Published online Nov 26, 2018. doi: 10.4330/wjc.v10.i11.234
Peer-review started: July 10, 2018
First decision: August 2, 2018
Revised: September 2, 2018
Accepted: October 11, 2018
Article in press: October 11, 2018
Published online: November 26, 2018
Processing time: 140 Days and 3.6 Hours
To investigate the incidence and risk factors for vancomycin concentrations less than 10 mg/L during cardiac surgery.
In this prospective study, patients undergoing cardiac surgery received a single dose of 1000 mg of vancomycin. Multiple arterial samples were drawn during surgery. Exclusion criteria were hepatic dysfunction; renal dysfunction; ongoing infectious diseases; solid or hematologic tumors; severe insulin-dependent diabetes; body mass index of < 17 or > 40 kg/m2; pregnancy or lactation; antibiotic, corticosteroid, or other immunosuppressive therapy; vancomycin or nonsteroidal anti-inflammatory drug therapy in the previous 2 wk; chemotherapy or radiation therapy in the previous 6 mo; allergy to vancomycin or cefazolin; drug abuse; cardiac surgery in the previous 6 mo; previous or scheduled organ transplantation; preoperative stay in the intensive care unit for more than 24 h; emergency procedure or lack of adequate preparation for surgery; and participation in another trial.
Over a 1-year period, 236 patients were enrolled, and a total of 1682 serum vancomycin concentrations (median 7/patient) were measured. No vancomycin levels under 10 mg/L were recorded in 122 out of 236 patients (52%), and 114 out of 236 patients (48%) were found to have at least 1 serum sample with a vancomycin level < 10 mg/L; 54 out of 236 patients (22.9%) had at least 5 serum samples with a vancomycin level lower than 10 mg/L. Vancomycin infusion was administered for 60 min in 97 out of 236 patients (41%). In 47 patients (20%), the duration of infusion was longer than 60 min, and in 92 patients (39%) the duration of infusion was shorter than 60 min. The maximum concentration and area under the concentration-time curve were significantly higher in patients with no vancomycin levels less than 10 mg/L (P < 0.001). The multivariate analysis identified female gender, body mass index (BMI) > 25 kg/m2, and creatinine clearance above 70 mL/min as risk factors for vancomycin levels less than 10 mg/L.
Results of this study identified female gender, BMI > 25 kg/m2, and creatinine clearance above 70 mL/min as risk factors for suboptimal vancomycin serum concentration during cardiac surgery; no relationship was found between infusion duration and vancomycin levels less than 10 mg/L. These findings call attention to the risk of facilitating the emergence of vancomycin-resistant methicillin-resistant Staphylococcus aureus strains.
Core tip: The aim of this study was to investigate the incidence and risk factors for vancomycin concentrations less than 10 mg/L during cardiac surgery. Over a 1-year period, 236 patients were enrolled, and a total of 1682 serum vancomycin concentrations were measured. A total of 48% of patients were found to have ≥ 1 sample with a vancomycin level < 10 mg/L. The maximum concentration and area under the concentration-time curve were significantly higher in patients with no vancomycin levels less than 10 mg/L (P < 0.001). The multivariate analysis identified female gender, body mass index > 25 kg/m2, and creatinine clearance above 70 mL/min as risk factors for vancomycin levels less than 10 mg/L.
- Citation: Cotogni P, Barbero C, Rinaldi M. Incidence and risk factors for potentially suboptimal serum concentrations of vancomycin during cardiac surgery. World J Cardiol 2018; 10(11): 234-241
- URL: https://www.wjgnet.com/1949-8462/full/v10/i11/234.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i11.234
Surgical site infections (SSIs) related to methicillin-resistant Staphylococcus aureus (MRSA) after cardiac surgery continue to cause substantial morbidity and mortality[1-3]. Therefore, the prevention of this feared complication, particularly in terms of antimicrobial prophylaxis, is a matter of discussion in the literature. The practice guidelines from the Society of Thoracic Surgeons on antibiotic prophylaxis in patients undergoing cardiac surgery suggests combining a β-lactam (cefazolin) with a glycopeptide (vancomycin) for antimicrobial prophylaxis in the scenario of an established “high incidence” of MRSA; a dose of 1 to 1.5 g or a weight-adjusted dose of 15 mg/kg of vancomycin administered intravenously over 1 h, with the infusion ending within 1 h from the incision of the skin, is recommended[4,5]. Likewise, the 2011 guidelines of the American College of Cardiology and the American Heart Association recommend that vancomycin should be initiated 2 h before cardiac surgery and administered by a slow infusion[6]. However, a detailed protocol of administration with dose and levels of vancomycin to reach and maintain during the surgical procedure is still not reported.
In addition, evidence highlights that in the current practice there is often a gap between the duration of administration or timing of antimicrobial prophylaxis recommended in the guidelines and what is practiced[7,8]; this may increase the risk of potentially suboptimal serum vancomycin levels during surgery, jeopardizing the efficacy of antimicrobial prophylaxis. Indeed, low serum vancomycin concentrations-lower than 10 mg/L - seem to be related with the emergence of vancomycin-resistant MRSA strains: vancomycin-resistant Staphylococcus aureus (VRSA), vancomycin-intermediate Staphylococcus aureus (VISA), and heteroresistant VISA (hVISA)[9-11]. To date, strains of VRSA, VISA, and hVISA have been reported from many countries, including the United States, Japan, Australia, France, Scotland, Brazil, Korea, Hong Kong, and others[10-12].
Our group has already analyzed intraoperative vancomycin pharmacokinetics (PK) in 236 patients undergoing cardiac surgery over a 1-year period[13]. In this study, and in the same study population, the incidence of potentially suboptimal vancomycin levels during cardiac surgery was investigated.
The primary objective of the present study was to investigate the incidence of vancomycin levels less than 10 mg/L during cardiac surgery. The secondary objective was to identify risk factors for intraoperative vancomycin levels less than 10 mg/L.
Over a 1-year period, a prospective study was carried out in the Department of Cardiovascular Surgery of a 1200-bed tertiary care university hospital, where approximately 850 cardiac operations are performed every year. The study design has been described previously[13]. The inclusion criteria included adult patients undergoing cardiac surgery, who were receiving a single 1000 mg vancomycin dose as prophylaxis, diluted in 100 mL 0.9% NaCl solution and administered by intravenous infusion over 60 min, with a skin incision made between 16 and 120 min after the end of the vancomycin infusion, as recommended by Garey et al[14]. The exclusion criteria included hepatic dysfunction (bilirubin ≥ 2 mg/dL); renal dysfunction [creatinine > 1.5 mg/dL or creatinine clearance (CrCl) ≤ 30 mL/min, estimated by the Cockcroft-Gault formula]; infectious diseases that required antibiotic therapy 2 wk prior to the procedure; solid or hematologic tumors; severe insulin-dependent diabetes; a body mass index (BMI) < 17 or > 40 kg/m2; pregnancy or lactation; antibiotic, corticosteroid, or other immunosuppressive therapy; vancomycin or non-steroidal anti-inflammatory drug therapy 2 wk prior to the procedure; chemotherapy or radiation therapy in the previous 6 mo; allergy to vancomycin or cefazolin; drug abuse; cardiac surgery in the previous 6 mo; previous or scheduled organ transplantation; a preoperative stay in the intensive care unit for more than 24 h; an emergency procedure or lack of adequate preparation for surgery; and participation in another trial.
Our protocol of antimicrobial prophylaxis is also designed for a single 1000 mg cefazolin dose, diluted in a 20 mL 0.9% NaCl solution, initiated 30 to 60 min before surgery and administered as a slow intravenous bolus. Three further doses of 1000 mg of cefazolin at 8-h intervals were given postoperatively, while no further doses of vancomycin were administered postoperatively. Since 2005, our protocol has allowed the choice to combine cefazolin with vancomycin for antimicrobial prophylaxis in patients undergoing cardiac surgery[13,15]. The rationale for using vancomycin was due to an increased prevalence of MRSA infections, which exceeded 60% hospital-wide, and to the identification of isolates in cardiac surgery patients with SSIs. The vancomycin protocol and timing of administration were chosen based upon recommendations of our Hospital Infection Control Committee and guidelines from the Society of Thoracic Surgeons[5,13,15].
A healthcare provider (i.e., physician, nurse or cardiovascular technician) was required to document the exact time the antibiotic infusion was initiated, as well as anesthesiologists or cardiac surgeons who recorded the exact time of the first skin incision and skin closure.
The study protocol was reviewed and approved by our Institutional Ethics Committee (approval No. 0078553), and patients provided written informed consent before their enrollment. The work was conducted in compliance with the Institutional Review Board/Human Subjects Research Committee requirements.
The vancomycin assay and PK analysis have been reported previously[13].Briefly, for the on-pump group, arterial samples were drawn from the arterial catheter before cardiopulmonary bypass (CPB) [end of infusion maximum concentration (Cmax) and skin incision (Cincision)], during CPB (5, 30, 60 min after the CPB start, and subsequently every 60 min to the CPB end: C5, C30, C60, C120, C180, and C240), and after CPB [wound closure (Cclosure)]. For the off-pump group, some arterial samples (i.e., C5, C30, C60, C120, C180, and C240) were drawn and time-matched to the CPB period of the on-pump group.
According to the Centers for Disease Control and Prevention guidelines, the definition of SSI requires positive culture results of surgical sites or drainage from the mediastinal area or evidence of infection during surgical re-exploration or fever, sternal instability, and positive blood culture results[16]. SSIs were classified as (1) superficial (infection above the sternum with no bone involvement); (2) deep (infection involving the sternum and organ/space such as mediastinitis); and (3) leg donor site infections[16].
The receiver operator characteristic (ROC) curve analysis was used to investigate the relationship between the duration of drug infusion and the occurrence of vancomycin concentrations under 10 mg/L. PK characteristics were compared between patients with vancomycin levels constantly above 10 mg/L and patients with at least 1 vancomycin level lower than 10 mg/L using the Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous ones. All reported P-values were obtained by the 2-sided exact method, at the conventional 5% significance level. A multivariate binary logistic model was used to predict risk factors for vancomycin levels less than 10 mg/L. Data were analyzed using R 3.4.0 (The R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org). The statistical methods of this study were reviewed by R Passera, a biomedical statistician.
Two hundred thirty-six cardiac surgery patients were enrolled in this study. The patients’ characteristics are shown in Table 1. During the study, 1682 serum vancomycin concentrations were measured, and 7 (median; range 6-9) blood samples per patient were collected. Vancomycin PK during cardiac surgery has been reported previously[13]. Out of the 1682 serum samples, vancomycin levels were lower than 10 mg/L in 443 cases, between 10 and 20 mg/L in 821 cases, between 20 and 30 mg/L in 192 cases, between 30 and 40 mg/L in 73 cases, between 40 and 50 mg/L in 50 cases, and higher than 50 mg/L in 103 cases.
| Characteristics | |
| Patients | 236 |
| Age, median (range) | 70 (25-86) |
| Male gender | 149 (63) |
| BMI, kg/m2, median (range) | 26 (18-40) |
| Diabetes | 46 (19) |
| COPD | 0 |
| Hypertension | 151 (64) |
| Smoke | 28 (12) |
| Surgical time, min, median (range) | 249 (119-593) |
| Surgical procedure | |
| CABG | 72 (30.5) |
| Valve | 113 (47.9) |
| CABG+Valve | 34 (14.4) |
| Other1 | 17 (7.2) |
| Off-pump CABG | 21 (8.9) |
| Left IMA | 53 (22.4) |
| Both IMA | 17 (7.2) |
| EUROscore add, median (range) | 5 (1-6) |
| EUROscore log, median (range) | 4.8 (1-7.74) |
| Mechanical ventilation, d, median (range) | 7 (2-912) |
| ICU stay, d, median (range) | 1 (1-24) |
| RBC transfusions, n, median (range) | 2 (0-9) |
Three SSIs were recorded (1.3%): one was a superficial wound infection, and 2 were deep wound infections; no SSIs were detected at the donor site. Pathogens isolated in SSIs included two gram-negative bacteria or fungi and one methicillin-sensitive Staphylococcus aureus.
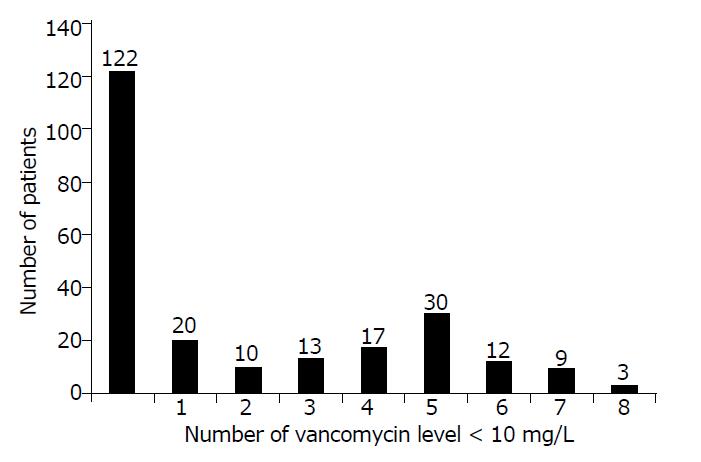
Figure 1 shows that, between the Cmax time and Cclose time, no vancomycin levels less than 10 mg/L were recorded in 122 out of 236 patients (52%) and that 114 out of 236 patients (48%) were found to have at least 1 sample with a vancomycin level < 10 mg/L. Fifty-four out of 236 patients (22.9%) had at least 5 serum samples with vancomycin levels lower than 10 mg/L.
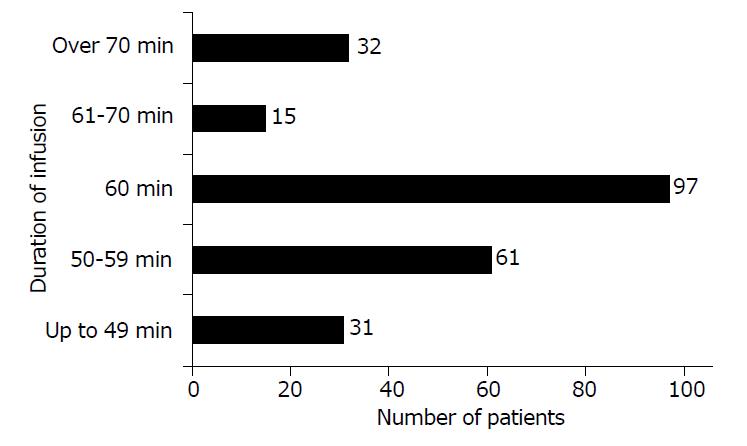
Vancomycin infusion was administered for 60 min in 97 out of 236 patients (41%). In 47 patients (20%), the duration of infusion was longer than 60 min, and in 92 patients (39%) the duration of infusion was shorter than 60 min (Figure 2).
The ROC curve analysis showed no influences of duration of drug infusion on the occurrence of vancomycin concentrations less than 10 mg/L. No significant relationships were found between the number of episodes of vancomycin levels less than 10 mg/L and the clusters of duration of infusion (P = 0.089).
No significant differences were observed in terms of the SSI rate between patients with vancomycin levels constantly above 10 mg/L (2 out of 3 cases) and patients with at least 1 level less than 10 mg/L (1 out of 3 cases).
Vancomycin PK parameters were estimated and compared between above versus under 10 mg/L patient groups (Table 2): Cmax and the area under the concentration-time (AUC) curve were significantly higher in patients with no vancomycin level under 10 mg/L, while the apparent total body clearance (Cl) and the apparent volume of distribution during the terminal phase (Vd) were significantly higher in patients with at least 1 episode of vancomycin concentration under 10 mg/L.
| Vancomycin levels < 10 mg/L (n = 114) | Vancomycin levels ≥ 10 mg/L (n = 122) | P-value | |
| Cmax (mg/L) | 33.2 (6.2-122.0) | 57.9 (14.6-210.0) | <0.001 |
| AUC (mg*h/L) | 119.2 (28.3-247.7) | 191.7 (95.8-467.8) | <0.001 |
| t1/2 (h) | 3.9 (1.6-9.2) | 4.0 (1.3-9.8) | 0.437 |
| CL (L/h) | 8.4 (4.0-35.3) | 5.2 (2.1-10.4) | <0.001 |
| Vd (L) | 47.2 (16.5-195.2) | 29.8 (12.4-63.3) | <0.001 |
The multivariate binary logistic model identified female gender, BMI higher than 25, and creatinine clearance above 70 mL/min as risk factors for vancomycin levels less than 10 mg/L (Tables 3 and 4).
| OR | 95%CI | P-value | |
| CPB (off-pump vs on-pump) | 0.51 | 0.20-1.30 | 0.156 |
| Vancomycin infusion (stopped vs non-stopped) | 0.6 | 0.17-2.10 | 0.421 |
| Age at surgery (> 70 yr vs ≤ 70 yr) | 0.54 | 0.32-0.92 | 0.021 |
| Gender (Female vs male) | 0.44 | 0.26-0.76 | 0.003 |
| BMI (> 25 vs ≤ 25) | 2.3 | 1.36-3.88 | 0.002 |
| Fluid balance (> 2000 mL vs ≤ 2000 mL) | 1.44 | 0.86-2.41 | 0.163 |
| CrCl (> 70 mL/min vs ≤ 70 mL/min) | 2.56 | 1.51-4.34 | <0.001 |
| Vancomycin dosage (> 15 mg/kg vs ≤ 15 mg/kg) | 0.38 | 0.21-0.66 | 0.001 |
| Vancomycin infusion duration | 0.237 | ||
| 50-59 min vs < 50 min | 0.5 | 0.21-1.21 | 0.123 |
| 60 min vs < 50 min | 0.63 | 0.28-1.42 | 0.26 |
| 61-70 min vs < 50 min | 0.48 | 0.14-1.69 | 0.254 |
| > 70 min vs < 50 min | 1.2 | 0.44-3.31 | 0.719 |
| OR | 95%CI | P-value | |
| Age at surgery (>70 yr vs ≤ 70 yr) | 0.69 | 0.36-1.28 | 0.245 |
| Gender (Female vs male) | 0.54 | 0.30-0.97 | 0.039 |
| BMI (> 25 vs ≤ 25) | 1.99 | 1.15-3.45 | 0.015 |
| CrCl (> 70 mL/min vs ≤ 70 mL/min) | 1.92 | 1.09-3.40 | 0.024 |
| Vancomycin dosage (>15 mg/kg vs ≤ 15 mg/kg) | 0.75 | 0.36-1.57 | 0.451 |
SSI is still one of the most serious complications after cardiac surgery, and one of the main strategies for prevention is the use of an appropriate antibiotic prophylaxis[17]. The spectrum of microorganisms related to SSIs varies among institutions; however, in the literature, MRSA and methicillin-resistant S. Epidermidis are the leading pathogens, and this brings attention to vancomycin as the prophylactic drug of choice[1-3].
Vancomycin has been one of the most investigated antimicrobial drugs as well as one of the most used antibiotics for the prevention and treatment of infections in cardiac surgery[10]. Several studies have investigated the association between vancomycin use as an antimicrobial prophylactic drug and the rate of SSIs in this surgical population. Different studies have analyzed vancomycin PK during cardiac surgery and the effects of CPB on serum vancomycin concentrations[13,18-20]. Other studies were carried out examining the timing of antibiotic prophylaxis, and in particular, the relationship between the end of vancomycin infusion and the first skin incision[14,15].
However, to date, no general agreement exists regarding guidelines for the dose and duration of antimicrobial prophylaxis administration, and, particularly, the level of vancomycin to reach and maintain during the surgical procedure for effective antimicrobial prophylaxis. Moreover, whether or not suboptimal intraoperative vancomycin levels are a cause of postoperative SSIs is still controversial. Studies have suggested that vancomycin operates in a concentration-independent fashion in which AUC is more effective than the drug level[10]. PK results of our study are in line with reports in the literature, even when administering vancomycin in the case of treating infections rather than antibiotic prophylaxis[10,21]; in particular, in our study, AUC was wider in the group of patients with no vancomycin levels less than 10 mg/L.
Larsson et al[22] simulated an in vitro model in which free vancomycin peak concentrations of 40, 20, 10, and 5 mg/L reported no significant difference in the corresponding bacterial kill curves for Staphylococcus aureus. On the other hand, to date, increasing evidence supports that Staphylococcus aureus exposure to trough serum concentrations of vancomycin lower than 10 mg/L can generate MRSA strains with vancomycin-resistant characteristics[5,10,11,23]. Sakoulas et al[24] have determined that the emergence of hVISA or VISA occurred when MRSA was exposed to suboptimal vancomycin concentrations (<10 mg/L); in this in vitro study the minimal inhibitory concentration (MIC) increased from 1 to 8 mg/L. Tsuji et al[25] evaluated Staphylococcus aureus accessory gene regulator groups I - IV exposed both to suboptimal and optimal vancomycin doses (1.5-10 mg/L) and reported that exposure to low vancomycin doses produced increases in the MIC to that of the VISA range.
In the present study, 114 out of 236 patients were found to have at least 1 value of vancomycin level lower than 10 mg/L between the Cmax time and Cclose time. The relatively small sample size and the low incidence of SSIs in this surgical population (1.3%, 3 out of 236 cases) make it difficult to obtain a significant relationship between vancomycin concentrations during surgery and the incidence of SSIs. However, this finding may be considered an indicator of the risk of selection of MRSA strains with vancomycin-resistant characteristics.
The multivariate analysis showed that female gender, BMI higher than 25, and creatinine clearance above 70 mL/min were risk factors for potentially suboptimal vancomycin concentrations. Regarding the BMI as a risk factor, our results are in line with other reports that highlight the efficacy of weight-based vancomycin dosing[26,27]. Recently, the European Medicines Agency claimed that the starting dose of vancomycin by infusion should be calculated according to the age and weight of the patient[28].
The study has many important characteristics. First of all, the results were obtained through a clinical trial and not from an analysis of a registry or database. Second, it was a prospective study. Third, only patients undergoing cardiac surgery were included. Fourth, information regarding antibiotic timing was gathered in “real-time” in the operating theater and not from the patient chart. Finally, the same protocol of antimicrobial prophylaxis was administered to all patients. Moreover, to the best of our knowledge, this is the first study investigating the incidence and risk factors for potentially suboptimal serum concentrations of vancomycin during cardiac surgery with such a large number of measured serum vancomycin concentrations (i.e., 1682).
This study has some limitations. First, no statistical analysis was performed on the number of patients enrolled since the study was planned by our statistician to be continuous over 12 mo. Second, it was a single-center trial. Third, we considered vancomycin levels of 10 mg/L as an arbitrary cut-off for potentially suboptimal serum concentrations when an antimicrobial prophylaxis has been administered, referring to the level reported in the literature in the case of antimicrobial therapy. Finally, a larger study should be carried out to investigate clinical variables; indeed, the number of subjects was appropriate for a pharmacokinetic study but insufficient to find statistically significant differences in the SSI rate between patient groups with vancomycin levels above or under 10 mg/L.
In conclusion, evidence in the literature suggests that the exposure to low vancomycin levels should be considered a risk factor in the selection of MRSA strains with vancomycin-resistant characteristics. The present study on vancomycin PK in cardiac surgery patients has reported an incidence of intraoperative potentially suboptimal concentrations of vancomycin in almost 50% of patients. Our data analysis shows that female gender, BMI higher than 25, and creatinine clearance above 70 mL/min were risk factors for potentially suboptimal concentrations of vancomycin. Overall, these findings call attention to the risk of potentially suboptimal serum concentrations of vancomycin during cardiac surgery. However, further studies are needed to better define the threshold level of serum intraoperative vancomycin concentration associated with the risk for the emergence of vancomycin resistance.
Based on evidence suggesting that Staphylococcus aureus exposure to low vancomycin concentrations can produce vancomycin-resistant strains, it is recommended that trough therapeutic serum concentrations of vancomycin are maintained above 10 mg/L.
There are no recommendations in the literature indicating target vancomycin concentrations to maintain intraoperatively for effective antimicrobial prophylaxis.
The aim of this prospective study was to evaluate the incidence and risk factors for vancomycin concentrations under 10 mg/L in adult patients undergoing cardiac surgery.
In this study, the frequency of suboptimal vancomycin levels intraoperatively was investigated in samples collected from cardiac surgery patients receiving a single 1000 mg vancomycin dose.
We found an incidence of intraoperative potentially suboptimal concentrations of vancomycin in almost 50% of these patients. The multivariate analysis identified female gender, body mass index > 25, and creatinine clearance above 70 mL/min as risk factors for vancomycin levels less than 10 mg/L.
Although we arbitrarily considered vancomycin levels of 10 mg/L as a cut-off, the findings of our study are interesting because they suggest a high incidence of potentially suboptimal serum concentrations in the case of antimicrobial prophylaxis.
Further studies will be necessary to define the cut-off of intraoperative vancomycin levels representing the optimal concentration of vancomycin for appropriate antimicrobial prophylaxis in patients undergoing cardiac surgery.
We thank Roberto Passera, PharmD, PhD, biomedical statistician at the Nuclear Medicine Unit, Molinette Hospital, University of Turin, who performed the pharmacokinetics and statistical analysis. We are grateful to the surgeons, anesthesiologists, cardiovascular perfusionists, and nurses of the Department of Cardiovascular Surgery for their contribution throughout the study period. We would also like to thank the technicians from our hospital’s Laboratory of Therapeutic Drug Monitoring for their support.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Greenway SC, Petix NR S- Editor: Wang JL L- Editor: A E- Editor: Wu YXJ
| 1. | Kanafani ZA, Arduino JM, Muhlbaier LH, Kaye KS, Allen KB, Carmeli Y, Corey GR, Cosgrove SE, Fraser TG, Harris AD. Incidence of and preoperative risk factors for Staphylococcus aureus bacteremia and chest wound infection after cardiac surgery. Infect Control Hosp Epidemiol. 2009;30:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Chen LF, Arduino JM, Sheng S, Muhlbaier LH, Kanafani ZA, Harris AD, Fraser TG, Allen K, Corey GR, Fowler VG Jr. Epidemiology and outcome of major postoperative infections following cardiac surgery: risk factors and impact of pathogen type. Am J Infect Control. 2012;40:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Yavuz SŞ, Tarçın O, Ada S, Dinçer F, Toraman S, Birbudak S, Eren E, Yekeler I. Incidence, aetiology, and control of sternal surgical site infections. J Hosp Infect. 2013;85:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR; Society of Thoracic Surgeons. The Society of Thoracic Surgeons Practice Guideline Series: Antibiotic Prophylaxis in Cardiac Surgery, Part I: Duration. Ann Thorac Surg. 2006;81:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, Jacobs M, Fernando H, Bridges C; Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg. 2007;83:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Society of Cardiovascular Anesthesiologists; Society of Thoracic Surgeons. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123-e210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 7. | Miliani K, L’Hériteau F, Astagneau P; INCISO Network Study Group. Non-compliance with recommendations for the practice of antibiotic prophylaxis and risk of surgical site infection: results of a multilevel analysis from the INCISO Surveillance Network. J Antimicrob Chemother. 2009;64:1307-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Lador A, Nasir H, Mansur N, Sharoni E, Biderman P, Leibovici L, Paul M. Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Appelbaum PC. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents. 2007;30:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1344] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 11. | Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, Grabsch EA, Roberts SA, Robson J, Read K. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis. 2004;38:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 498] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Cotogni P, Passera R, Barbero C, Gariboldi A, Moscato D, Izzo G, Rinaldi M. Intraoperative vancomycin pharmacokinetics in cardiac surgery with or without cardiopulmonary bypass. Ann Pharmacother. 2013;47:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Garey KW, Dao T, Chen H, Amrutkar P, Kumar N, Reiter M, Gentry LO. Timing of vancomycin prophylaxis for cardiac surgery patients and the risk of surgical site infections. J Antimicrob Chemother. 2006;58:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Cotogni P, Barbero C, Passera R, Fossati L, Olivero G, Rinaldi M. Violation of prophylactic vancomycin administration timing is a potential risk factor for rate of surgical site infections in cardiac surgery patients: a prospective cohort study. BMC Cardiovasc Disord. 2017;17:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250-78; quiz 279-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2944] [Cited by in RCA: 2795] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 17. | Cotogni P, Barbero C, Rinaldi M. Deep sternal wound infection after cardiac surgery: Evidences and controversies. World J Crit Care Med. 2015;4:265-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Vuorisalo S, Pokela R, Syrjälä H. Is single-dose antibiotic prophylaxis sufficient for coronary artery bypass surgery? An analysis of peri- and postoperative serum cefuroxime and vancomycin levels. J Hosp Infect. 1997;37:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Miglioli PA, Merlo F, Grabocka E, Padrini R. Effects of cardio-pulmonary bypass on vancomycin plasma concentration decay. Pharmacol Res. 1998;38:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Ortega GM, Martí-Bonmatí E, Guevara SJ, Gómez IG. Alteration of vancomycin pharmacokinetics during cardiopulmonary bypass in patients undergoing cardiac surgery. Am J Health Syst Pharm. 2003;60:260-265. [PubMed] |
| 21. | Jeffres MN, Isakow W, Doherty JA, McKinnon PS, Ritchie DJ, Micek ST, Kollef MH. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006;130:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Larsson AJ, Walker KJ, Raddatz JK, Rotschafer JC. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J Antimicrob Chemother. 1996;38:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38:448-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 24. | Sakoulas G, Eliopoulos GM, Moellering RC Jr, Novick RP, Venkataraman L, Wennersten C, DeGirolami PC, Schwaber MJ, Gold HS. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis. 2003;187:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Tsuji B, Rybak M. The influence of Staphylococcus aureus accessory gene regulator (agr) function on the development of vancomycin heteroresistance in an in vitro pharmacodynamic model. In: Abstracts of the Fifteenth European Congress of Clinical Microbiology and Infectious Diseases. The 15th European Congress of Clinical Microbiology and Infectious Diseases; 2005; European Society for Clinical Microbiology and Infectious Disease, 2005: 1590. |
| 26. | Crabtree TD, Codd JE, Fraser VJ, Bailey MS, Olsen MA, Damiano RJ Jr. Multivariate analysis of risk factors for deep and superficial sternal infection after coronary artery bypass grafting at a tertiary care medical center. Semin Thorac Cardiovasc Surg. 2004;16:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2003;23:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | European Medicines Agency. EMA recommends changes to prescribing information for vancomycin antibiotics - Changes aim to ensure appropriate use in context of fight against antimicrobial resistance. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2017/05/news_detail_002748.jsp&mid=WC0b01ac058004d5c1. |