Peer-review started: January 8, 2018
First decision: January 23, 2018
Revised: January 23, 2018
Accepted: February 5, 2018
Article in press: February 5, 2018
Published online: February 26, 2018
Processing time: 49 Days and 17 Hours
The red blood cell distribution width (RDW) is a simple, rapid, inexpensive and straightforward hematological parameter, reflecting the degree of anisocytosis in vivo. The currently available scientific evidence suggests that RDW assessment not only predicts the risk of adverse outcomes (cardiovascular and all-cause mortality, hospitalization for acute decompensation or worsened left ventricular function) in patients with acute and chronic heart failure (HF), but is also a significant and independent predictor of developing HF in patients free of this condition. Regarding the biological interplay between impaired hematopoiesis and cardiac dysfunction, many of the different conditions associated with increased heterogeneity of erythrocyte volume (i.e., ageing, inflammation, oxidative stress, nutritional deficiencies and impaired renal function), may be concomitantly present in patients with HF, whilst anisocytosis may also directly contribute to the development and worsening of HF. In conclusion, the longitudinal assessment of RDW changes over time may be considered an efficient measure to help predicting the risk of both development and progression of HF.
Core tip: The red blood cell distribution width is a simple, rapid, inexpensive and straightforward hematological parameter, reliably reflecting the degree of anisocytosis in vivo. The current epidemiological and biological evidence suggests that longitudinal assessment of red blood cell distribution width over time may be considered an efficient measure to help predicting the risk of both development and progression of heart failure.
- Citation: Lippi G, Turcato G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width in heart failure: A narrative review. World J Cardiol 2018; 10(2): 6-14
- URL: https://www.wjgnet.com/1949-8462/full/v10/i2/6.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i2.6
As a complex clinical syndrome, heart failure (HF) is characterized by certain symptoms and signs such as dyspnea and fatigue, which impair exercise tolerance, fluid retention, and may provoke pulmonary and/or splanchnic congestion, ankle swelling, peripheral edema, elevated jugular venous pressure, and pulmonary crackles[1,2]. These are principally due to structural and/or functional cardiac abnormalities, which result in an impaired cardiac output and/or elevated intracardiac pressures[1]. The classification of the different types of HF is based on left ventricle ejection fraction (LVEF) as follows: (1) HF with preserved LVEF (HFpEF), i.e., patients with normal LVEF (≥ 50%); (2) HF with reduced EF (HFrEF), i.e., patients with reduced LVEF (< 40%); (3) HF with midrange EF (HFmrEF), i.e., patients with an LVEF in the range of 40%-49%[1].
The etiology of HF is varied, including a wide range of pathologies both cardiovascular and non-cardiovascular. Many patients will suffer different diseases at the same time, which ultimately trigger the HF. Nonetheless, a history of ischemic heart disease (IHD) and myocardial infarction or revascularization is very common among patients with HF[1]. Thus, among the most important causes of death in patients with HF are cardiovascular diseases, mainly sudden death and worsening HF[3,4]. It seems that HFpEF and HFrEF have different etiological profiles, since patients with HFpEF are more often older, women and have a history of hypertension and atrial fibrillation (AF). However, a history of myocardial infarction is uncommonly found in HFpEF patients[5].
The prevalence of HF in developed countries is considered to be around 1%-2% of the adult general population[1]. The incidence increases with age, up to ≥ 10% among people > 70 years of age[6]. For instance, 20% of American population ≥ 40 years of age will develop HF[7] and nearly 5.1 million people in the United States already have clinical signs and symptoms of HF, with a prevalence that seems to be constantly increasing[8], so that approximately 33% of men and 28% of women ≥ 55 years will develop HF worldwide[9]. Using the conventional definition, the percentage of patients with HFpEF ranges from 22% to 73%[1]. Likewise, the incidence of HF may be decreasing, more for HFrEF than for HFpEF[10,11]. Inequalities in the epidemiology of HF have been also reported. A high risk of developing HF has been reported in black populations[12], whilst the incidence seems the lowest among white women[13] and the highest among black men[14], with a higher 5-year mortality[15]. Non-Hispanic black males have a higher prevalence (4.5%) than females (3.8%), whilst non-Hispanic white males also have a higher prevalence (2.7%) than females (1.8%)[8].
Many prognostic biomarkers of death and/or hospitalization in patients with HF have been studied and identified[1]. Unfortunately, their clinical uses remains limited due to the challenges in stratifying the risk of HF patients. Furthermore, multiple prognostic risk scores have been developed in HF[4,16,17], and may be helpful to predict death in these patients. However, they are less useful to predict HF hospitalizations[16,17]. In fact, several studies only reported a moderate accuracy of these models to predict mortality, whilst they were basically less accurate for predicting hospitalization[16,17].
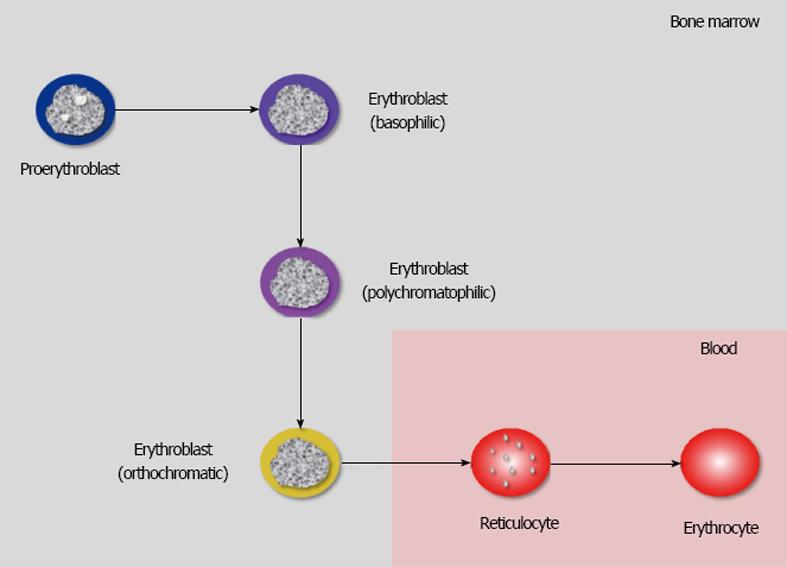
The erythrocytes, also known as red blood cells (RBCs), are non-nuclear corpuscular elements of blood produced in the bone from erythroid colony-forming unit-erythroid (CFU-E) progenitors, which undergo a complex process of maturation (also known as erythropoiesis) into proerythroblasts, erythroblasts, reticulocytes and, finally, into mature erythrocytes[18]. The ensuing conversion of erythroblasts into reticulocytes and erythrocytes is accompanied by the loss of the nucleus, which makes erythrocytes virtually terminal elements (Figure 1).
The entire process of erythropoiesis is regulated by several transcription factors, chromatin modifiers, cytokines, and hormones, the most important of which is erythropoietin (Epo), which not only stimulates the proliferation and differentiation of hematopoietic precursors but is also essential for survival of newly generated RBCs[18]. Physiological erythropoiesis mainly occurs in the bone marrow, so that proerythroblasts and erythroblasts (in their different stages of maturation, i.e., basophilic, polychromatophilic and orthochromatic) are normally absent in the bloodstream, whilst the number of reticulocytes is typically < 1% of the total RBC population[19]. The leading function of RBCs is carrying oxygen throughout the bloodstream, from the lungs to the peripheral tissues, mainly bound to hemoglobin, the most important protein contained within the erythrocytes. The total number of mature RBCs in adult human blood is usually comprised between 4.7 × 1012/L-6.1 × 1012/L in men and 4.2 × 1012/L-5.4 × 1012/L in women, respectively, with a mean survival time in blood of approximately 100-120 d. A reduction of RBC number below these conventional thresholds is known as “anemia”, which is usually diagnosed when the level of hemoglobin in blood falls below 130 g/L in men and 120 g/L in women, respectively[18].
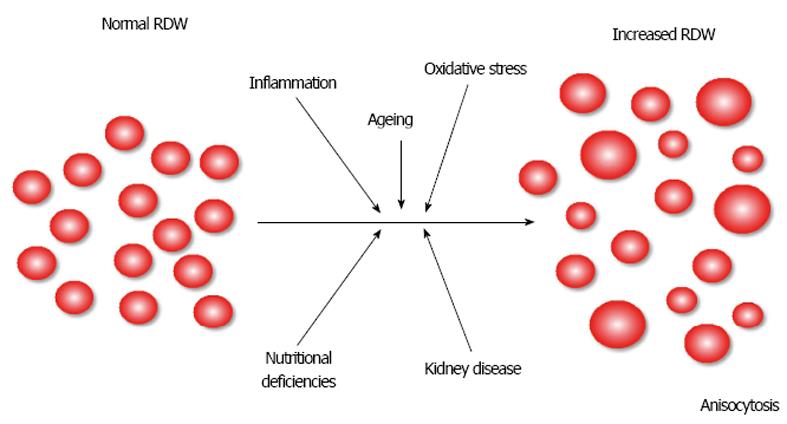
A typical mature erythrocyte appears as a disc-shaped element with a pale-staining central area, a diameter comprised between 6-8 μm and a total volume (also known as mean corpuscular volume (MCV)] comprised between 80-100 fL. RBC with abnormal volumes, either reduced or increased, are conventionally called microcytic or macrocytic, respectively[19]. RBCs display a physiological size heterogeneity in adult human blood, which is usually measured in terms of RBC distribution width (RDW). This simple and straightforward parameter can thus be expressed both in absolute value, as the standard deviation (SD) of erythrocyte volumes (RDW-SD), or as the coefficient of variation (RDW-CV) of erythrocyte volumes [i.e., (RDW-SD)/ (MCV)*100]. The normal range of RDW-CV is 11.5-14.5% but often varies according to the technique used for its assessment by the different commercially available hematological analyzers[20]. Although a decreased RDW value is very uncommon and has no clinically significance[21], an increase of this parameter is called anisocytosis and has many important consequences on the future risk of adverse cardiovascular events and mortality in the general population[22], as well as in patients with HF or in those at risk of developing HF[23], as more comprehensively discussed in the next sections of this article.
Anisocytosis can hence be essentially defined as a DW value exceeding the analyzer-dependent threshold[24]. More practically, it can be defined as the presence of erythrocytes with a large size heterogeneity in peripheral venous blood, as simplified in Figure 2. The partial or complete derangement of many biological pathways, mainly including aging, inflammation, oxidative stress, nutritional deficiencies, and impaired renal function, has been straightforwardly associated with disrupted erythropoiesis resulting in variable degrees of anisocytosis (Table 1)[25]. Therefore, the aim of this article is to provide an overview of the epidemiological and biological evidence linking anisocytosis and HF.
| Conditions | MCV | RDW |
| Chronic diseases anemia | ↓ | N |
| Heterozygous thalassemia | ↓ | N |
| Iron deficiency | ↓ | ↑ |
| β-thalassemia | ↓ | ↑ |
| Sickle cell trait | ↓ | N |
| Haemolytic anemia | N/↓ | ↑ |
| Hereditary spherocytosis | N/↓ | ↑ |
| Sickle cell disease | N | ↑ |
| Haemorrhage | N | N |
| Blood transfusions | N | ↑ |
| Chronic liver disease | N/↑ | ↑ |
| Aplastic anemia | ↑ | N |
| Folate deficiency | ↑ | ↑ |
| Vitamin B12 deficiency | ↑ | ↑ |
| Myelodysplastic syndrome | ↑ | ↑ |
The very first large prospective study which explored the clinical significance of measuring RDW in patients with HF was published in 2007 by Felker et al[26]. Briefly, the authors measured RDW values at enrollment in 2679 chronic HF patients recruited from the North American Candesartan in HF: Assessment of Reduction in Mortality and Morbidity (CHARM) study (validation cohort), who were followed-up for at least 2 years for collecting data on death or hospitalization for managing worsened HF. The data obtained in this cohort were then validated in a replication dataset consisting of additional 2140 HF patients enrolled from the Duke Databank in 1969, and with follow-up data completely available for more than 96% patients. In the final multivariable analysis, including all significant clinical and laboratory parameters, each 1 SD increase of RDW in the CHARM Cohort was associated with 17% higher risk of cardiovascular death or hospitalization for HF [hazard ratio (HR), 1.17; 95% confidence interval (95%CI), 1.10-1.25] and 12% higher risk (HR = 1.12; 95%CI: 1.03-1.20) of all-cause mortality. In the validation cohort, each 1 SD increase of RDW was also associated as with 29% enhanced risk (HR = 1.29; 95%CI: 1.16-1.43) of all-cause mortality.
The following year, Tonelli et al[27] published the results of another large prospective study, based on 4111 participants of the Cholesterol and Recurrent Events study, free of HF at baseline, who had their RDW value measured at enrollment and were then followed-up for a median period of approximately 60 mo. In a multivariable model adjusted for all significant clinical and laboratory parameters, each 1% increase in RDW value was associated with 14% increased all-cause mortality (HR = 1.14; 95%CI: 1.05-1.24). Importantly, each 1% increase in RDW value was also associated with 15% higher risk (HR = 1.15; 95%CI: 1.05-1.26) of developing symptomatic HF on follow-up.
These earlier findings were then replicated in a vast number of prospective, retrospective and cross-sectional studies, which were meta-analyzed by Huang et al[28], Shao et al[29] and, more recently, by Hou et al[30] (Table 2).
| Ref. | Variable | Outcome measure | Baseline RDW |
| Huang et al[28], 2014 | 1% increase in RDW value | Risk of future death in patients with HF | HR, 1.10 (95%CI: 1.07-1.13) |
| Risk of hospitalization in patients with HF | HR, 1.09 (95%CI: 1.03-1.16) | ||
| Shao et al[29], 2015 | 1% increase in RDW value | Risk of future MACE in patients with HF | HR, 1.19 (95%CI: 1.08-1.30) |
| Risk of future death in patients with HF | HR, 1.12 (95%CI: 1.08-1.16) | ||
| Hou et al[30], 2017 | 1% increase in RDW value | General risk of HF | HR, 1.11 (95%CI: 1.05-1.17) |
| HR, 1.11 (95%CI: 1.04-1.14) |
More specifically, in the meta-analysis by Huang and collaborators[28], each 1% increase in RDW value was associated with 10% enhanced risk of future mortality events (HR = 1.10; 95%CI: 1.07-1.13) in patients with HF. No substantial difference was observed between retrospective (n = 4; HR, 1.09 and 95%CI: 1.02-1.17) and prospective (n = 5; HR = 1.10 and 95%CI: 1.05-1.15) studies, whilst a greater risk was observed in studies with follow-up >2 years (n = 5; HR = 1.13 and 95%CI: 1.09-1.16) than in those with shorter follow-up (n = 4; HR = 1.04; 95%CI: 1.02-1.06). In the ensuing meta-analysis of Shao et al[29], each 1% increase in RDW value was associated with 19% enhanced risk of major adverse cardiovascular events (HR = 1.19; 95%CI: 1.08-1.30), with 12% higher risk of death (HR = 1.12; 95%CI: 1.08-1.16), as well as with 9% higher risk of hospitalization (HR = 1.09, 95%CI: 1.03-1.16) in patients with HF. Notably, the association between RDW value and death was found slightly stronger in patients with chronic HF (HR = 1.13, 95%CI: 1.08-1.18) than in those with acute HF (HR = 1.09; 95%CI: 1.04-1.15). More recently, the meta-analysis of Hou et al[30] showed that each 1% increase in RDW value was associated with 11% higher risk of death (HR = 1.11; 95%CI: 1.04-1.14) in patients with HF, whilst each 1% increase in RDW value was associated with 11% higher risk of HF in patients with preexisting cardiovascular disease (HR = 1.11; 95%CI: 1.05-1.17).
Although the notion that baseline RDW assessment may help to predict both unfavorable outcomes in patients with acute or chronic HF as well as the risk of developing HF in patients without this condition seems now quite straightforward (Table 2), an alternative concept is strongly emerging, indicating that serial assessment of RDW over time may be more clinically meaningful and informative than the admission value (Table 3).
| Ref. | Study design | Outcome measure | Variable | Baseline RDW | Longitudinal RDW change |
| Cauthen et al[31], 2012 | Retrospective, 6159 patients with chronic HF | 1-year all-cause mortality | 1% increase in RDW at diagnosis or during 1 year of follow-up | RR, 1.09 (95%CI: 1.01-1.17) | RR, 1.21 (95%CI: 1.08-1.34) |
| Makhoul et al[32], 2013 | Prospective, 614 patients with acute decompensated HF followed-up during hospital stay | All-cause mortality during hospital stay | 1% increase in RDW value at admission or during hospital stay | HR, 1.15 (95%CI: 1.08-1.21) | HR, 1.23 (95%CI: 1.09-1.38) |
| Núñez et al[33], 2014 | Prospective, 1702 patients with HF followed-up for 18 mo | All-cause mortality during follow-up | RDW ≥ 15% at admission or during follow-up | Anemic patients: HR, 1.04 (95%CI: 1.00-1.07)Non-anemic patients: HR, 1.11 (95%CI: 1.05-1.19) | Anemic patients: HR, 1.08 (95%CI: 1.04-1.13)Non-anemic patients: HR, 1.31 (95%CI: 1.22-1.42) |
| Ferreira et al[35], 2016 | Retrospective, 502 patients with acute decompensated HF | Hospitalization for acute decompensated HF or 180-d cardiovascular death | RDW ≥ 15% at admission and delta RDW > 0 at discharge | OR, 1.29 (95%CI: 0.71-2.33) | OR, 2.47 (95%CI: 1.35-4.51) |
| Muhlestein et al[34], 2016 | Prospective, 6414 patients with HF followed-up during hospital stay | 30-d all-cause mortality | 1% increase in RDW value at admission and during hospital stay | HR, 1.09 (95%CI: 1.07-1.12) | HR, 1.09 (95%CI: 1.03-1.16) |
| Uemura et al[36], 2016 | Prospective, 229 patients with acute decompensated HF followed-up followed for 692 d | All-cause mortality during follow-up | RDW ≥ 14.5% at admission and positive change of RDW at discharge | HR, 1.08 (95%CI: 0.99-1.19) | HR, 1.19 (95%CI: 1.01-1.41) |
| Turcato et al[37], 2017 | Retrospective, 588 patients with acute decompensated HF | 30-d all caused mortality | ΔRDW > 0.4% at 48 and 96 h | - | OR, 3.04 (95%CI: 1.56-5.94) and 3.65 (95%CI: 2.02-6.15) |
The first study which assessed the significance of longitudinal RDW changes in patients with HF was published by Cauthen et al[31]. The authors retrospectively analyzed data from 6159 ambulatory chronic HF patients, with the aim of exploring the potential association between clinical outcomes and RDW changes over a 1-year follow-up period. Although each 1% increase in baseline RDW value was independently associated with 9% enhanced risk of 1-year all-cause mortality [relative risk (RR) = 1.09; 95%CI: 1.01-1.17], this association was found to be much stronger considering longitudinal RDW variations (RR for each 1% increase in RDW during follow-up, 1.12; 95%CI: 1.08-1.34).
In the study performed in 2013 by Makhoul et al[32], the population consisted of a total number of 614 patients with acute decompensation of HF, who had RDW measured at baseline and throughout hospital stay, and who were then followed-up for 1 year. Interestingly, each 1% increase in RDW value measured at baseline was independently associated with a 15% higher risk (HR = 1.15; 95%CI: 1.08-1.21) of all-cause mortality, but this association was even stronger using longitudinal changes of RDW, since each 1% increase in RDW value during hospital stay was associated with 23% higher risk (HR = 1.23; 95%CI: 1.09-1.38) of all-cause mortality.
In 2014, Núñez et al[33] also studied 1702 patients discharged after being diagnosed with acute HF, and who had their RDW assessed during a median follow-up period of 18 mo. The baseline RDW value was found to be independently associated with all-cause mortality both in anemic (HR = 1.04; 95%CI: 1.00-1.07) and non-anemic patients (HR = 1.11; 95%CI: 1.05-1.19), but an even stronger association was found between the last longitudinally updated RDW (i.e., the mean of RDW values measured during follow-up) and death, both in anemic (HR = 1.08; 95%CI: 1.04-1.13) and non-anemic (1.31; 95%CI: 1.22-1.42) patients.
In an ensuing article, Muhlestein et al[34] published the results of a prospective study based on 6414 patients hospitalized for HF, who had RDW measured within 24 h from admission and at least one more time during hospitalization. As predictable, each 1% increase in RDW measured at baseline was independently associated with a 9% higher risk of 30-d all-cause mortality (HR = 1.09; 95%CI: 1.07-1.12), but a similar risk was also observed for each 1% increase in RDW during hospitalization (HR = 1.09; 95%CI: 1.03-1.16). Interestingly, the risk of 30-d all-cause death was considerably magnified (i.e., HR, 2.02) when data of both the baseline value and longitudinal changes of RDW were combined in the predictive model.
Ferreira et al[35] carried out a retrospective study based on 2 independent cohorts of patients admitted to the emergency department with acute decompensation of HF, the first (i.e., the derivation cohort) consisting of 170 patients and the second (i.e., the validation cohort) consisting of 332 patients. RDW was measured at admission and at hospital discharge, with calculation of the ratio between these two values (i.e., ΔRDW). In the final model, a RDW value >15% at admission was independently associated with a 29% higher risk [odds ratio (OR), 1.29; 95%CI: 0.71-2.33] of composite outcome (hospitalization for acute decompensated HF or 180-d cardiovascular death), whilst such risk was found to be substantially higher for patients with ΔRDW > 0 (OR = 2.47; 95%CI: 1.35-4.51). Even more importantly, the combination of RDW value > 15% at admission and ΔRDW > 0 yielded a substantially higher risk of composite outcome than the two measures alone (OR = 3.40; 95%CI: 1.63-7.08).
Uemura et al[36] studied 229 patients hospitalized for acute decompensated HF, who had their RDW measured at admission and at hospital discharge, and who were then followed-up for a median period of 692 d. Although an increased baseline value of RDW at admission (i.e., ≥ 14.5%) was slightly but non-significantly associated with all-cause mortality (HR, 1.08; 95%CI: 0.99-1.19), patients exhibiting a positive change (i.e., an increase) of RDW between admission and discharge had a 19% higher risk of all-cause mortality on follow-up (HR = 1.19; 95%CI: 1.01-1.41).
More recently, Turcato et al[37] carried out a retrospective study including 588 patients hospitalized for acute decompensation of HF. RDW values were measured at admission and also after 48 h and 96 h of hospitalization. Interestingly, a ΔRDW > 0.4% calculated between the value at admission and those obtained after 48 h and 96 h of hospital stay was independently associated with a over 3-fold higher risk of 30-d mortality (OR of 48 h ΔRDW, 3.04; 95%CI: 1.56-5.94 and OR of 96 h ΔRDW, 3.65; 95%CI: 2.02-6.15).
Finally, Xanthopoulos et al[38] studied 218 patients who were admitted to the emergency department for acute HF, and who had their RDW measured at admission, at discharge and at 4, 8 and 12 mo afterward. Follow-up for all-cause mortality or rehospitalization was 12 mo. Each 1% increase in RDW value at admission was independently associated with the composite endpoint both in non-diabetic (HR = 1.14; 95%CI: 1.01-1.29) and diabetic (1.35; 95%CI: 1.12-1.62) patients. Notably, the longitudinal changes of RDW showed a significant interaction with diabetes (β coefficient, -0.002; P = 0.042), thus highlighting that metabolic imbalances may actually have an impact on longitudinal changes of RDW. According to these findings, anisocytosis may hence be considered not only a bystander but also a potential underlying biological mechanism explaining the adverse long-term effects of diabetes on the risk of hospitalization and mortality in patients with HF[39].
Regarding the physiopathological interplay between anisocytosis and HF, many of the different conditions impairing hematopoiesis, and thus potentially leading to a larger size heterogeneity of RBC volumes (Figure 2), may be concomitantly present in patients with HF.
Convincing evidence has accumulated that both cell- and cytokine-mediated inflammatory pathways actively contribute to development and progression of HF[40]. An important interplay has also been recognized between inflammation and anisocytosis since inflammation is frequently associated with bone marrow dysfunction and an increase of circulating premature erythrocytes[41]. As regards oxidative stress, an excess production of reactive oxygen species (ROS) has been associated with both adverse cardiac remodeling[42] and deranged hematopoiesis, ultimately leading to anisocytosis[43]. Nutritional deficiencies are commonplace in many forms of anemia characterized by different degrees of anisocytosis[44], but they are also deeply involved in onset and progression of HF[45]. The progressive impairment of renal function is one of the leading causes of anemia and anisocytosis, especially in the elderly[46], but is also an important determinant of adverse outcomes in patients with HF[47]. Lastly, anisocytosis gradually increases with aging as a result of multiple metabolic dysfunctions[48], but advanced age is also a strong contributing factor for cardiac dysfunction[49]. Therefore, the current evidence suggests that anisocytosis and HF may share many pathogenetic mechanisms, which may explain why both conditions may develop and progress in parallel, thus making RDW a reliable marker of cardiac dysfunction.
Nevertheless, anisocytosis may also play a direct role in the onset and progressive worsening of HF. The erythrocyte size heterogeneity mirrors a reduced (often severely impaired) function of this essential corpuscular blood elements. In conditions of high anisocytosis, RBCs are often characterized by lower deformability and decreased oxygen-carrier capacity, thus contributing to reduced oxygenation of many peripheral tissues and cells (including cardiomyocytes), whilst abnormal erythrocytes may also actively participate in the pathogenesis of cardiac fibrosis through promotion or amplification of inflammation, cardiomyocyte stress and apoptosis[20].
The RDW is a simple, rapid, inexpensive and straightforward hematological parameter, which is now automatically generated by all commercially available hematological analyzers together with the complete blood cells count (CBC). Increased RDW values in venous blood samples truly mirror the degree of anisocytosis in vivo, and can hence be used for diagnostic, prognostic and even therapeutic decisions in many acute and chronic pathological conditions[50].
The currently available scientific evidence convincingly suggests that RDW measurement not only predicts the risk of adverse outcomes (cardiovascular and all-cause mortality, hospitalization for acute decompensation or cardiac dysfunction) in patients with HF but is also a significant and independent predictor of developing HF in patients free of this condition at the time of baseline assessment (Table 2). Nevertheless, the longitudinal assessment of RDW changes over time (i.e., during a hospital stay or shortly afterward) may be an even more effective measure than the baseline value for predicting adverse outcomes in patients with chronic, acute and even acutely decompensated HF (Table 3). The longitudinal assessment of RDW has another important advantage, emerging from its insensitivity to the analyzer used for its measurement. In fact, longitudinal changes either assessed as differences or ratios between the first and the following measurements, may help overcoming the still unresolved issue of poor harmonization of RDW measures[24], which still hampers the identification of an universally valid diagnostic or predictive threshold. It is also noteworthy in the two studies combining RDW values at admission and their subsequent variations during follow-up[34,35], the diagnostic efficiency of this combination was found to be much better than either measure alone for predicting adverse outcomes in HF patients.
In conclusion, we suggest that the serial measurement of RDW, and especially the combination of admission value with subsequent changes during in-hospital or home care, may be seen as an affordable and efficient tool to help assessing the prognosis of patients with HF and for reliably predicting the risk of adverse events.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amiya E, Anan R, Nunez-Gil IJJ, Teragawa H S- Editor: Cui LJ L- Editor: A E- Editor: Yan JL
| 1. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10348] [Cited by in RCA: 9335] [Article Influence: 1037.2] [Reference Citation Analysis (3)] |
| 2. | Writing Committee Members, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1565] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 3. | Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L; Heart Failure Association of the European Society of Cardiology (HFA). EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15:808-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 579] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 4. | Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 926] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 5. | Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 548] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 6. | Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1267] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 7. | Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 8. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2893] [Cited by in RCA: 3392] [Article Influence: 282.7] [Reference Citation Analysis (0)] |
| 9. | Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 787] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 10. | Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 603] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 11. | Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3102] [Article Influence: 163.3] [Reference Citation Analysis (1)] |
| 12. | Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138-2145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 491] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 13. | Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1172] [Cited by in RCA: 1196] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 14. | Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1182] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 15. | Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 16. | Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 17. | Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail. 2014;2:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118:6258-6268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 19. | Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013;3:a011601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 20. | Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 710] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 21. | Fischbach FT, Dunning MB. A manual of laboratory and diagnostic tests. Philadelphia: Wolters Kluwer Health 2015; . |
| 22. | Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, Bandinelli S, Phillips CS, Yu B, Connelly S. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 23. | Lippi G, Cervellin G. Risk assessment of post-infarction heart failure. Systematic review on the role of emerging biomarkers. Crit Rev Clin Lab Sci. 2014;51:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Lippi G, Pavesi F, Bardi M, Pipitone S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem. 2014;47:1100-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Lippi G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width and cardiovascular disorders. Does it really matter which comes first, the chicken or the egg? Int J Cardiol. 2016;206:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 696] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 27. | Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008;117:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 603] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 28. | Huang YL, Hu ZD, Liu SJ, Sun Y, Qin Q, Qin BD, Zhang WW, Zhang JR, Zhong RQ, Deng AM. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. PLoS One. 2014;9:e104861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 29. | Shao Q, Li L, Li G, Liu T. Prognostic value of red blood cell distribution width in heart failure patients: a meta-analysis. Int J Cardiol. 2015;179:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Hou H, Sun T, Li C, Li Y, Guo Z, Wang W, Li D. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci Rep. 2017;7:43420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Cauthen CA, Tong W, Jain A, Tang WH. Progressive rise in red cell distribution width is associated with disease progression in ambulatory patients with chronic heart failure. J Card Fail. 2012;18:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Makhoul BF, Khourieh A, Kaplan M, Bahouth F, Aronson D, Azzam ZS. Relation between changes in red cell distribution width and clinical outcomes in acute decompensated heart failure. Int J Cardiol. 2013;167:1412-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Núñez J, Núñez E, Rizopoulos D, Miñana G, Bodí V, Bondanza L, Husser O, Merlos P, Santas E, Pascual-Figal D. Red blood cell distribution width is longitudinally associated with mortality and anemia in heart failure patients. Circ J. 2014;78:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Muhlestein JB, Lappe DL, Anderson JL, Muhlestein JB, Budge D, May HT, Bennett ST, Bair TL, Horne BD. Both initial red cell distribution width (RDW) and change in RDW during heart failure hospitalization are associated with length of hospital stay and 30-day outcomes. Int J Lab Hematol. 2016;38:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Ferreira JP, Girerd N, Arrigo M, Medeiros PB, Ricardo MB, Almeida T, Rola A, Tolppanen H, Laribi S, Gayat E. Enlarging Red Blood Cell Distribution Width During Hospitalization Identifies a Very High-Risk Subset of Acutely Decompensated Heart Failure Patients and Adds Valuable Prognostic Information on Top of Hemoconcentration. Medicine (Baltimore). 2016;95:e3307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Uemura Y, Shibata R, Takemoto K, Uchikawa T, Koyasu M, Watanabe H, Mitsuda T, Miura A, Imai R, Watarai M. Elevation of red blood cell distribution width during hospitalization predicts mortality in patients with acute decompensated heart failure. J Cardiol. 2016;67:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Turcato G, Zorzi E, Prati D, Ricci G, Bonora A, Zannoni M, Maccagnani A, Salvagno GL, Sanchis-Gomar F, Cervellin G. Early in-hospital variation of red blood cell distribution width predicts mortality in patients with acute heart failure. Int J Cardiol. 2017;243:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Xanthopoulos A, Giamouzis G, Melidonis A, Kitai T, Paraskevopoulou E, Paraskevopoulou P, Patsilinakos S, Triposkiadis F, Skoularigis J. Red blood cell distribution width as a prognostic marker in patients with heart failure and diabetes mellitus. Cardiovasc Diabetol. 2017;16:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Dauriz M, Mantovani A, Bonapace S, Verlato G, Zoppini G, Bonora E, Targher G. Prognostic Impact of Diabetes on Long-term Survival Outcomes in Patients With Heart Failure: A Meta-analysis. Diabetes Care. 2017;40:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 40. | Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of Inflammation in Heart Failure. Curr Atheroscler Rep. 2017;19:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 41. | Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 42. | Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181-H2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 870] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 43. | Friedman JS, Lopez MF, Fleming MD, Rivera A, Martin FM, Welsh ML, Boyd A, Doctrow SR, Burakoff SJ. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood. 2004;104:2565-2573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 45. | Sciatti E, Lombardi C, Ravera A, Vizzardi E, Bonadei I, Carubelli V, Gorga E, Metra M. Nutritional Deficiency in Patients with Heart Failure. Nutrients. 2016;8:pii E442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest. 2008;68:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 47. | Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 48. | Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med. 2014;52:e197-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Vigen R, Maddox TM, Allen LA. Aging of the United States population: impact on heart failure. Curr Heart Fail Rep. 2012;9:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Lippi G, Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med. 2014;52:1247-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |