiac amyloid are different: native T1 mapping and ECV detect different biology. J Cardiovasc Magn Reson. 2014;16:341. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Williams LK, Forero JF, Popovic ZB, Phelan D, Delgado D, Rakowski H, Wintersperger BJ, Thavendiranathan P. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson. 2017;19:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 76. | Oda S, Utsunomiya D, Nakaura T, Yuki H, Kidoh M, Morita K, Takashio S, Yamamuro M, Izumiya Y, Hirakawa K, Ishida T, Tsujita K, Ueda M, Yamashita T, Ando Y, Hata H, Yamashita Y. Identification and Assessment of Cardiac Amyloidosis by Myocardial Strain Analysis of Cardiac Magnetic Resonance Imaging. Circ J. 2017;81:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Pandey T, Alapati S, Wadhwa V, Edupuganti MM, Gurram P, Lensing S, Jambhekar K. Evaluation of Myocardial Strain in Patients With Amyloidosis Using Cardiac Magnetic Resonance Feature Tracking. Curr Probl Diagn Radiol. 2017;46:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Meng L, Lin K, Collins J, Markl M, Carr JC. Automated Description of Regional Left Ventricular Motion in Patients With Cardiac Amyloidosis: A Quantitative Study Using Heart Deformation Analysis. AJR Am J Roentgenol. 2017;209:W57-W63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Legou F, Tacher V, Damy T, Planté-Bordeneuve V, Rappeneau S, Benhaiem N, Rosso J, Itti E, Luciani A, Kobeiter H, Rahmouni A, Deux JF. Usefulness of T2 ratio in the diagnosis and prognosis of cardiac amyloidosis using cardiac MR imaging. Diagn Interv Imaging. 2017;98:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Kotecha T, Martinez-Naharro A, Treibel TA, Francis R, Nordin S, Abdel-Gadir A, Knight DS, Zumbo G, Rosmini S, Maestrini V, Bulluck H, Rakhit RD, Wechalekar AD, Gilbertson J, Sheppard MN, Kellman P, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Myocardial Edema and Prognosis in Amyloidosis. J Am Coll Cardiol. 2018;71:2919-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |