Published online Oct 26, 2018. doi: 10.4330/wjc.v10.i10.145
Peer-review started: May 13, 2018
First decision: June 14, 2018
Revised: June 30, 2018
Accepted: August 11, 2018
Article in press: August 11, 2018
Published online: October 26, 2018
Processing time: 165 Days and 17.1 Hours
To examine the efficacy and safety of the 6 French (6F) Rotarex®S catheter system in patients with acute limb ischemia (ALI) involving thromboembolic occlusion of the proximal and mid-crural vessels.
The files of patients in our department with ALI between 2015 and 2017 were examined. In seven patients, the Rotarex®S catheter was used in the proximal segment of the crural arteries. Data related to the clinical examination, Doppler sonography, angiography and follow-up from these patients were further used for analysis.
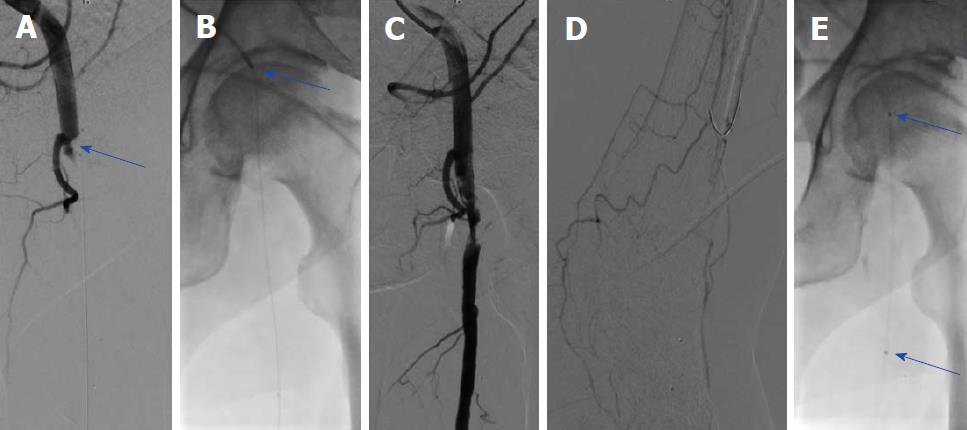
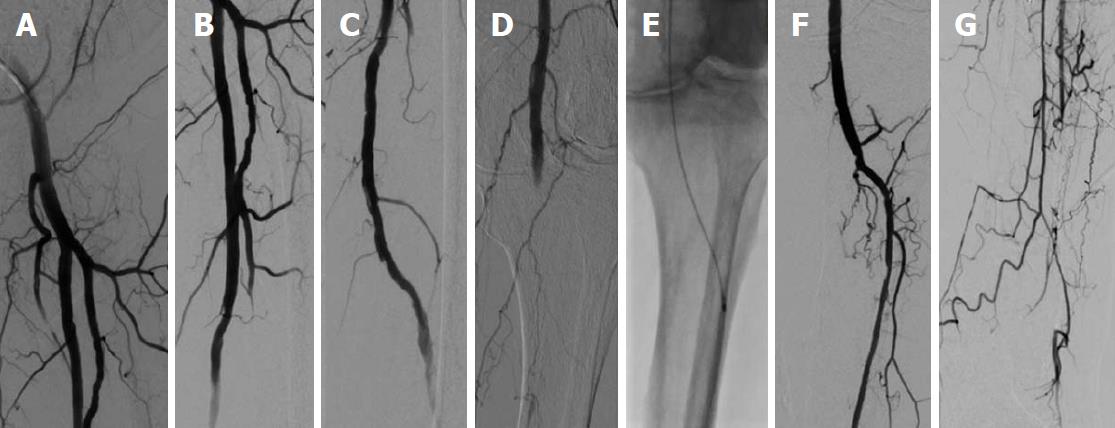
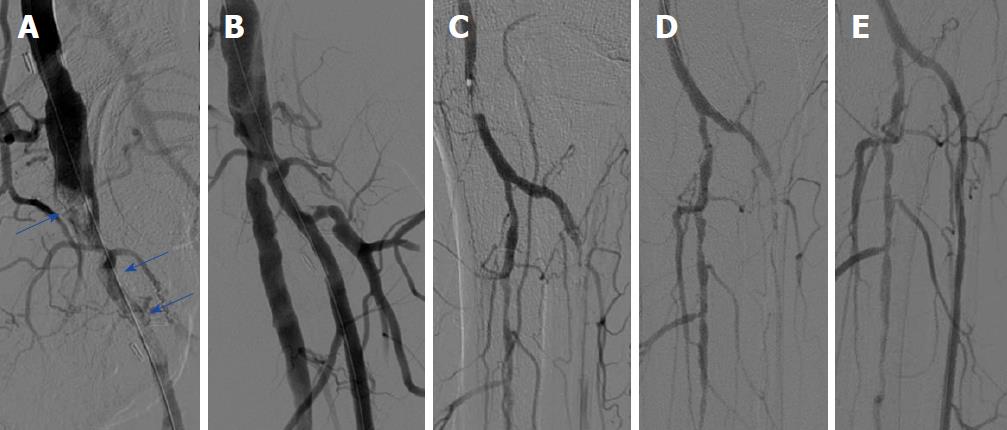
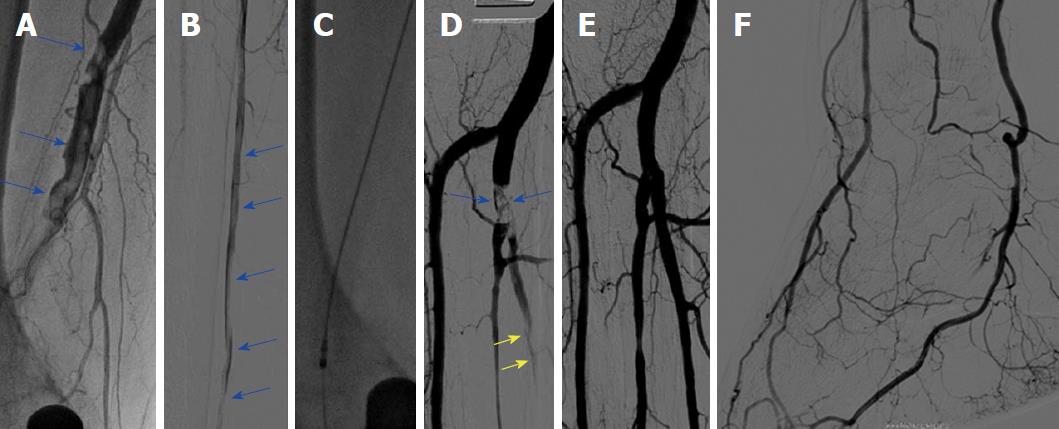
Two patients (29%) had thrombotic occlusion of the common femoral artery, and the remaining five exhibited thrombosis of the superficial femoral artery and popliteal artery. Mechanical thrombectomy was performed in all cases using a 6F Rotarex®S catheter. Additional Rotarex®S catheter thrombectomy due to remaining thrombus formation with no reflow was performed in the anterior tibial artery in two of seven cases (29%), in the tibiofibular tract and posterior tibial artery in two of seven cases (29%) and in the tibiofibular tract and fibular artery in the remaining three of seven cases (43%). Ischemic symptoms resolved promptly in all, and none of the patients experienced a procedural complication, such as crural vessel dissection, perforation or thrombus embolization.
Mechanical debulking using the 6F Rotarex®S catheter system may be a safe and effective treatment option in case of thrombotic or thromboembolic occlusion of the proximal and mid-portion of crural arteries.
Core tip: Herein, we report on seven consecutive patients with acute limb ischemia, who were treated by an endovascular approach, using the 6 French (6F) Rotarex®S catheter system for local mechanical thrombectomy. The procedures were effective in all cases, restoring flow and abolishing ischemic symptoms without causing any complications. Thus, mechanical debulking using the 6F Rotarex®S catheter system may be a safe and effective treatment option in the case of thrombotic occlusion of the proximal and mid-portion of crural arteries, obviating the need for local thrombolysis, which is associated with an increased risk for major bleeding.
- Citation: Giusca S, Raupp D, Dreyer D, Eisenbach C, Korosoglou G. Successful endovascular treatment in patients with acute thromboembolic ischemia of the lower limb including the crural arteries. World J Cardiol 2018; 10(10): 145-152
- URL: https://www.wjgnet.com/1949-8462/full/v10/i10/145.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i10.145
Acute limb ischemia (ALI) constitutes a medical emergency defined as a severely reduced perfusion of the leg resulting from a total or subtotal arterial occlusion, with symptoms debuting < 14 d prior to presentation. It has an incidence of around 140/million/year and a prevalence of 1%-3%[1,2]. Depending on the severity of symptoms, patients can be grouped according to the Rutherford classification of lower extremity ischemia[3]. Although significant advances have been made in the treatment of ALI, most of the studies still report an amputation rate of 10%-30% at 30 d[4-6]. Patients with thromboembolic ALI are especially at high risk for major amputation and death due to sepsis and multi-organ dysfunction. Such patients are usually older than 75 years and show further co-morbidities, including atrial fibrillation and history of heart failure[7].
Previous studies demonstrated the superiority of catheter-directed thrombolysis (CDT) compared to surgical treatment in regard to amputation-free survival in patients presenting with ALI[6,8]. However, this technique has its clear limitations in patients with an increased bleeding risk. Therefore, percutaneous mechanical thrombectomy systems have emerged in the last years as a valid therapeutic option in patients with ALI[9]. One such system, the Rotarex®S mechanical debulking catheter (Straub Medical, Wangs, Switzerland) is based on mechanical fragmentation and simultaneous aspiration of occlusion material, thus transporting the debris out of the patient. Several studies have shown a very high success rate of Rotarex®S alone or in combination with drug-coated balloons in terms of establishing vessel patency in patients with ALI[5]. However, operators should be cautious when using the 6 French (6F) Rotarex®S catheter in arteries below-the-knee because this catheter system is limited to vessel diameters of ≥ 3mm and might cause dissection or perforation when used in smaller diameter arteries. Herein, we present the clinical safety and effectiveness of the 6F Rotarex®S system in a miniseries of seven patients with acute lower limb ischemia affecting their crural arteries.
The files of 102 patients with thrombotic occlusions of the lower extremities between January 2015 and December 2017 at the Department of Cardiology and Vascular Medicine, Academic Teaching Hospital Weinheim were examined. In seven patients, the Rotarex®S catheter was used in the proximal segment of the crural arteries, and the data from these patients were further used for the analysis. In the remaining 95 patients, the Rotarex®S catheter was used for mechanical thrombectomy in iliac and femoropopliteal arteries. The study was approved by the local ethics committee of the University Hospital Heidelberg (S-100/2017). Retrospective data were collected in accordance with the Declaration of Helsinki.
All patients received a bolus of 2500 U of heparin after placement of a 6F sheath introducer in the femoral artery. During interventional treatment, all patients also received heparin to reach an activated clotting time of > 300 s. If necessary, patients also received 500 mg aspirin during and 300 mg clopidogrel during or after the interventional procedure. If additional thrombolysis was deemed necessary, a bolus of 10 mg recombinant tissue plasminogen activator (rtPA) was administrated after placement of the dedicated thrombolysis catheter (Unifuse catheter, AngioDynamics, Netherlands). Postprocedural rtPA was continuously administered at an infusion rate of 1 mg/h for 6-18 h, adding heparin to achieve partial thromboplastin time of 50-60 s.
Continuous variables are presented as numbers, providing the corresponding range of each variable. Categorical variables are represented as percentages. Measures of the vessel diameters were conducted using ImageJ software (version 1.50, NIH, Bethesda, MD, United States).
We present a mini-series of seven patients (Patient A-G). Baseline characteristics of our patients are provided in Table 1. Patients were referred to our department with symptoms of ALI with new onset of pain, paleness and pulselessness during the last 17 ± 13 h. Duplex sonography revealed thrombotic occlusion of the common femoral artery (CFA) in two of seven cases (29%), and of the distal superficial femoral artery (SFA) and of the popliteal artery in the remaining five of seven cases (71%). The localization of arterial occlusion was confirmed in all cases by digital subtraction angiography. Mechanical thrombectomy was performed in all cases using a 6F Rotarex®S catheter and was combined by local lysis in five of seven cases (71%). Rotarex®S catheter thrombectomy was performed in the CFA and in the SFA in two of seven cases (29%), and in the SFA and in the popliteal artery in the remaining five of seven cases (71%). Additional Rotarex®S catheter thrombectomy due to remaining thrombus formation with no reflow in the crural arteries was performed in the anterior tibial artery in two of seven cases (29%), in the tibiofibular tract and posterior tibial artery in two of seven cases (29%) and in the tibiofibular tract and fibular artery in the remaining three of seven cases (43%)(Table 2).
| Patient A | Patient B | Patient C | Patient D | Patient E | Patient F | Patient G | All patients | |
| Sex | Male | Male | Male | Male | Male | Male | Male | All male (100%) |
| Age (yr) | 89 | 72 | 55 | 67 | 85 | 67 | 80 | 74 ± 11 |
| Cardiovascular risk factors | Hypertension | Hypertension | Hypertension | Hypertension | Hypertension | Hypertension | Hypertension | Hypertension (100%) |
| Hyperlipidemia | Hyperlipidemia | Hyperlipidemia | Hyperlipidemia | Hyperlipidemia | Hyperlipidemia | Hyperlipidemia | Hyperlipidemia (100%) | |
| Type 2 DM | Type 2 DM | Type 2 DM | Smoking | Type 2 DM | Smoking | Type 2 DM (57%) | ||
| Smoking | Smoking (43%) | |||||||
| PAD history | No | Surgical endatherectomy of the left common femoral artery 2012 | No | Prior Angioplasty and stent placement in the left popliteal artery 2015 | No | Prior Angioplasty and stent placement in the left popliteal artery 2016 | No | 3/7 (43%) |
| LV function | Moderately reduced | Normal | Normal | Normal | Severely reduced | Mildly reduced | Normal | Reduced in 3/7 (43%) |
| Symptoms onset | For 12 h | For 16 h | For 2 h | For 12 h | For 36 h | For 6 h | For 36 h | 17 ± 13 |
| CAD history | 3 vessel CAD | 3 vessel CAD | No | 3 vessel CAD | 3 vessel CAD | 3 vessel CAD | 3 vessel CAD | 6/7 (86%) |
| GABG 2005 | ||||||||
| Baseline medication | Aspirin | Aspirin | Aspirin | Aspirin | Aspirin | Aspirin | Aspirin | Aspirin (100%) |
| ß-blocker | ß-blocker | ACE inhibitor | ß-blocker | ß-blocker | ß-blocker | ß-blocker | ß-blocker (86%) | |
| ACE inhibitor | ACE inhibitor | Statin | ACE inhibitor | ACE inhibitor | ACE inhibitor | ACE inhibitor | ACE inhibitor 100%) | |
| Statin | Statin | Statin | Statin | Statin | Statin | Statin (100%) | ||
| Diuretics | Diuretics | Diuretics | Diuretics | Diuretics (57%) | ||||
| Other comorbidities | Reduced renal function with estimated GFR of -40 mL/min/1.73 m² | Reduced renal function with estimated GFR of -50 mL/min/1.73 m² | None | Reduced renal function with estimated GFR of -45 mL/min/1.73 m² | Reduced renal function with estimated GFR of -55 mL/min/1.73 m² | Reduced renal function with estimated GFR of -50 mL/min/1.73 m² | Reduced renal function with estimated GFR of -40 mL/min/1.73 m² | Reduced renal function (86%) |
| Atrial fibrillation | Atrial fibrillation | Atrial fibrillation | Atrial fibrillation | Atrial fibrillation | Atrial fibrillation (71%) | |||
| Heart failure | Heart failure NYHA III | Heart failure NYHA II | Heart failure (43%) | |||||
| NYHA III |
| Patient A | Patient B | Patient C | Patient D | Patient E | Patient F | Patient G | |
| Duplex sonography findings | Thrombotic CFA occlusion | Thrombotic CFA occlusion | Thrombotic occlusion of the distal SFA | Thrombotic occlusion of the popliteal artery | Thrombotic occlusion of the distal SFA | Thrombotic occlusion of the distal SFA | Thrombotic occlusion of the popliteal artery |
| DSA findings | Thrombotic CFA occlusion | Thrombotic CFA occlusion | Thrombotic occlusion of the distal SFA and of the popliteal artery | Thrombotic occlusion of the popliteal artery | Thrombotic occlusion of the distal SFA and of the popliteal artery | Thrombotic occlusion of the distal SFA and of the popliteal artery | Thrombotic occlusion of the popliteal artery |
| Treated crural vessels | Proximal and mid tibial anterior artery | Proximal and mid tibial anterior artery | Proximal and mid posterior tibial artery | Tibiofibular tract and posterior tibial artery | Tibiofibular tract and fibular artery | Tibiofibular tract | Tibiofibular tract and fibular artery |
| Rotarex catheter | 6F | 6F | 6F | 6F | 6F | 6F | 6F |
| Local lysis | Yes | No | No | Yes | Yes | Yes | Yes |
| Second look DSA | Yes | No | No | Yes | Yes | Yes | Yes |
In all seven cases, 6F Rotarex®S catheter thrombectomy resulted in vessel patency, whereas no vessel dissections or perforations were observed. Compared to the remaining 95 patients who received Rotarex®S catheter thrombectomy in iliac and femoropopliteal vessels, it should be noted that Rotarex®S efficacy was present in 93 of 95 cases (98%), whereas vessel dissection or perforation was observed in two of 95 cases (2%), which in both cases was treated using an endovascular approach by prolonged balloon inflation and by placement of a stent, respectively.
The size of the proximal crural arteries varied between 3.2 and 4.0 mm, whereas the size of the mid-portion of the crural arteries varied between 2.5 and 3.5 mm. An overview of the diameters of the proximal and mid-portions of the crural arteries of our patients, where mechanical thrombectomy was performed, can be appreciated in Table 3.
| Proximal anterior tibial artery | Mid anterior tibial artery | |
| Patient A | 3.2 mm* | 2.8 mm |
| Patient B | 3.4 mm | 2.7 mm |
| Proximal posterior tibial artery | Mid posterior tibial artery | |
| Patient C | 3.5 mm | 3.0 mm |
| Tibiofibular tract | Proximal posterior tibial artery | |
| Patient D | 3.5 mm | 3.0 mm |
| Tibiofibular tract | Proximal fibular artery | |
| Patient E | 4.0 mm | 2.5 mm |
| Tibiofibular tract | ||
| Patient F | 3.5 mm | |
| Tibiofibular tract | Proximal fibular artery | |
| Patient G | 4 mm | 3.5 mm |
Ischemic symptoms promptly resolved in all patients after the index procedure. Duplex sonography on the following day exhibited patency of all of the treated crural arteries. In addition, further clinical course was uneventful in all seven patients, who were discharged within three days after mechanical thrombectomy. Five of seven (71%) patients were diagnosed with atrial fibrillation and were put on triple anticoagulation with 100 mg aspirin, 75 mg clopidogrel and oral anticoagulation for 4 wk, and were then continued with oral anticoagulation. The remaining two patients were treated with 100 mg aspirin and 75 mg clopidogrel for 3 mo and were then put on 100 mg aspirin daily. Representative images of our patients (Patient A-C) can be appreciated in Figures 1-4.
ALI is a serious medical condition that requires rapid diagnosis and prompt initiation of appropriate treatment. Depending on the clinical presentation and anatomy of the lesion, either an endovascular approach or a surgical therapy may be chosen. CDT is the classical method employed in the treatment of ALI. Mechanical thrombectomy techniques, on the other hand, represent a relatively new treatment in patients with ALI. Various devices using different mechanisms of action, (i.e., fragmentation, aspiration or rheolytic thrombectomy) were shown to be useful alone or associated with the use of additional thrombolytics or local thrombolysis (combined mechanical and pharmacologic thrombectomy) for the management of patients with ALI. The main advantage of mechanical thrombectomy consists of the reduction of thrombotic burden, which reduces or even avoids the need for local thrombolysis. This is of major importance, particularly in patients with contraindication to thrombolysis due to high bleeding risks.
Many mechanical thrombectomy devices are currently used for the endovascular treatment of ALI. The ThromCat XT catheter device consists of an atraumatic tip and a flexible steel helix and can provide an effective aspiration capacity of 0.63 mL/s even in vessels with relative large diameters[10]. However, due to the small aspiration ports of this catheter system, it is limited to the treatment of fresh arterial occlusions, as it is difficult for the system to aspirate partially organized thrombotic material. The AngioJet (Possis Medical, Minneapolis, Minnesota, United States) on the other hand, is a combined pharmacologic and mechanical thrombectomy system, which is dedicated to peripheral interventions and uses active aspiration and Power Pulse™ lytic delivery to remove the thrombus and restore blood flow[11]. It should be noted that only observational, non-randomized data are available for such devices, including the Rotarex®S system, whereas no direct comparisons for different thrombectomy devices have been reported so far.
The Rotarex®S system is a purely mechanical endovascular thrombectomy device (Straub Medical AG, Switzerland)[12,13]. The system consists of an external drive system, which is connected to the Rotarex®S catheter via a magnetic clutch. Inside the catheter tube, a helix transmits the rotation from the drive system to the catheter head, which can rotate with up to 60000 rpm, thus creating a powerful vortex to debulk all detachable occlusion material from the vessel. The fragmented debris is subsequently aspirated through side slits in the catheter head. The inner helix simultaneously creates a strong suction force, following the Archimedes principle, and finally transports the fragmented material into an external collecting bag. The Rotarex®S catheter is currently available in three sizes, including 6F, 8F and 10F, and is inserted over a dedicated 0.018 guidewire. The aspiration efficacy is approximately 0.75 mL/s for the 6F system, which can be safely used in vessels with a diameter of ≥ 3 mm to 5 mm.
Several studies have demonstrated the efficacy of this system in the treatment of patients with ALI[14-18]. In this regard, a high success rate of > 98% was reported in a recent study, which elegantly demonstrated that purely mechanical thrombectomy by the Rotarex®S system was safer and more effective than thrombolysis, which was associated with higher rates of major bleedings, longer hospitalization durations and higher costs[19]. Potential complications associated with the Rotarex®S endovascular system is peripheral embolization of thrombotic debris in peripheral foot arteries (in most of the cases after additional balloon angioplasty and not directly related to Rotarex®S thrombectomy) and vessel dissection or perforation in smaller vessels. Particularly in vessels smaller than 3 mm in diameter, perforation may occur due to complete filling of the vessel by the catheter, which may eventually suck the vessel wall into the side windows of the catheter head. Although such complications can in most cases be treated by prolonged balloon inflation or by stent placement without requiring surgical action[16,20], the use of the 6F Rotarex®S system is not currently generally recommended for crural arteries in the current literature[21]. In this regard, the use of the Rotarex®S has been reported only in a relatively small number of patients with ALI involving below the knee vessels (n = 4 in the study of Stanek et al[18]).
To the best of our knowledge the present study is the first in the current literature, which in detail describes the efficacy and safety of the 6F Rotarex®S system in a miniseries of seven patients, who were all treated for ALI in the proximal or mid-part of relatively big crural arteries with good angiographic and clinical results. Although no vascular complications in terms of dissection or perforation occurred in the crural arteries, the use of the 6F Rotarex®S debulking system should be performed with caution in crural arteries.
In conclusion, mechanical debulking using the 6F Rotarex®S catheter system may be a safe and effective treatment option in case of thrombotic or thromboembolic occlusion of the proximal and mid portion of crural arteries in patients presenting with ALI, especially when local thrombolysis needs to be avoided due to increased bleeding risk.
Endovascular treatment of acute limb ischemia (ALI) is increasingly gaining importance in older and multimorbid patients, compared to conventional surgical techniques. The Rotarex®S debulking system is one such endovascular device, which can be used for catheter-assisted thrombectomy in ALI. However, the use of the 6 French (6F) Rotarex®S system is not generally recommended for crural arteries in the current literature.
Limited data exist to date on the efficacy and safety of the 6F Rotarex®S system for thrombectomy in crural arteries.
Our study aimed to examine whether the 6F Rotarex®S system can be used effectively and safely for endovascular thrombectomy of crural arteries in patients with ALI.
Retrospective analysis of all patients who were referred to our department for endovascular thrombectomy dues to ALI between January 2015 and December 2017.
We identified seven patients who underwent endovascular Rotarex®S catheter thrombectomy in crural arteries due to remaining thrombus formation with no reflow. In two cases, thrombectomy was performed in the anterior tibial artery, in another two cases, in the posterior tibial artery and in the remaining three cases, in the fibular artery. In all seven cases, treatment resulted in restoration of the blood flow to the foot arteries, resolving ischemic symptoms. Vessel dissection or perforation did not occur in any of the seven cases..
Endovascular thrombectomy using the 6F Rotarex®S catheter system may be safe and effective for the treatment of thrombotic occlusion of the proximal and mid portion of crural arteries. In particular, patients with high bleeding risk may profit from such a “mechanical only” treatment option without the need for additional thrombolysis.
Larger prospective trials are necessary in the future to examine the efficacy and safety of the 6F Rotarex®S catheter system in smaller arteries of the lower limb.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P- Reviewer: Chang ST, Petix NR, Teragawa H, Said SA, Ueda H, Anan R S- Editor: Dou Y L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4051] [Cited by in RCA: 4116] [Article Influence: 228.7] [Reference Citation Analysis (0)] |
| 3. | Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2587] [Cited by in RCA: 2571] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 4. | Faglia E, Clerici G, Clerissi J, Gabrielli L, Losa S, Mantero M, Caminiti M, Curci V, Lupattelli T, Morabito A. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: data of a cohort study of 564 patients. Eur J Vasc Endovasc Surg. 2006;32:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Lichtenberg MKW, Stahlhoff WF. Endovascular-first strategy for acute and subacute limb ischaemia: Potential benefits of a pure mechanical thrombectomy approach Comment on Stanek et al, p. 49–56. Vasa. 2016;45:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 469] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Wasilewska M, Gosk-Bierska I. Thromboembolism associated with atrial fibrillation as a cause of limb and organ ischemia. Adv Clin Exp Med. 2013;22:865-873. [PubMed] |
| 8. | Ouriel K, Shortell CK, DeWeese JA, Green RM, Francis CW, Azodo MV, Gutierrez OH, Manzione JV, Cox C, Marder VJ. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 371] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Lukasiewicz A. Treatment of acute lower limb ischaemia. Vasa. 2016;45:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Deák Z, Strube H, Sadeghi-Azandaryani M, Reiser MF, Treitl M. Rotational thrombectomy of acute peripheral vascular occlusions using the ThromCat XT device: techniques, indications and initial results. Diagn Interv Radiol. 2011;17:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Walker TG. Acute limb ischemia. Tech Vasc Interv Radiol. 2009;12:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Stanek F, Ouhrabkova R, Prochazka D. Mechanical thrombectomy using the Rotarex catheter--safe and effective method in the treatment of peripheral arterial thromboembolic occlusions. Vasa. 2010;39:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Stanek F, Ouhrabkova R, Prochazka D. Mechanical thrombectomy using the Rotarex catheter in the treatment of acute and subacute occlusions of peripheral arteries: immedite results, long-term follow-up. Int Angiol. 2013;32:52-60. [PubMed] |
| 14. | Schmitt HE, Jäger KA, Jacob AL, Mohr H, Labs KH, Steinbrich W. A new rotational thrombectomy catheter: system design and first clinical experiences. Cardiovasc Intervent Radiol. 1999;22:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Bérczi V, Deutschmann HA, Schedlbauer P, Tauss J, Hausegger KA. Early experience and midterm follow-up results with a new, rotational thrombectomy catheter. Cardiovasc Intervent Radiol. 2002;25:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Duc SR, Schoch E, Pfyffer M, Jenelten R, Zollikofer CL. Recanalization of acute and subacute femoropopliteal artery occlusions with the rotarex catheter: one year follow-up, single center experience. Cardiovasc Intervent Radiol. 2005;28:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Zeller T, Frank U, Bürgelin K, Schwarzwälder U, Horn B, Flügel PC, Neumann FJ. Long-term results after recanalization of acute and subacute thrombotic occlusions of the infra-aortic arteries and bypass-grafts using a rotational thrombectomy device. Rofo. 2002;174:1559-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Stanek F, Ouhrabkova R, Prochazka D. Percutaneous mechanical thrombectomy in the treatment of acute and subacute occlusions of the peripheral arteries and bypasses. Vasa. 2016;45:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Kronlage M, Printz I, Vogel B, Blessing E, Müller OJ, Katus HA, Erbel C. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Des Devel Ther. 2017;11:1233-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Eisele T, Muenz BM, Korosoglou G. Successful Endovascular Repair of an Iatrogenic Perforation of the Superficial Femoral Artery Using Self-Expanding Nitinol Supera Stents in a Patient with Acute Thromboembolic Limb Ischemia. Case Rep Vasc Med. 2016;2016:7376457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Wissgott C, Kamusella P, Richter A, Klein-Wiegel P, Steinkamp HJ. [Mechanical rotational thrombectomy for treatment thrombolysis in acute and subacute occlusion of femoropopliteal arteries: retrospective analysis of the results from 1999 to 2005]. Rofo. 2008;180:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |