Published online Oct 26, 2019. doi: 10.4330/wjc.v11.i10.236
Peer-review started: February 12, 2019
First decision: April 11, 2019
Revised: August 31, 2019
Accepted: September 15, 2019
Article in press: September 15, 2019
Published online: October 26, 2019
Processing time: 257 Days and 18.1 Hours
The prevalence of cardiovascular diseases, especially heart failure, continues to rise worldwide. In heart failure, increasing levels of circulating atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are associated with a worsening of heart failure and a poor prognosis.
To test whether a high concentration of BNP would inhibit relaxation to ANP.
Pulmonary arteries were dissected from disease-free areas of lung resection, as well as pulmonary artery rings of internal diameter 2.5–3.5 mm and 2 mm long, were prepared. Pulmonary artery rings were mounted in a multiwire myograph, and a basal tension of 1.61gf was applied. After equilibration for 60 min, rings were pre-constricted with 11.21 µmol/L PGF2α (EC80), and concentration response curves were constructed to vasodilators by cumulative addition to the myograph chambers.
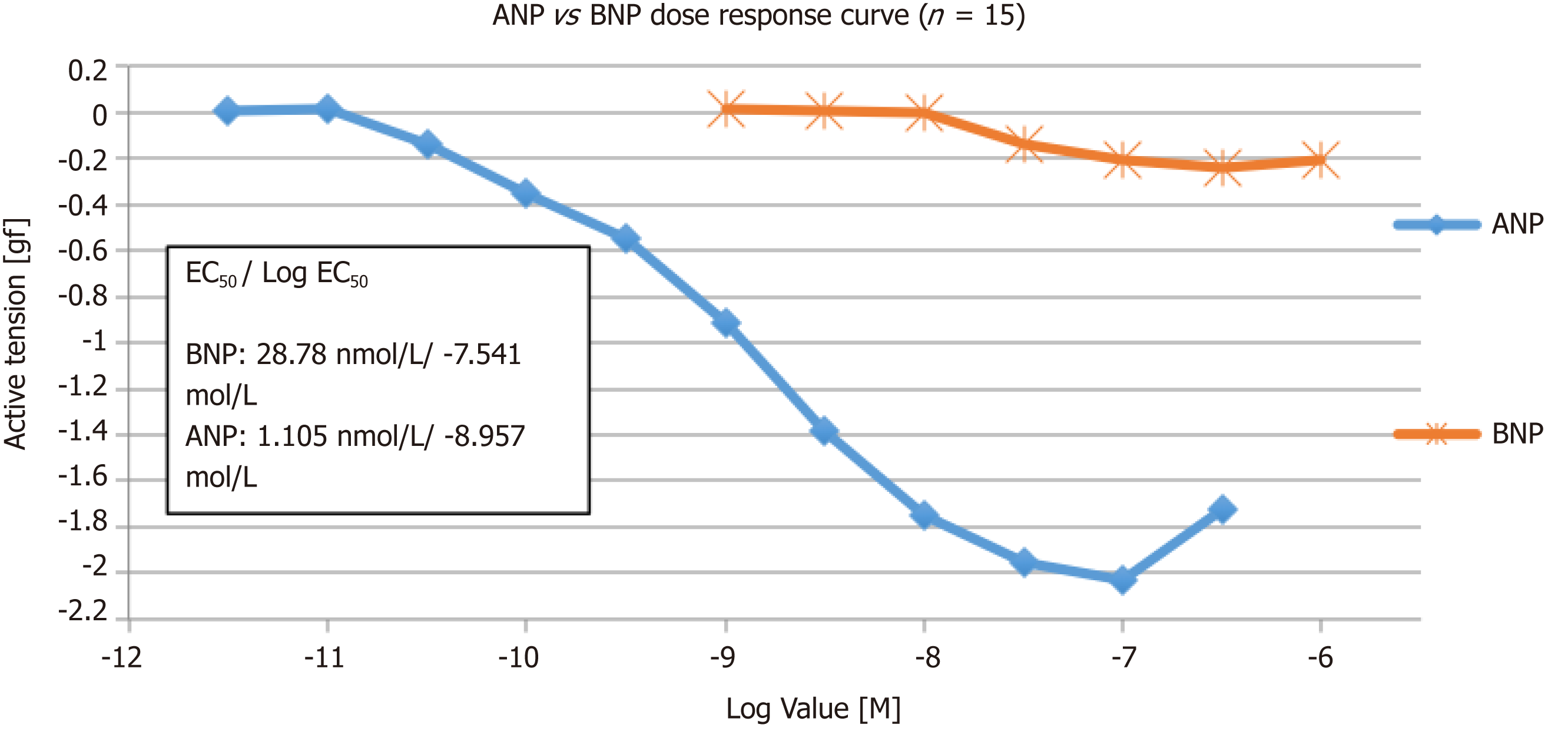
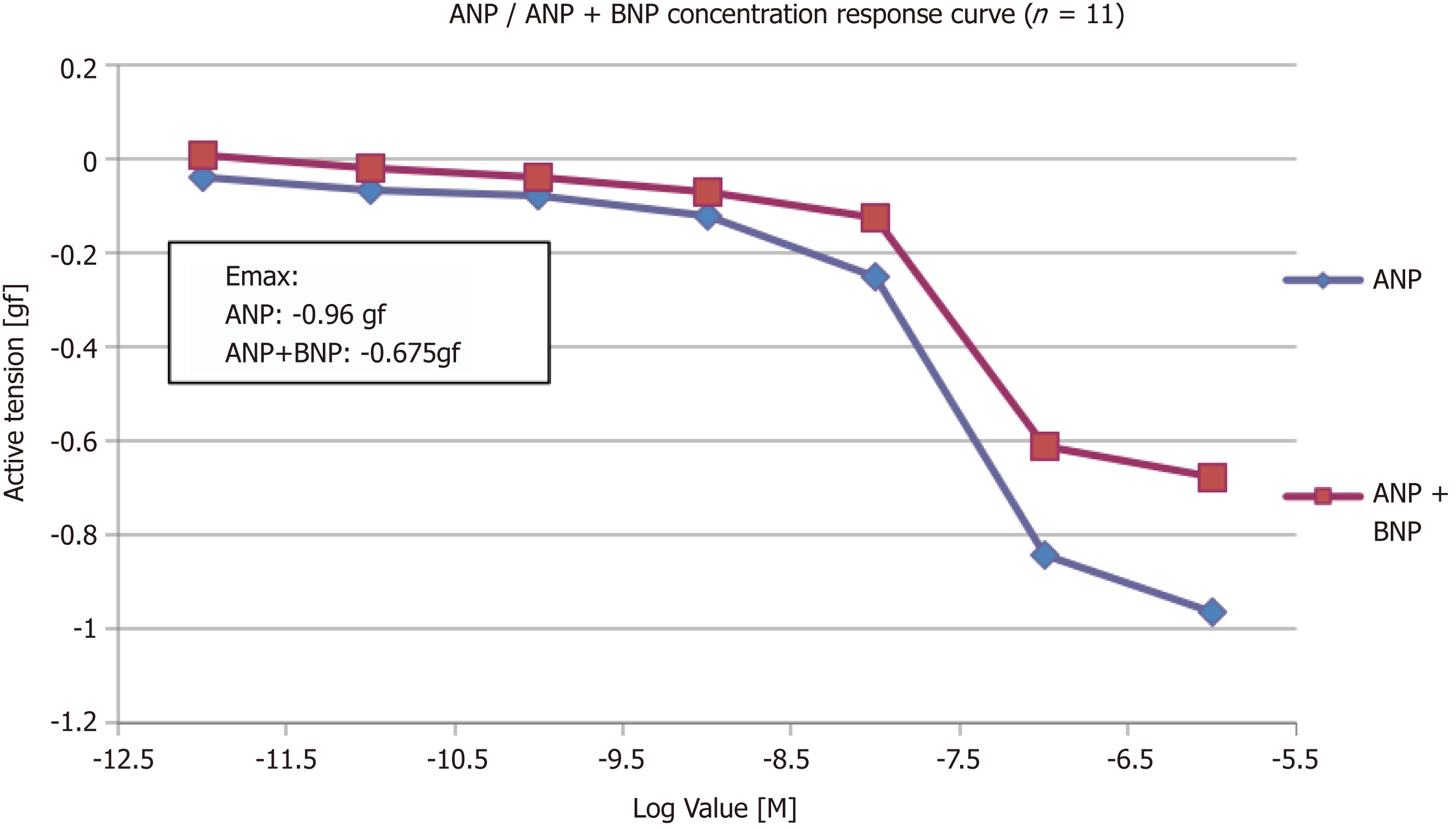
Although both ANP and BNP were found to vasodilate the pulmonary vessels, ANP is more potent than BNP. pEC50 of ANP and BNP were 8.96 ± 0.21 and 7.54 ± 0.18, respectively, and the maximum efficacy (Emax) for ANP and BNP was -2.03 gf and -0.24 gf, respectively. After addition of BNP, the Emax of ANP reduced from -0.96gf to -0.675gf (P = 0.28).
BNP could be acting as a partial agonist in small human pulmonary arteries, and inhibits relaxation to ANP. Elevated levels of circulating BNP could be responsible for the worsening of decompensated heart failure. This finding could also explain the disappointing results seen in clinical trials of ANP and BNP analogues for the treatment of heart failure.
Core tip: This study demonstrated that both atrial natriuretic peptide and brain natriuretic peptide (BNP) vasodilate isolated human pulmonary artery rings, and that BNP acts as a partial agonist and inhibits the effects of atrial natriuretic peptide. The finding that the addition of BNP inhibits the effects of atrial natriuretic peptide suggests that BNP does act as a partial agonist, and could be advancing the progression to decompensated heart failure.
- Citation: Hussain A, Bennett RT, Tahir Z, Isaac E, Chaudhry MA, Qadri SS, Loubani M, Morice AH. Differential effects of atrial and brain natriuretic peptides on human pulmonary artery: An in vitro study. World J Cardiol 2019; 11(10): 236-243
- URL: https://www.wjgnet.com/1949-8462/full/v11/i10/236.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i10.236
Decompensated heart failure is a worldwide health issue that is associated with considerable morbidity and mortality[1,2]. Despite the development of several device- and medical-based therapies over the past few decades, the rate of rehospitalisation and early death has not significantly improved[3].
The natriuretic peptides (NPs) family consists of three structurally interrelated vasoactive peptides, and was initially discovered by de Bold et al[4] in 1981. The family includes atrial natriuretic peptide (ANP), brain natriuretic peptides (BNPs) and C-type natriuretic peptide (CNP), which are mainly secreted by cardiac myocytes in response to wall stress[5,6]. ANP and BNP act via guanylyl cyclase-linked natriuretic peptide receptor-A (NPR-A), whereas CNP activates the related cyclase natriuretic peptide receptor-B (NPR-B)[7]. ANP and BNP exert their beneficial effects by reducing systemic and pulmonary vascular resistance, and by increasing natriuresis and diuresis[8]. In addition to their haemodynamic effects, NPs attenuate vascular smooth muscle proliferation and cardiac hypertrophy[9,10]. They also inhibit the synthesis of growth factors, by counteracting the effects of the renin-angiotensin system, which is involved in the development of pulmonary hypertension[11].
In vitro studies on pulmonary arterial rings and isolated lung models have shown that ANP and BNP infusion induced pulmonary vasodilation by reducing pulmonary vascular resistance[12,13]. However, in heart failure, increasing levels of circulating ANP and BNP are associated with a worsening of heart failure and a poor prognosis[14]. The aim of this study is to evaluate whether BNP acts as a partial agonist, and inhibits the effects of ANP.
Local research ethics committee and institutional (Hull & East Yorkshire Hospitals NHS Trust) Research and Development Department approval was obtained for the use of lung specimens and surplus lung tissue from patients undergoing elective lobe or lung resection for cancer. Patients gave written consent for the use of surplus tissue for research purposes.
In accordance with the recommendations of the human tissue act (2004) 127 and the conditions of the local ethics committee approval, the donor patient was anonymous to the researcher.
Excess segments of pulmonary artery were obtained from patients undergoing lobectomy, and the sample was immediately transferred to the lab in Krebs-Henseleit solution after resection. After the removal of connective tissue, the pulmonary artery (PA) sample was divided into 2 mm long rings. The small pulmonary vessels with an internal diameter of 2-4 mm were used for these experiments.
A multiwire myograph system was used for the measurement of isometric tension. Under physiological conditions (37oC, 21%O2), PA rings were mounted in Krebs Henseleit solution. A resting tension of 1.61 gf was applied, which was calculated from earlier experiments[15], and the vessels were left to equilibrate for 60-90 min. After equilibration, vessels were pre-constricted with 11.21 µmol/L PGF2α (EC80, calculated from earlier experiments[16]), and concentration response curves were constructed to ANP and BNP by cumulative addition to the myograph chambers.
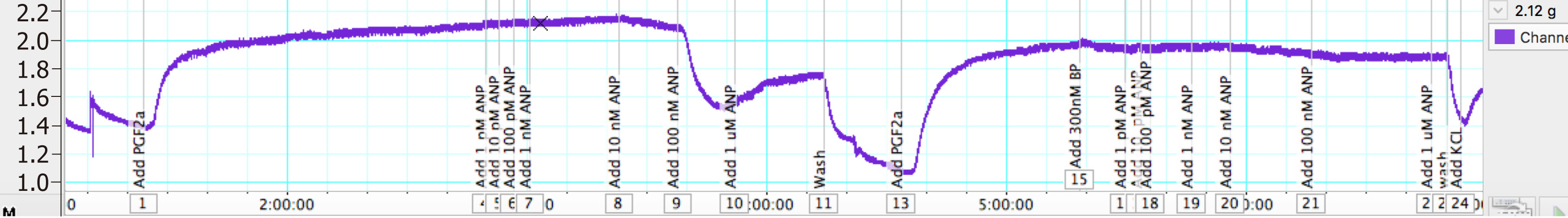
In another set of experiments, once the vessels tension reached a plateau after pre-constriction with PGF2α, 300 nmol/L of BNP was added and the vessels were left for 30 min. When a stable resting tension was achieved, concentration response curves were constructed to ANP. Vessels were then washed for 30 min, and the whole experiment was repeated again without the addition of BNP.
Active tension was calculated in gram force (gf) as maximum tension at plateau (gf) – resting tension (gf). The maximum efficacy (Emax) for each agent was determined in gf and expressed as gf/mm internal diameter of each vessel (to take into account the variability in PA ring diameter). The integrity of the endothelium was confirmed with 1μM Acetylcholine, and KCl was added to check viability. Vessels that did not constrict with KCl were excluded from the study. Figure 1 shows the schematic representation of myograph setup for measuring isometric tension.
A 5% CO2/balance air was sourced from BOC Limited (Guilford, Surrey, United Kingdom). The agents used were (supplier in parentheses) ANP (Tocris Bioscience, part of Bio-Techne, Abingdon, United Kingdom), BNP (Tocris Bioscience) Acetylcholine (Sigma-Aldrich, St. Louis, MO, United States) and PGF2α (Tocris Bioscience). Stock solutions of drugs were prepared using the solvents recommended by the suppliers, and control responses to solvents were obtained when necessary. Fresh serial dilutions were made using the appropriate solvent for each experiment. All other reagents were obtained from Thermo Fisher Scientific unless otherwise stated.
Data are presented as mean ± SD, and n represents the number of individual PA rings used in an experiment. Agonist EC50 concentrations (the concentration required to elicit 50% of the maximum response) were determined using nonlinear regression to fit a standard slope model using the statistical analysis function of GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA, United States). More details can be found at http://www.graphpad.com/guides/prism/6/curve-fitting/index.htm). Agonist potency is presented as pEC50 (the negative logarithm of the molar EC50 concentration). Significance was taken as P < 0.05.
A total of 35 PA rings were obtained from 15 patients. The internal diameter of PAs ranged from 2.5–3.5 mm. Nine rings were not included, as they did not respond to KCl.
ANP and BNP caused a concentration-dependent relaxation of PAs pre-constricted to PGF2α, with a pEC50 of 8.96 ± 0.21 and 7.54 ± 0.18 for ANP and BNP, respectively (Figure 2). The maximum efficacy (Emax) for ANP and BNP was -2.03 gf and -0.24 gf, respectively.
Another set of experiments was conducted to determine whether a high concentration of BNP would inhibit relaxation to ANP. After addition of BNP, the Emax of ANP was reduced by 30% from -0.96 gf to -0.675 gf (P = 0.28, n = 11) (Figure 3).
All vessels vasodilate in response to ANP. Increasing the concentration of ANP from 3pmol/L–1 μmol/L were used on 8 PA rings. Maximal relaxation was seen at 100 nmol/L (log -7.0 mol/L), and the EC20, EC50 and EC80 were 0.17 nmol/L, 1.105 nmol/L and 7.01 nmol/L, respectively. The hill slope was -0.75 ± 0.5.
In order to evaluate the effect of BNP on pulmonary vessels, 7 PA rings and a concentration of BNP from 1 nmol/L–1 µmol/L was used. As the concentration went above 10 nmol/L, vessels start to vasodilate and the maximum vasodilatory response was seen at 300 nmol/L (log -6.5 mol/L). The EC20, EC50 and EC80 were 13.3 nmol/L, 28.7 nmol/L and 61.5 nmol/L, respectively. The hill slope was -1.818 ± 2.55.
In another set of experiments, the cumulative vasodilator effect of ANP and BNP on pulmonary vascular tone was investigated. Sixteen PA rings from seven patients, and an increasing concentration of ANP from 1 pmol/L–1 µmol/L, was used. Five rings were excluded, as they did not respond to KCl. When a stable resting tension was achieved, vessels were pre-constricted to 11.21 μmol/L PGF2α (EC80). When a stable plateau relaxation was achieved, the effect of ANP on active tension was determined by cumulative addition to the myograph chambers.
The PA rings were washed for 60 min, and were pre-constricted again with 11.21 μmol/L PGF2α (EC80). A single dose of 300 nm of BNP was added and left for 30 min. Once a plateau was achieved by cumulative addition to the myograph chambers, the concentration response curve of ANP was performed. The addition of BNP reduced the Emax of ANP by 30% (from -0.96 gf to -0.675 gf).
In this study, we demonstrated for the first time that (1) both ANP and BNP vasodilate isolated human PA rings; and (2) that BNP acts as a partial agonist and inhibits the effects of ANP. The finding that the addition of BNP inhibits the effects of ANP suggests that BNP does act as a partial agonist, and could be advancing the progression to decompensated heart failure.
The circulating concentration of ANP, BNP and CNP is low in healthy individuals, but it is elevated in heart failure patients, although to variable degrees (e.g., CNP elevated to a lower extent than its counterparts)[17,18]. In patients with HF, circulating concentration of BNP exceeds that of ANP; this consistency of response and high dynamic range makes bioassays for plasma BNP more useful than ANP[19,20]. This might be due to the fact that BNP is also a marker of cardiac remodelling[21]. Previous studies have shown that in heart failure (HF) patients, BNP and NT-pro BNP (N-terminal pro b-type NP) are independent predictors of cardiovascular mortality, worsening HF and need for hospitalization[22-24]. Although BNP and NT-pro BNP have prognostic value, their therapeutic value is inconclusive in HF patients[25].
In the early 21st century, the United States Food and Drug Administration (FDA) approved the use of nesiritide (recombinant endogenous BNP) for heart failure patients[26]. However, several subsequent studies demonstrated that Nesiritide is associated with worsening renal function and increased risk of death[27]. A randomized, double blind, placebo-controlled, ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) trial concluded that nesiritide showed no substantial improvement in dyspnoea or clinical outcomes[28]. Another double-blinded, multicentre, randomized clinical trial, ROSE-AHF (Renal Optimization Strategies Evaluation - Acute Heart Failure) enrolled 360 patients. The study was designed to evaluate the use of low dose nesiritide, with the view that there would be less side effects and substantial therapeutic effects. However, the study failed to provide significant evidence in support of the routine use of nesiritide in heart failure patients[29].
Although NPs are always attractive therapeutic targets for heart failure treatment, their use is limited by inadequate clinical efficacy. It is thought that the activity of neprilysin, a protease produced by the kidney that cleaves various vasoactive compounds including BNP, is increased in heart failure[30]. In heart failure, increasing levels of circulating ANP and BNP are associated with worsening heart failure and a poor prognosis. This raised the suspicion that BNP might act as a partial agonist and inhibit the effects of ANP, as shown in this study. These findings could also explain the disappointing results seen in clinical trials of ANP and BNP analogues for the treatment of heart failure. Further studies are needed to confirm the findings of this study, which raises the possibility that selective BNP antagonists could be of greater clinical benefit than BNP agonists for the treatment of heart failure.
Our study had several limitations. It was a laboratory-based project that was carried out in a control setting, which may not truly reflect the in vivo environment. The therapeutic dose and the dose provided in the experiments may differ. We also used the pre-constrictor PGF2α, and since the potency of the agent depends on the pre-constrictor, other pre-constrictors need to be analysed and compared. The full potential of the study needs to be backed by a double-blinded randomized control trial.
The prevalence of cardiovascular diseases, especially heart failure, continues to rise worldwide. In heart failure, increasing levels of circulating atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are associated with worsening heart failure and poor prognosis.
ANP and BNP play an important role in homeostasis, but trials with BNP and ANP infusion showed disappointing results for unknown reasons.
The aim of this study was to evaluate whether BNP acts as a partial agonist and inhibits the effect of ANP.
In this study, the effect of natriuretic peptides (ANP and BNP) on human pulmonary arteries was evaluated by cumulative addition to the myograph.
Both ANP and BNP act as pulmonary vasodilators, although ANP was found to be more potent and efficacious than BNP. Also, the addition of BNP reduced the efficacy of ANP.
The study confirms that BNP inhibits the effects of ANP, and acts as a partial agonist. These findings also explained the disappointing results associated with the ANP and BNP infusion trials.
Further studies are needed to validate the results of this study, and to evaluate the possibility of the clinical beneficial role of BNP antagonists for heart failure treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bełtowski J S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Xing YX
| 1. | Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1204] [Article Influence: 80.3] [Reference Citation Analysis (1)] |
| 2. | Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L; EuroHeart Survey Investigators; Heart Failure Association, European Society of Cardiology. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 883] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 3. | Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303:2141-2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 573] [Cited by in RCA: 585] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 4. | de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2358] [Cited by in RCA: 2212] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 5. | Pandey KN. Guanylyl cyclase / atrial natriuretic peptide receptor-A: role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol. 2011;89:557-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;341-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 8. | Del Ry S, Cabiati M, Clerico A. Natriuretic peptide system and the heart. Front Horm Res. 2014;43:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Itoh H, Pratt RE, Dzau VJ. Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest. 1990;86:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 258] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Hutchinson HG, Trindade PT, Cunanan DB, Wu CF, Pratt RE. Mechanisms of natriuretic-peptide-induced growth inhibition of vascular smooth muscle cells. Cardiovasc Res. 1997;35:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hu RM, Levin ER, Pedram A, Frank HJ. Atrial natriuretic peptide inhibits the production and secretion of endothelin from cultured endothelial cells. Mediation through the C receptor. J Biol Chem. 1992;267:17384-17389. [PubMed] |
| 12. | Jin H, Yang RH, Chen YF, Jackson RM, Oparil S. Atrial natriuretic peptide attenuates the development of pulmonary hypertension in rats adapted to chronic hypoxia. J Clin Invest. 1990;85:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Klinger JR, Warburton RR, Pietras L, Hill NS. Brain natriuretic peptide inhibits hypoxic pulmonary hypertension in rats. J Appl Physiol (1985). 1998;84:1646-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Wright GA, Struthers AD. Natriuretic peptides as a prognostic marker and therapeutic target in heart failure. Heart. 2006;92:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Hussain A, Bennett RT, Chaudhry MA, Qadri SS, Cowen M, Morice AH, Loubani M. Characterization of optimal resting tension in human pulmonary arteries. World J Cardiol. 2016;8:553-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Hussain A, Bennett R, Haqzad Y, Qadri S, Chaudhry M, Cowen M, Loubani M, Morice A. The differential effects of systemic vasoconstrictors on human pulmonary artery tension. Eur J Cardiothorac Surg. 2017;51:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Charles CJ, Prickett TC, Espiner EA, Rademaker MT, Richards AM, Yandle TG. Regional sampling and the effects of experimental heart failure in sheep: differential responses in A, B and C-type natriuretic peptides. Peptides. 2006;27:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Del Ry S, Passino C, Maltinti M, Emdin M, Giannessi D. C-type natriuretic peptide plasma levels increase in patients with chronic heart failure as a function of clinical severity. Eur J Heart Fail. 2005;7:1145-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Maisel A. B-type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next? Circulation. 2002;105:2328-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 756] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 21. | Gaggin HK, Januzzi JL. Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. 2013;1832:2442-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 22. | Balion C, Santaguida PL, Hill S, Worster A, McQueen M, Oremus M, McKelvie R, Booker L, Fagbemi J, Reichert S, Raina P. Testing for BNP and NT-proBNP in the diagnosis and prognosis of heart failure. Evid Rep Technol Assess (Full Rep). 2006;1-147. [PubMed] |
| 23. | Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 515] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 24. | Vrtovec B, Delgado R, Zewail A, Thomas CD, Richartz BM, Radovancevic B. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation. 2003;107:1764-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Balion C, Don-Wauchope A, Hill S, Santaguida PL, Booth R, Brown JA, Oremus M, Ali U, Bustamam A, Sohel N, McKelvie R, Raina P. Use of Natriuretic Peptide Measurement in the Management of Heart Failure [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013; 13(14)-EHC118-EF. [PubMed] |
| 26. | Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 695] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 27. | Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 435] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 28. | van Deursen VM, Hernandez AF, Stebbins A, Hasselblad V, Ezekowitz JA, Califf RM, Gottlieb SS, O'Connor CM, Starling RC, Tang WH, McMurray JJ, Dickstein K, Voors AA. Nesiritide, renal function, and associated outcomes during hospitalization for acute decompensated heart failure: results from the Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure (ASCEND-HF). Circulation. 2014;130:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Dávila-Román VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM; NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 30. | Knecht M, Pagel I, Langenickel T, Philipp S, Scheuermann-Freestone M, Willnow T, Bruemmer D, Graf K, Dietz R, Willenbrock R. Increased expression of renal neutral endopeptidase in severe heart failure. Life Sci. 2002;71:2701-2712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |