Published online Aug 26, 2018. doi: 10.4330/wjc.v10.i8.62
Peer-review started: April 10, 2018
First decision: May 16, 2018
Revised: May 22, 2018
Accepted: June 30, 2018
Article in press: June 30, 2018
Published online: August 26, 2018
Processing time: 139 Days and 14.1 Hours
Increasing life expectancy is expected to lead to a corresponding increase in the prevalence of aortic valve disease (AVD). Further, the number of indications for transcatheter aortic valve replacement (TAVR) as a treatment option for AVD is expanding, with a growing role for echocardiography in its management. In this review we summarize the current literature on some newer echocardiographic modalities and the parameters they generate, with a particular focus on their prognostic and clinical value beyond conventional methods in the management of aortic stenosis, TAVR, and aortic regurgitation. Speckle tracking and 3D echocardiography are now increasingly being used in the management of AVD. For instance, global longitudinal strain, the best-studied speckle tracking echocardiographic parameter, can detect subtle subclinical cardiac dysfunction in patients with AVD that is not apparent using traditional echocardiographic techniques. The emerging technique of 3D full volume color Doppler echocardiography provides more accurate measurement of the severity of aortic regurgitation than 2D-proximal isovelocity surface area. These novel techniques are promising for evaluating and risk stratifying patients to optimize surgical interventions, predict recovery, and improve clinical outcomes.
Core tip: Reduced strain is now established for early diagnosis, prognosis, and risk stratification, predicting post-op recovery and showing associations with mortality. Decreased global longitudinal strain (GLS) is a robust parameter to diagnose subclinical left ventricular dysfunction before the left ventricular ejection fraction deteriorates and the patient develops symptoms. GLS also correlates with disease severity and helps to identify patients with excess risk of cardiovascular events and death who are likely to benefit from earlier surgical intervention. The high accuracy and reproducibly of 3D echocardiography has made the precise assessment of volume and AR possible and, therefore, the early recognition of its severity.
- Citation: Tiwari N, Patel K. Newer echocardiographic techniques for aortic-valve imaging: Clinical aids today, clinical practice tomorrow. World J Cardiol 2018; 10(8): 62-73
- URL: https://www.wjgnet.com/1949-8462/full/v10/i8/62.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i8.62
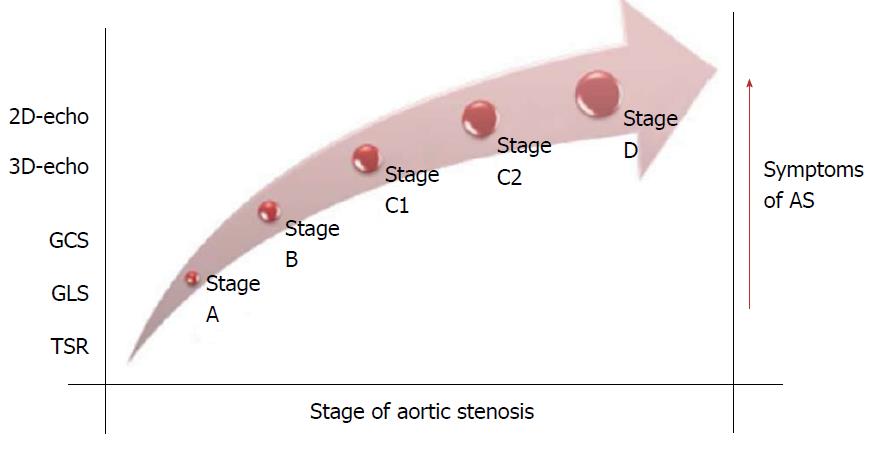
The prevalence of aortic valve disease (AVD) continues to rise in line with increasing life expectancy. Aortic stenosis (AS) is the most common valvular heart disease, affecting 12.4% of patients over 75 years in North America and Europe[1], while the incidence of aortic regurgitation (AR) increases with age and affects 4%-5% of the population overall[2]. The introduction of minimally invasive techniques such as transcatheter aortic valve replacement (TAVR) has revolutionized the management of AVD. Current AVD management mainly represents a “horse has bolted” approach since symptoms have already developed or the left ventricle is damaged, and consequently the medical and socio-economic impact is greater than if the disease was managed earlier in its course or prevented. Here we discuss the role of newer echocardiographic techniques in the management of AS and AR. For each, we first discuss the current guidelines before outlining the modalities that are expected to change clinical practice in the future.
In developed countries, AS is most commonly caused by calcific degenerative valve disease, with congenitally abnormal valves (commonly bicuspid) with superimposed calcification and rheumatic valve disease being less common causes[3]. With advances in technologies, AS is increasingly recognized as a complex disease with different patient subgroups and pathophysiologies. In order to individualize treatment, accurate and timely sub classification is important.
According to American College of Cardiology (ACC)/American Heart Association (AHA) 2014 guidelines[3], severe AS is defined as a peak aortic velocity of > 4 m/s, which corresponds to a mean aortic valve gradient of > 40 mmHg and calculated valve area of 1.0 cm2 or less[3]. Valvular heart disease guidelines from major societies like the ACC/AHA (2014)[3], European Society of Cardiology (ESC; 2012)[4], and Canadian Cardiovascular Society (2004)[5] recommend aortic valve replacement (AVR) as a class I indication in symptomatic patients with severe AS, asymptomatic patients with severe AS and left ventricular ejection fraction (LVEF) < 50%, and asymptomatic patients with severe AS who are undergoing other cardiac surgery.
Aortic stenosis leads to pressure overload in the left ventricle (LV). To maintain cardiac output, there is compensatory LV hypertrophy, which, although seemingly beneficial, is in fact maladaptive and results in cardiac fibrosis, heart failure, and ultimately increased mortality[6]. Over the last decade, new perspectives on the pathophysiology of AS have resulted in a new, four flow gradient pattern classification system that challenges the previous misconception that patients with AS and normal ejection fraction (EF) inevitably have normal flow. Patients are first divided based on left ventricular flow state-normal flow (NF) vs low flow (LF), where low flow is defined as LV stroke volume of < 35 mL/m2[7], and then based on pressure gradient-low gradient (LG) vs high gradient (HG), where low gradient is defined as mean trans-aortic pressure gradient of < 40 mmHg. This makes the four categories NF/LG, NF/HG, LF/LG, and LF/HG[7-10], as shown in Table 1.
| High gradient (mean > 40 mmHg) | Low gradient (mean < 40 mmHg) | |
| Normal flow (SV > 35 mL/m2) | ||
| Prevalence | 30%-62.7% | 15.3%-38% |
| Prognosis (2 yr survival rates) | 44% ± 6% | 83% ± 6% (best prognosis) |
| % Undergoing surgery | 80% (highest rates of surgery) | 53% |
| Low flow (SV < 35 mL/m2) | ||
| Prevalence | 8%-13.2% | 8.8%-24% |
| Prognosis (2 yr survival rates) | 30% ± 12% | 27% ± 13%-worst prognosis |
| % Undergoing surgery | 68% | 36%-lowest rates of surgery |
The LF/LG subgroup occurs in patients with low forward stroke volume and is associated with a worse prognosis and higher mortality than patients with high gradient severe AS[11]. Despite this, this group was noted to undergo the lowest rates of surgical intervention of the four categories[8]. Prognostic stratification using the above categories is therefore essential to prevent delay in timely intervention.
It is also important to distinguish pseudo-AS from true AS in the LF/LG subgroup. Pseudo-AS is seen in patients with mild to moderate AS due to incorrect calculation of lower aortic valve area (AVA) from poor forward flow causing incomplete valve opening, an inherent pitfall in the continuity equation. Therefore, ACC/AHA guidelines for assessing valvular heart disease recommend low dose (up to 20 mg/kg per minute) dobutamine stress echocardiography (DSE) to distinguish true from pseudo-AS in patients with LF/LG AS and decreased LVEF (< 40%)[3]. In pseudo-AS, gradient increases will correspond to proportionate increases in AVA, whereas in true AS, the AVA will remain diminished (AVA ≤ 1 cm2) even with progressive dobutamine dosage. An absence of contractile reserve, i.e., a failure to increase stroke volume by 20%, also indicates a worse prognosis. However, DSE’s high dependence on flow response results in high inter-patient variability, especially in those with restrictive physiology. Mal-alignment of the Doppler signal, erroneous measurement of LVOT diameter, poor quality images due to severe calcification in and around the AV, and extremes of body size (i.e., very low body surface area or morbidly obese patients) challenge the reliability of DSE.
Impaired LVEF in AS is associated with higher operative mortality[12], worse long-term prognosis[13], and less than 50% of patients recover to normal LVEF following AV replacement[12]. Even in the presence of symptoms, the majority of patients with severe AS have a normal EF[14]. Therefore, more sensitive parameters have been developed to identify patients likely to benefit from surgical treatment despite a preserved EF.
Impaired myocardial contractility often precedes the observable decrease in LVEF[6]. Early markers and predictors of myocardial dysfunction would therefore be highly desirable to recognize the subset of patients that would benefit from early intervention. A newer technique for strain imaging called speckle-tracking echocardiography (STE) is highly reproducible, angle-independent, and more sensitive for detecting global and regional LV wall function than routine echocardiographic indices like tissue Doppler imaging and conventional imaging techniques like multi-slice computed tomography (MSCT) and cardiac MRI (cMR)[15,16].
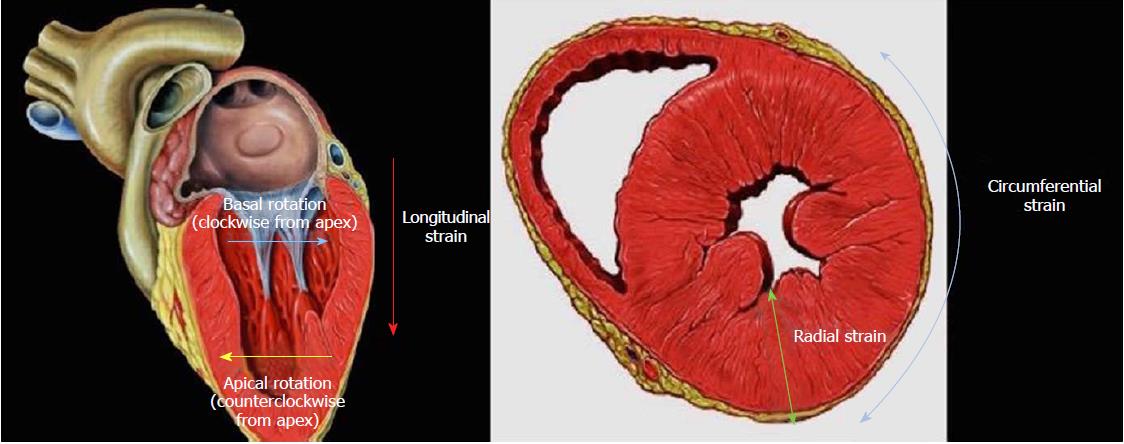
LV strain essentially assesses changes in myocardial fiber length relative to their resting phase. Strain rate indicates the speed at which this deformation occurs. An echocardiographic image is a grayscale image derived from the speckles produced by ultrasound waves scattered by body tissue. STE measures myocardial deformation (strain) based on speckle displacement and assesses the parameters of strain, strain rate, twist/torsion, and rotation (see definitions, Table 2 and Figure 1). We summarize current clinical data supporting the use of STE parameters in the prognostication and management of AS below.
| Parameter | Definition |
| Strain | Change in myocardial fiber length relative to its resting phase |
| Global longitudinal strain | Percentage change in LV fiber length in the longitudinal axis |
| Global circumferential strain | Percentage change in LV circumference in the short axis view |
| Global radial strain | Percentage change in LV wall thickness in the short axis view |
| Strain rate | Rate at which change in myocardial fiber length relative to its resting phase occurs |
| Twist/Torsion/Rotation | Myofiber geometry in the LV myocardium changes from a right-handed helix in the subendocardium to a left-handed helix in the subepicardium, and this results in twisting during systole with the apex rotating counterclockwise and the base in a clockwise direction |
Longitudinal strain: Longitudinal strain refers to the percentage change in LV fiber length in the longitudinal axis. Global longitudinal strain (GLS) is the most studied parameter to date, and it reflects contraction of longitudinally arranged subendocardial fibers. Severe AS hinders myocardial perfusion, particularly in the sub-endocardium, due to higher wall stress and impaired coronary blood flow[17]. Hence, decreased longitudinal shortening is the first impairment seen in patients with AS[18]. As the severity of AS increases, GLS decreases[19].
As the ventricle is non-spheroidal, pressure increases lead to the basal and mid LV segments being exposed to higher wall stress than the apical segments[20] according to Laplace’s law, as the heart base has a larger curvature and flatter contour compared to the middle and apical segments[21,22]. As would be expected, these segments respond better after unloading with AVR compared to the apical segments.
In a retrospective study of 395 patients with moderate to severe AS and preserved EF, GLS was an independent predictor of mortality[23]; this was subsequently confirmed in a prospective study of 142 patients[24]. GLS also shows incremental prognostic benefit over traditional parameters like AV gradient, stroke volume index[8,25], and valvuloarterial impedance[26,27]. Therefore, GLS can be used to detect subtle subclinical dysfunction that may not be apparent using current standard echocardiographic parameters. Lee et al[28] reported a higher percentage of two-year cardiac events (re-admission for heart failure or death) in asymptomatic severe AS patients with impaired GLS (-16.5%; 77% sensitivity and 67% specificity) who were managed conservatively. This suggests that incorporating GLS into current risk models might further optimize timing for AVR. In two prospective studies with severe AS and preserved EF (103 patients[29] and 340 patients[9], respectively), GLS was noted to be particularly worse in patients with LF AS compared to patients with NF AS, again supporting the hypothesis that the low flow state represents a more advanced form of the disease.
In asymptomatic moderate and severe AS patients with preserved LVEF, reduced GLS worse than -15% was a significant risk factor for cardiac hospitalization, AVR, cardiovascular death[30,31], all-cause mortality at one year[24], and, interestingly, increased risk of post-operative atrial fibrillation independent of left atrial size and age[32]. Other studies have established that regional deformation analysis is also important, with an additional parameter called basal longitudinal strain (BLS) less than -13% associated with adverse outcomes including heart failure, MI, all-cause mortality, and symptomatic status in asymptomatic severe AS. In fact, BLS has the strongest association with symptomatic status of all the longitudinal strain parameters[33]. Kempny et al[34] studied 101 patients undergoing TAVR and showed that pre-echocardiographic assessment of GLS is associated with post-operative symptom improvement.
Circumferential strain: The percentage change in LV circumference in the short axis view is called circumferential strain, which reflects contraction of the circumferentially arranged mid-layer fibers. Following a decrease in longitudinal strain, circumferential fibers compensate for the loss in longitudinal function. Hence, impairment of both longitudinal and circumferential strain suggests more extensive myocardial damage. Global circumferential strain (GCS) is considered an important prognostic factor in patients with symptomatic AS, and conservatively treated patients with impaired GCS have higher two-year all cause mortality and re-admission for heart failure than those who do not[28].
Radial strain: The percentage change in wall thickness in the short axis view is known as the radial strain. As noted above, impaired subendocardial perfusion is an early feature of AS due to increased wall stress and impaired coronary blood flow. Impaired endocardial radial strain is observed in patients with AS and preserved LVEF and correlates with AS severity; epicardial radial strain is, however, preserved[35]. Hyodo et al[36] suggested the use of a novel parameter called the bilayer ratio (subendocardial to subepicardial strain ratio), the value of which decreases as the severity of AS increases given the decrease in subendocardial thickness due to ischemia and compensatory increase in subepicardial thickness. This was deemed to be superior to longitudinal strain, which only takes the subendocardium into account.
Twist/torsion and rotation: Myofiber geometry in the LV myocardium changes from a right-handed helix in the subendocardium to a left-handed helix in the subepicardium. This configuration results in twisting during systole, with the apex rotating counterclockwise and the base in a clockwise direction. When both layers contract simultaneously, a larger radius of rotation for the outer epicardial myofiber layer results in mechanical predominance of the epicardial fibers in the overall direction of rotation. LV twist is proportionate to the severity of AS[37], which is thought to be a consequence of subendocardial dysfunction reducing inhibition of longitudinal muscle fibers. Conversely, untwisting rate is delayed and decreased[38].
Santoro et al[39] also observed an increase in LV twist in LV hypertrophy with preserved EF and suggested it as a marker of early systolic dysfunction. The LV twist to circumferential shortening ratio (TSR) is also considered a reliable marker of subendocardial dysfunction[40], and van Dalen et al[37] demonstrated an increase in TSR in AS. Apical rotation (ApRot) is also known to increase in severe AS[38,39]. Some studies have shown that ApRot is positively associated with the presence of symptoms[41], while others[42] have found that patients with low ApRot have higher rates of syncopal events, a similar rate of overall symptoms, and that increased ApRot is an independent predictor of mortality in severe AS with preserved EF. Asymptomatic patients with severe AS and increased ApRot have similar survival to symptomatic patients, but the measurement is useful for identifying patients who might benefit from early evaluation for aortic valve replacement (AVR)[42].
The widespread emergence of 3D echocardiography has also introduced the possibility of measuring 3D strain (3D-STE). 2D-STE assumes geometric LV morphology, needs multiple, high-quality image acquisition, and there is the possibility of mistracking speckles that move out of the scanning plane. 3D-STE, using the block-matching method in full-volume datasets, has been developed to overcome these shortcomings, and it tracks the 3D motion of the acoustic speckles but is independent of speckles moving out of the scanning plane and free of geometric presumptions. 3D-STE represents a more accurate model than 2D-STE because all strain parameters are simultaneously obtained from one volume image[43] in contrast to 2D-STE, in which the long and short axes are measured at different points in time.
As expected, compared to LVEF, 3D-STE is a more sensitive and accurate assessment of early LV dysfunction[44]. In a study of 104 asymptomatic patients with severe AS and preserved EF, 3D-GLS, 3D-GRS, and 2D-GLS were all found to be useful predictors of major adverse cardiac events (MACE). 3D-GLS was also an independent predictor of MACE after correcting for LV mass index and mean pressure gradient[45]. However, poor temporal resolution and the need for a high frame rate hamper its current widespread use. Further prospective studies utilizing a 3D-GLS-guided approach are imperative to assess its role in predicting adverse cardiovascular outcomes.
In addition to playing a role in early diagnostics and patient stratification, newer imaging modalities like MSCT, cMR, and 3D trans-esophageal echocardiography are increasingly being used as part of the treatment of AS. AS is only treatable by valve replacement, traditionally surgically (SAVR) but now also with balloon-expandable or self-expanding valves via TAVR. With the recent United States Food and Drug Administration (FDA) approval of the Sapien XT and Sapien 3 transcatheter heart valves in patients at intermediate risk for open-heart surgery, TAVR will increasingly be used beyond only high-risk and inoperable cases. Pre-procedural, intra-procedural, and post-procedural echocardiography are recommended as part of TAVR evaluation[46]. These are described in detail below (Figure 2).
Pre-TAVR, transthoracic echocardiography (TTE) helps to determine the degree of stenosis, valvular cusp size, motion, the location and amount of calcification, the aortic root and annular anatomy, mitral valve pathology, the presence of a bulging inter-ventricular septum at the level of the aortic root, baseline LV and RV function, and pulmonary artery pressure[6]. With modern echocardiograms, Doppler echocardiographic interrogation of aortic valve gradients is superior and preferred to other imaging modalities, although misalignment of the Doppler signal with the AS jet of over 20 degrees and the pressure recovery phenomenon in the ascending aorta diameter < 30 mm are known limitations.
The effective orifice area is determined using the left ventricular outflow tract (LVOT) diameter on TTE or transesophageal echocardiography (TEE). It is important to measure this 1-2 mm apical to the aortic annulus, and a difference of > 2 mm between the aortic annulus and LVOT should prompt repeat measurement[6]. A basal inter-ventricular septal bulge may also lead to inaccurate measurements and superior displacement of the deployed valve, so this also needs pre-operative determination[6]. On the other hand, a thin septum is also important to recognize pre-operatively to avoid post-operative ventricular septal rupture.
It is well established that multi-slice computer tomography (MSCT) overestimates the LVOT by 20% and 3D echocardiogram underestimates by 20% given the elliptical shape of the LVOT. Aortic valve area (AVA) measured by CT for severe AS needs a higher cut-off (1.2 cm2) compared to AVA measured by echocardiography (1 cm2)[47]. It is challenging to obtain the appropriate cross-sectional view by 2D-TEE due to the movement of the aortic annulus along the long axis and its tilting movement during the cardiac cycle. Volumetric 3D-TEE overcomes this problem by incorporating the entire AV valve. As anticipated, in a prospective study of 60 patients, Nakai et al[48] showed that 3D-TEE is more accurate that 2D-TEE for calculating the AVA. Further, 3D-TEE provides a more accurate reconstruction of the aortic root and better measurement of the distance between the annulus and coronary ostia, most importantly the left coronary ostium.
One of the most important roles for echocardiography is to assist in choosing the appropriate size of prosthetic heart valve. Undersizing of the prosthetic causes paravalvular aortic regurgitation (PVR), while oversizing of artificial valves, especially in the setting of a calcified LVOT, is associated with higher risk of aortic rupture and periaortic hematoma as the native calcific valves are retained behind the artificial valve. Measurement of LVOT diameter by TTE has long been used and validated for calculation of the aortic valve area and AS severity, but TTE measurements should not be relied upon to decide the prosthetic valve size. There is an ongoing discussion about which method is most accurate and reproducible for valve sizing; Tsang et al[49] compared cMR, MSCT, and 3D-TEE in vivo and in vitro and showed that cMR was most accurate and reproducible. Several studies have suggested that MSCT is superior to 3D-TEE[49,50], while others[51] have noted good agreement between annulus perimeter and the area measured by 3D-TEE and MSCT. Altiok et al[52] also showed highly consistent measurements of sagittal and coronal diameters on 3D-TEE and MSCT.
The distribution and extent of calcification should also be determined preoperatively by echocardiography as it is a useful predictor of procedural success. It is also crucial to identify patients with obliteration of the sinus of Valsalva (SOV) or smaller or shorter SOV height, as these patients require shorter prosthetic valves. 3D-TEE has also emerged as a valuable tool (comparable to MSCT) for accurately measuring left main coronary artery to annulus distance and length of coronary cusp[53].
Intra-TAVR, fluoroscopy is advocated by the 2012 ACCF/AATS/SCAI/STS expert consensus document[54] on TAVR regardless of type of access. However, in a prospective study of 100 patients undergoing transapical transcatheter aortic valve implantation, Bagur et al[55] noted similar acute and 30-d outcomes for patients managed intra-operatively with angiography and TEE. Current American and European guidelines advocate the use of 2D and 3D-TEE support during TAVR.
The first step of the TAVR procedure is to place the pacing wire in the right ventricle, with echocardiography used to confirm its position and exclude perforation of the ventricle or pericardial effusion. Next, a stiff wire is placed into the left ventricle, with echocardiography ensuring its stability at the apex and lack of entanglement with mitral apparatus and again excluding perforation and pericardial effusion. The aortic root must be continually visualized during balloon aortic valvuloplasty, after which coronary artery patency is established, LV wall motion assessed, and position of the calcified coronary leaflets noted. The TAVR valve is then introduced. It is important to ensure that the native leaflets are covered by the TAVR valve. In trans-apical valve placement, the puncture site is visualized. It is also important to ensure angulation of the valve away from the inter-ventricular septum and the right ventricle.
TEE during TAVR is usually performed under general anesthesia, which requires intubation and hence increases the patient’s risk profile. TTE seems to be a reasonable alternative to TEE but factors against TTE include relatively poor image quality, inability to position the patient, and potential compromise of operative field sterility with the trans-apical or trans-aortic approach. Intracardiac echocardiography (ICE) can overcome some of these issues, and ICE has been used in the closure of inter-atrial defects and in some electrophysiological interventions. For TAVR monitoring, the ICE catheter is introduced through the femoral vein and advanced to the superior cavo-right atrial junction. Apart from TR jet velocity, ICE allows placement of guide wires and catheters carrying the valve, valve deployment, and continuous monitoring for pericardial effusion. However, it has yet to be optimized for accurately assessing paravalvular regurgitation (PVR) given its narrow sector angle and poor resolution. Further, ICE requires venous access, which carries a risk of hematoma and bleeding complications. Since the ICE catheter shares the same RV space, there is a risk of dislodging the pacemaker leads used for rapid pacing during valve deployment[56]. 3D-ICE imaging currently has a limited field of view of only 22°, making the measurement of annular size difficult.
Post-TAVR, valve deployment, position, shape, and leaflet motion must be confirmed. Then, hemodynamic measurements need to be performed to assess valve function. Effective orifice area, Doppler velocity index, and mean and peak trans-valvular gradients should be measured. Effective orifice area is determined using the LVOT diameter, which is measured from the outer to outer stent diameter at the lower edge or inner to inner stent diameter at the upper edge if the valve is low. LV and RV function and pulmonary artery pressures are also noted as a part of the post-operative evaluation.
TEE and TTE are routinely used in clinical practice to detect immediate post-operative complications like malposition, valvular regurgitation or PVR, mitral valve damage, aorta to right atrium fistulae, cardiac shunts secondary to inter-ventricular septal damage, pericardial effusion, and cardiac tamponade following free wall or annular rupture, coronary artery patency, and left ventricular wall akinesis due to inadvertent coronary ostial closure. More than a moderate degree of PVR is clearly associated with increased short- and long-term mortality[57]; however, data on mild PVR and outcomes are conflicting. The exact incidence of PVR varies widely across studies due to the differences in the parameters and criteria used to grade PVR; different schemes used to classify PVR severity; and a lack of standard assessments for PVR. To address this issue, a classification of PVR has recently been proposed that divides severity into five categories: trace, mild, mild-to-moderate; moderate; moderate-to-severe, and severe[25].
Doppler is the gold standard for evaluating PVR. Both TTE and TEE may be required, as the PVR jets located posteriorly and anteriorly are often shadowed in TTE and TEE views, respectively, as a result of the shadowing caused by native aortic valve calcifications and the prosthetic stent. It is always important to use color Doppler with echocardiography in both the long and short axis views. Further, jets must be quantified in terms of number, width, path, and convergence. The 2012 Valve Academic Research Consortium (VARC) 2 defines moderate PVR as the circumferential extent of the PVR estimated in the parasternal short axis at 10%-30% and severe PVR at > 30%[58]. Tiny para-valvular jets usually regress spontaneously over a period of 10-15 min[59], as do those appearing with self-expandable balloon aortic valves as the frame expands[59]. Therefore, it is important to wait for a while before intervening. With multiple jets, eccentricity, presence of calcification, and changing loading conditions, the accurate assessment of PVR severity can be difficult using conventional echo methods. 3D-TTE has been shown to be superior for assessing PVR utilizing 3D vena contracta and 3D regurgitant volumes[60].
TTE can be used to determine prosthesis location in the long axis view compared to LVOT location. In the short axis view, TTE can determine if the proper circular shape has been assumed. There is a now a trend toward using only TTE in appropriately selected patients. In a retrospective study of 111 patients, Sengupta et al[61] demonstrated a significant difference in the procedural time with non-inferiority in terms of procedural success, extent of PVR, additional valve implantation, and complications such as peri-procedural stroke rate or death. However, prospective data supporting TTE use during TAVR are still lacking.
Echocardiography should be performed prior to discharge (and after 30 d) to establish a new baseline of replaced valve function including mean transaortic gradient, valve area, and PVR. As data on the long-term functioning of TAVR are not robust, annual TTE follow up to assess valvular and ventricular function should be undertaken.
Several studies have reported reverse remodeling (improvement in strain parameters) in the minutes, 72 h, and month following TAVR[36,62-64]. In a prospective study, Swan et al[65] demonstrated immediate improvement (within minutes) in circumferential and radial strain following TAVR. Kim et al[66] conducted a multilayer strain study and demonstrated a significant improvement in longitudinal but not circumferential strain following TAVR as early as one week. A study of 68 LF LG severe AS patients showed significant improvements in GLS at 6 and 12 months after TAVR[67]. Interestingly, post TAVR changes in strain pattern do not appear to be influenced by pacemaker-induced rhythm or post-procedure new left bundle branch block[64]. Kempny et al[34] reported a correlation between improvement in longitudinal strain and symptomatic improvement following TAVR, while Løgstrup et al[68] (n = 100, mean EuroScore: 10.5 ± 2.8) noted a correlation between improvement in GLS and decrease in mortality rate following TAVR. Poulin et al[69] (n = 102 patients) reported that improvements in longitudinal systolic and diastolic deformation were significantly lower in patients with prosthesis-patient mismatch at follow-up.
The most common causes of AR in developed countries are aortic root dilation, calcific valve disease, and bicuspid aortic valve, with rheumatic AR the less common etiology. Less common causes include infective endocarditis and aortic dissection. The inability of valve leaflets to remain coapted during diastole leads to blood flow back into the left ventricle. This leads to increase in end-diastolic volume and elevated wall stress, eventually leading to compensatory eccentric hypertrophy from volume overload. In contrast to AS (where both pressure and volume overload occurs), this eccentric LV change predominantly affects the circumferentially arranged fibers leading to more severe impairment in GCS as compared to GLS.
ACC/AHA 2014 guidelines define severe AR as a Doppler jet width ≥ 65% of LVOT, vena contracta > 0.6 cm, regurgitant volume ≥ 60 mL/beat, regurgitant fraction of ≥ 50%, and effective orifice area of ≥ 0.3 cm2[3]. Guidelines from the ACC/AHA (2014)[3], European Society of Cardiology (ESC; 2012)[4], and Canadian Cardiovascular Society (2004)[5] on the management of valvular heart disease recommend AVR as a class I indication in symptomatic patients with severe AR, asymptomatic patients with severe AR and LVEF of < 50%, and patients with severe AR who are undergoing other cardiac surgery. AVR is also recommended as a Class IIa indication for patients with severe AR, normal LVEF ≥ 50% but severe LV dilation [LV end-systolic diameter (LVESD) of > 50 mm], or indexed LVESD of > 25 mm/m2. It is also reasonable to pursue AVR in patients with moderate AR undergoing cardiac surgery. However, severe dilation and decreased LVEF represent the late stage of the disease, and other parameters to identify subtle LV dysfunction early in the course of disease are desired.
STE has also been used in the assessment of AR. Stefani et al[70] examined 60 patients including young athletes with bicuspid aortic valves and mild AR with matched controls and found a reduced longitudinal peak systolic strain in LV basal segments. A single center retrospective study of 314 patients with chronic moderate to severe AR noted that global longitudinal strain was independently predictive of mortality (at a threshold of -12.5%)[71]. Di Salvo et al[72] showed that patients with moderate to severe AR with a progressive pattern and symptom development had significantly reduced longitudinal strain compared to those with stable disease (-17.8% ± 3.9% vs -22.7% ± 2.7%, P = 0.001) despite having similar LVEFs. In a longitudinal study of 64 patients with moderate to severe AR, Olsen et al[73] noted that patients with reduced GLS, strain rate, and early diastolic strain were more likely to have progressive disease (symptom development or worsening LVEF) in patients managed conservatively and also poorer outcomes following surgery. In a study of 90 patients, Iida et al[74] demonstrated that the LVEF may continue to be normal despite a significant lowering of longitudinal strain due to compensatory increases in wall thickening in the subepicardium.
Gabriel et al[75] reported a correlation between reduced longitudinal strain rate and subclinical LV dysfunction on exercise echocardiography in patients with moderate to severe AR. Similarly, Marciniak et al[76] also found that longitudinal and radial strain rates were a sensitive indicator of subclinical dysfunction in severe AR. Interestingly, Onishi et al[77] noted that LV radial systolic strain rate was predictive of LVEF post surgery. In moderate to severe AR with preserved EF, Ewe et al[78] noted impairment in all three strains (longitudinal, circumferential, and radial) in symptomatic compared to asymptomatic patients. Also, impaired baseline GLS (per 1% decrease, HR = 1.21, P = 0.04) or GCS (per 1% decrease, HR= 1.22, P = 0.04) was predictive of the need for surgery in asymptomatic patients. Li et al[44] noted that circumferential strain is a more sensitive marker for AR/volume overload compared to longitudinal strain for AS/pressure overload. Similarly, Broch et al[79] showed that in asymptomatic patients with moderate to severe AR and preserved LVEF, GCS was higher and thought to contribute to reduced GLS.
Decreased LV apical rotation and torsion have been detected in moderate to severe AR with preserved EF compared to normal healthy subjects[80], while others[81] have noted lower LV torsion in the severe AR group compared to the moderate AR group but no difference in the apical rotation compared to moderate AR and control patients. Finally, in a prospective study by Chen et al[82] for severe AR and reduced EF, there were significant improvements in GLS and GCS at three months following AVR when measured using 3D-STE.
Although many different 2D echocardiography parameters can be used to quantify AR, it is still challenging. Not only are there subtle differences in the scan plane with altered size of the vena contracta jet, but also its shape is not always circular but sometimes irregular or ellipsoid. 3D-echocardiography can be used to directly measure the vena contracta area (3D-VCA). Sato et al[83] showed that the 3D-VCA was 32 mm2 in severe AR (sensitivity: 89%; specificity: 98%). 2D-derived proximal isovelocity surface area (PISA) and regurgitant volume (Rvol) suffer from geometric assumptions, angle correction, and problems with assessing multiple jets. Choi et al[84] reported 3D full volume color Doppler echocardiography to be more accurate then 2D-PISA for assessment of all AR grades, especially with eccentric or multiple jets. Perez de Isla et al[85] reported that 3D color Doppler echocardiography was both accurate and reproducible for AR evaluation with a high correlation with gold standard cMR. Real-time 3D-TEE was also able to reveal specific anatomical differences between type I (annular dilation) and type II (prolapsed) AR[86].
Reduced strain is now established for early diagnosis, prognosis, and risk stratification, predicting post-op recovery and showing associations with mortality. Decreased GLS is a robust parameter to diagnose subclinical LV dysfunction before the LVEF deteriorates and the patient develops symptoms. GLS also correlates with disease severity and helps to identify patients with excess risk of cardiovascular events and death who are likely to benefit from earlier surgical intervention. The high accuracy and reproducibly of 3D echocardiography has made the precise assessment of volume and AR possible and, therefore, the early recognition of its severity.
Newer echocardiographic techniques have significantly enhanced our understanding of the pathophysiology of aortic valve diseases and are being increasingly employed to assess disease severity and to risk-stratify patients. Early detection of LV dysfunction is paramount in these patients to allow timely intervention before irreversible impairment occurs. Novel non-invasive imaging techniques such as speckle tracking echocardiography and 3D imaging have shown promise in the quantification of subclinical myocardial damage in vivo. With development of higher processing power echocardiograms, newer generation probes, and with increasing familiarity and experience with these techniques, these are likely to become the new norm.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Anan R, Barik R, Schoenhagen P, Ueda H S- Editor: Ma YJ L- Editor: A E- Editor: Tan WW
| 1. | Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 944] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 2. | Lebowitz NE, Bella JN, Roman MJ, Liu JE, Fishman DP, Paranicas M, Lee ET, Fabsitz RR, Welty TK, Howard BV. Prevalence and correlates of aortic regurgitation in American Indians: the Strong Heart Study. J Am Coll Cardiol. 2000;36:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW; American College of Cardiology; American College of Cardiology/American Heart Association; American Heart Association. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148:e1-e132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 712] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 4. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Lung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M; ESC Committee for Practice Guidelines (CPG); Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42:S1-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 1024] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 5. | Jamieson WR, Cartier PC, Allard M, Boutin C, Burwash IG, Butany J, de Varennes B, Del Rizzo D, Dumesnil JG, Honos G. Surgical management of valvular heart disease 2004. Can J Cardiol. 2004;20 Suppl E:7E-120E. [PubMed] |
| 6. | Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 421] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 7. | Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 713] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 8. | Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Adda J, Mielot C, Giorgi R, Cransac F, Zirphile X, Donal E, Sportouch-Dukhan C, Réant P, Laffitte S, Cade S. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle-tracking echocardiography: a multicenter study. Circ Cardiovasc Imaging. 2012;5:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Lancellotti P, Magne J, Donal E, Davin L, O’Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 11. | Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Vaquette B, Corbineau H, Laurent M, Lelong B, Langanay T, de Place C, Froger-Bompas C, Leclercq C, Daubert C, Leguerrier A. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart. 2005;91:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Lund O, Flø C, Jensen FT, Emmertsen K, Nielsen TT, Rasmussen BS, Hansen OK, Pilegaard HK, Kristensen LH. Left ventricular systolic and diastolic function in aortic stenosis. Prognostic value after valve replacement and underlying mechanisms. Eur Heart J. 1997;18:1977-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2255] [Cited by in RCA: 2264] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 15. | Herrmann S, Störk S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlöhner S, Voelker W, Ertl G. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Marwick TH. Methods used for the assessment of LV systolic function: common currency or tower of Babel? Heart. 2013;99:1078-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4052] [Cited by in RCA: 3989] [Article Influence: 114.0] [Reference Citation Analysis (1)] |
| 18. | Pibarot P, Dumesnil JG. Longitudinal myocardial shortening in aortic stenosis: ready for prime time after 30 years of research? Heart. 2010;96:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Miyazaki S, Daimon M, Miyazaki T, Onishi Y, Koiso Y, Nishizaki Y, Ichikawa R, Chiang SJ, Makinae H, Suzuki H. Global longitudinal strain in relation to the severity of aortic stenosis: a two-dimensional speckle-tracking study. Echocardiography. 2011;28:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Regen DM, Anversa P, Capasso JM. Segmental calculation of left ventricular wall stresses. Am J Physiol. 1993;264:H1411-H1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Büchi M, Hess OM, Murakami T, Krayenbuehl HP. Left ventricular wall stress distribution in chronic pressure and volume overload: effect of normal and depressed contractility on regional stress-velocity relations. Basic Res Cardiol. 1990;85:367-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Heng MK, Janz RF, Jobin J. Estimation of regional stress in the left ventricular septum and free wall: an echocardiographic study suggesting a mechanism for asymmetric septal hypertrophy. Am Heart J. 1985;110:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, Grimm RA, Griffin BP, Desai MY. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, Burrell LM, Srivastava PM. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2012;13:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 26. | Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 27. | Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging. 2012;5:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | Lee HF, Hsu LA, Chan YH, Wang CL, Chang CJ, Kuo CT. Prognostic value of global left ventricular strain for conservatively treated patients with symptomatic aortic stenosis. J Cardiol. 2013;62:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Lee SP, Kim YJ, Kim JH, Park K, Kim KH, Kim HK, Cho GY, Sohn DW, Oh BH, Park YB. Deterioration of myocardial function in paradoxical low-flow severe aortic stenosis: two-dimensional strain analysis. J Am Soc Echocardiogr. 2011;24:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Lafitte S, Perlant M, Reant P, Serri K, Douard H, DeMaria A, Roudaut R. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur J Echocardiogr. 2009;10:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Lancellotti P, Donal E, Magne J, Moonen M, O’Connor K, Daubert JC, Pierard LA. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Levy F, Debry N, Labescat AL, Meimoun P, Malaquin D, Marechaux S, Rusinaru D, Jeu A, Ennezat PV, Castel AL. Echocardiographic prediction of postoperative atrial fibrillation after aortic valve replacement for aortic stenosis: a two-dimensional speckle tracking left ventricular longitudinal strain multicentre pilot study. Arch Cardiovasc Dis. 2012;105:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Carstensen HG, Larsen LH, Hassager C, Kofoed KF, Dalsgaard M, Kristensen CB, Jensen JS, Mogelvang R. Tissue Velocities and Myocardial Deformation in Asymptomatic and Symptomatic Aortic Stenosis. J Am Soc Echocardiogr. 2015;28:969-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Kempny A, Diller GP, Kaleschke G, Orwat S, Funke A, Radke R, Schmidt R, Kerckhoff G, Ghezelbash F, Rukosujew A. Longitudinal left ventricular 2D strain is superior to ejection fraction in predicting myocardial recovery and symptomatic improvement after aortic valve implantation. Int J Cardiol. 2013;167:2239-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Ohara Y, Fukuoka Y, Tabuchi I, Sahara S, Hosogi S, Nishimoto M, Yamamoto K. The impairment of endocardial radial strain is related to aortic stenosis severity in patients with aortic stenosis and preserved LV ejection fraction using two-dimensional speckle tracking echocardiography. Echocardiography. 2012;29:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Hyodo E, Arai K, Koczo A, Shimada YJ, Fujimoto K, Di Tullio MR, Homma S, Gillam LD, Hahn RT. Alteration in subendocardial and subepicardial myocardial strain in patients with aortic valve stenosis: an early marker of left ventricular dysfunction? J Am Soc Echocardiogr. 2012;25:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | van Dalen BM, Tzikas A, Soliman OI, Heuvelman HJ, Vletter WB, Ten Cate FJ, Geleijnse ML. Assessment of subendocardial contractile function in aortic stenosis: a study using speckle tracking echocardiography. Echocardiography. 2013;30:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | van Dalen BM, Tzikas A, Soliman OI, Kauer F, Heuvelman HJ, Vletter WB, ten Cate FJ, Geleijnse ML. Left ventricular twist and untwist in aortic stenosis. Int J Cardiol. 2011;148:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Santoro A, Alvino F, Antonelli G, Zacà V, Benincasa S, Lunghetti S, Mondillo S. Left ventricular twisting modifications in patients with left ventricular concentric hypertrophy at increasing after-load conditions. Echocardiography. 2014;31:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Delhaas T, Kotte J, van der Toorn A, Snoep G, Prinzen FW, Arts T. Increase in left ventricular torsion-to-shortening ratio in children with valvular aortic stenosis. Magn Reson Med. 2004;51:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Carasso S, Mutlak D, Lessick J, Reisner SA, Rakowski H, Agmon Y. Symptoms in severe aortic stenosis are associated with decreased compensatory circumferential myocardial mechanics. J Am Soc Echocardiogr. 2015;28:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Holmes AA, Taub CC, Garcia MJ, Shan J, Slovut DP. Increased apical rotation in severe aortic stenosis is associated with reduced survival: a speckle-tracking study. J Am Soc Echocardiogr. 2015;28:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Pérez de Isla L, Balcones DV, Fernández-Golfín C, Marcos-Alberca P, Almería C, Rodrigo JL, Macaya C, Zamorano J. Three-dimensional-wall motion tracking: a new and faster tool for myocardial strain assessment: comparison with two-dimensional-wall motion tracking. J Am Soc Echocardiogr. 2009;22:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Li CM, Li C, Bai WJ, Zhang XL, Tang H, Qing Z, Li R. Value of three-dimensional speckle-tracking in detecting left ventricular dysfunction in patients with aortic valvular diseases. J Am Soc Echocardiogr. 2013;26:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Nagata Y, Takeuchi M, Wu VC, Izumo M, Suzuki K, Sato K, Seo Y, Akashi YJ, Aonuma K, Otsuji Y. Prognostic value of LV deformation parameters using 2D and 3D speckle-tracking echocardiography in asymptomatic patients with severe aortic stenosis and preserved LV ejection fraction. JACC Cardiovasc Imaging. 2015;8:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 46. | Otto CM, Kumbhani DJ, Alexander KP, Calhoon JH, Desai MY, Kaul S, Lee JC, Ruiz CE, Vassileva CM. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2017;69:1313-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 389] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 47. | Clavel MA, Malouf J, Messika-Zeitoun D, Araoz PA, Michelena HI, Enriquez-Sarano M. Aortic valve area calculation in aortic stenosis by CT and Doppler echocardiography. JACC Cardiovasc Imaging. 2015;8:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 48. | Nakai H, Takeuchi M, Yoshitani H, Kaku K, Haruki N, Otsuji Y. Pitfalls of anatomical aortic valve area measurements using two-dimensional transoesophageal echocardiography and the potential of three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2010;11:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Tsang W, Bateman MG, Weinert L, Pellegrini G, Mor-Avi V, Sugeng L, Yeung H, Patel AR, Hill AJ, Iaizzo PA. Accuracy of aortic annular measurements obtained from three-dimensional echocardiography, CT and MRI: human in vitro and in vivo studies. Heart. 2012;98:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Jilaihawi H, Doctor N, Kashif M, Chakravarty T, Rafique A, Makar M, Furugen A, Nakamura M, Mirocha J, Gheorghiu M. Aortic annular sizing for transcatheter aortic valve replacement using cross-sectional 3-dimensional transesophageal echocardiography. J Am Coll Cardiol. 2013;61:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 51. | Khalique OK, Kodali SK, Paradis JM, Nazif TM, Williams MR, Einstein AJ, Pearson GD, Harjai K, Grubb K, George I. Aortic annular sizing using a novel 3-dimensional echocardiographic method: use and comparison with cardiac computed tomography. Circ Cardiovasc Imaging. 2014;7:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 52. | Altiok E, Koos R, Schröder J, Brehmer K, Hamada S, Becker M, Mahnken AH, Almalla M, Dohmen G, Autschbach R. Comparison of two-dimensional and three-dimensional imaging techniques for measurement of aortic annulus diameters before transcatheter aortic valve implantation. Heart. 2011;97:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Tamborini G, Fusini L, Gripari P, Muratori M, Cefalù C, Maffessanti F, Alamanni F, Bartorelli A, Pontone G, Andreini D. Feasibility and accuracy of 3DTEE versus CT for the evaluation of aortic valve annulus to left main ostium distance before transcatheter aortic valve implantation. JACC Cardiovasc Imaging. 2012;5:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Holmes DR Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD, Harrington RA, Bhatt DL, Ferrari VA, Fisher JD, Garcia MJ, Gardner TJ, Gentile F, Gilson MF, Hernandez AF, Jacobs AK, Kaul S, Linderbaum JA, Moliterno DJ, Weitz HH; American Heart Association; American Society of Echocardiography; European Association for Cardio-Thoracic Surgery; Heart Failure Society of America; Mended Hearts; Society of Cardiovascular Anesthesiologists; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collabration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Thorac Cardiovasc Surg. 2012;144:e29-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Bagur R, Rodés-Cabau J, Doyle D, De Larochellière R, Villeneuve J, Lemieux J, Bergeron S, Côté M, Bertrand OF, Pibarot P. Usefulness of TEE as the primary imaging technique to guide transcatheter transapical aortic valve implantation. JACC Cardiovasc Imaging. 2011;4:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Bartel T, Edris A, Velik-Salchner C, Müller S. Intracardiac echocardiography for guidance of transcatheter aortic valve implantation under monitored sedation: a solution to a dilemma? Eur Heart J Cardiovasc Imaging. 2016;17:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Athappan G, Patvardhan E, Tuzcu EM, Svensson LG, Lemos PA, Fraccaro C, Tarantini G, Sinning JM, Nickenig G, Capodanno D. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 611] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 58. | Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 885] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 59. | Abdel-Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tölg R, Zachow D, Guerra E, Massberg S, Schäfer U. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 531] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 60. | Altiok E, Frick M, Meyer CG, Al Ateah G, Napp A, Kirschfink A, Almalla M, Lotfi S, Becker M, Herich L. Comparison of two- and three-dimensional transthoracic echocardiography to cardiac magnetic resonance imaging for assessment of paravalvular regurgitation after transcatheter aortic valve implantation. Am J Cardiol. 2014;113:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Sengupta PP, Wiley BM, Basnet S, Rajamanickman A, Kovacic JC, Fischer GW, Kini AS, Sharma SK. Transthoracic echocardiography guidance for TAVR under monitored anesthesia care. JACC Cardiovasc Imaging. 2015;8:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Schattke S, Baldenhofer G, Prauka I, Zhang K, Laule M, Stangl V, Sanad W, Spethmann S, Borges AC, Baumann G. Acute regional improvement of myocardial function after interventional transfemoral aortic valve replacement in aortic stenosis: a speckle tracking echocardiography study. Cardiovasc Ultrasound. 2012;10:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Giannini C, Petronio AS, Talini E, De Carlo M, Guarracino F, Grazia M, Donne D, Nardi C, Conte L, Barletta V. Early and late improvement of global and regional left ventricular function after transcatheter aortic valve implantation in patients with severe aortic stenosis: an echocardiographic study. Am J Cardiovasc Dis. 2011;1:264-273. [PubMed] |
| 64. | Grabskaya E, Becker M, Altiok E, Dohmen G, Brehmer K, Hamada-Langer S, Kennes L, Marx N, Hoffmann R. Impact of transcutaneous aortic valve implantation on myocardial deformation. Echocardiography. 2011;28:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Swan A, Prakash R, Chew DP, Perry R, Sinhal A, Selvanayagam JB, Joseph MX. Instantaneous Decrease in Left Ventricular Afterload during Transcatheter Aortic Valve Implantation Results in Immediate Changes in Left Ventricular Strain. Echocardiography. 2016;33:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Kim HJ, Lee SP, Park CS, Park JB, Kim YJ, Kim HK, Sohn DW. Different responses of the myocardial contractility by layer following acute pressure unloading in severe aortic stenosis patients. Int J Cardiovasc Imaging. 2016;32:247-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Kamperidis V, Joyce E, Debonnaire P, Katsanos S, van Rosendael PJ, van der Kley F, Sianos G, Bax JJ, Ajmone Marsan N, Delgado V. Left ventricular functional recovery and remodeling in low-flow low-gradient severe aortic stenosis after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2014;27:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 68. | Løgstrup BB, Andersen HR, Thuesen L, Christiansen EH, Terp K, Klaaborg KE, Poulsen SH. Left ventricular global systolic longitudinal deformation and prognosis 1 year after femoral and apical transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2013;26:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Poulin F, Yingchoncharoen T, Wilson WM, Horlick EM, Généreux P, Tuzcu EM, Stewart W, Osten MD, Woo A, Thavendiranathan P. Impact of Prosthesis-Patient Mismatch on Left Ventricular Myocardial Mechanics After Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 2016;5:pii: e002866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Stefani L, De Luca A, Maffulli N, Mercuri R, Innocenti G, Suliman I, Toncelli L, Vono MC, Cappelli B, Pedri S. Speckle tracking for left ventricle performance in young athletes with bicuspid aortic valve and mild aortic regurgitation. Eur J Echocardiogr. 2009;10:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Park SH, Yang YA, Kim KY, Park SM, Kim HN, Kim JH, Jang SY, Bae MH, Lee JH, Yang DH. Left Ventricular Strain as Predictor of Chronic Aortic Regurgitation. J Cardiovasc Ultrasound. 2015;23:78-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Di Salvo G, Rea A, Mormile A, Limongelli G, D’Andrea A, Pergola V, Pacileo G, Caso P, Calabrò R, Russo MG. Usefulness of bidimensional strain imaging for predicting outcome in asymptomatic patients aged ≤ 16 years with isolated moderate to severe aortic regurgitation. Am J Cardiol. 2012;110:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Olsen NT, Sogaard P, Larsson HB, Goetze JP, Jons C, Mogelvang R, Nielsen OW, Fritz-Hansen T. Speckle-tracking echocardiography for predicting outcome in chronic aortic regurgitation during conservative management and after surgery. JACC Cardiovasc Imaging. 2011;4:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 74. | Iida N, Seo Y, Ishizu T, Nakajima H, Atsumi A, Yamamoto M, Machino-Ohtsuka T, Kawamura R, Enomoto M, Kawakami Y. Transmural compensation of myocardial deformation to preserve left ventricular ejection performance in chronic aortic regurgitation. J Am Soc Echocardiogr. 2012;25:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Gabriel RS, Kerr AJ, Sharma V, Zeng IS, Stewart RA. B-type natriuretic peptide and left ventricular dysfunction on exercise echocardiography in patients with chronic aortic regurgitation. Heart. 2008;94:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Marciniak A, Sutherland GR, Marciniak M, Claus P, Bijnens B, Jahangiri M. Myocardial deformation abnormalities in patients with aortic regurgitation: a strain rate imaging study. Eur J Echocardiogr. 2009;10:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Onishi T, Kawai H, Tatsumi K, Kataoka T, Sugiyama D, Tanaka H, Okita Y, Hirata K. Preoperative systolic strain rate predicts postoperative left ventricular dysfunction in patients with chronic aortic regurgitation. Circ Cardiovasc Imaging. 2010;3:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Ewe SH, Haeck ML, Ng AC, Witkowski TG, Auger D, Leong DP, Abate E, Ajmone Marsan N, Holman ER, Schalij MJ. Detection of subtle left ventricular systolic dysfunction in patients with significant aortic regurgitation and preserved left ventricular ejection fraction: speckle tracking echocardiographic analysis. Eur Heart J Cardiovasc Imaging. 2015;16:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Broch K, de Marchi SF, Massey R, Hisdal J, Aakhus S, Gullestad L, Urheim S. Left Ventricular Contraction Pattern in Chronic Aortic Regurgitation and Preserved Ejection Fraction: Simultaneous Stress-Strain Analysis by Three-Dimensional Echocardiography. J Am Soc Echocardiogr. 2017;30:422-430.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Enache R, Popescu BA, Piazza R, Muraru D, Călin A, Beladan CC, Roşca M, Nicolosi GL, Ginghină C. Left ventricular shape and mass impact torsional dynamics in asymptomatic patients with chronic aortic regurgitation and normal left ventricular ejection fraction. Int J Cardiovasc Imaging. 2015;31:1315-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Mizariene V, Bucyte S, Zaliaduonyte-Peksiene D, Jonkaitiene R, Vaskelyte J, Jurkevicius R. Left ventricular mechanics in asymptomatic normotensive and hypertensive patients with aortic regurgitation. J Am Soc Echocardiogr. 2011;24:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Chen Y, Zhang Z, Cheng L, Fan L, Wang C, Shu X. The Early Variation of Left Ventricular Strain after Aortic Valve Replacement by Three-Dimensional Echocardiography. PLoS One. 2015;10:e0140469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Sato H, Ohta T, Hiroe K, Okada S, Shimizu K, Murakami R, Tanabe K. Severity of aortic regurgitation assessed by area of vena contracta: a clinical two-dimensional and three-dimensional color Doppler imaging study. Cardiovasc Ultrasound. 2015;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Choi J, Hong GR, Kim M, Cho IJ, Shim CY, Chang HJ, Mancina J, Ha JW, Chung N. Automatic quantification of aortic regurgitation using 3D full volume color doppler echocardiography: a validation study with cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2015;31:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Perez de Isla L, Zamorano J, Fernandez-Golfin C, Ciocarelli S, Corros C, Sanchez T, Ferreirós J, Marcos-Alberca P, Almeria C, Rodrigo JL. 3D color-Doppler echocardiography and chronic aortic regurgitation: a novel approach for severity assessment. Int J Cardiol. 2013;166:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Shibayama K, Watanabe H, Sasaki S, Mahara K, Tabata M, Fukui T, Takanashi S, Sumiyoshi T, Tomoike H, Shiota T. Impact of regurgitant orifice height for mechanism of aortic regurgitation. JACC Cardiovasc Imaging. 2013;6:1347-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |