Published online Aug 26, 2016. doi: 10.4331/wjbc.v7.i3.223
Peer-review started: February 27, 2016
First decision: March 24, 2016
Revised: April 19, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 26, 2016
Processing time: 177 Days and 4.2 Hours
The three-dimensional (3D) organization of the eukaryotic genome is critical for its proper function. Evidence suggests that extensive chromatin loops form the building blocks of the genomic architecture, separating genes and gene clusters into distinct functional domains. These loops are anchored in part by a special type of DNA elements called chromatin boundary elements (CBEs). CBEs were originally found to insulate neighboring genes by blocking influences of transcriptional enhancers or the spread of silent chromatin. However, recent results show that chromatin loops can also play a positive role in gene regulation by looping out intervening DNA and “delivering” remote enhancers to gene promoters. In addition, studies from human and model organisms indicate that the configuration of chromatin loops, many of which are tethered by CBEs, is dynamically regulated during cell differentiation. In particular, a recent work by Li et al has shown that the SF1 boundary, located in the Drosophila Hox cluster, regulates local genes by tethering different subsets of chromatin loops: One subset enclose a neighboring gene ftz, limiting its access by the surrounding Scr enhancers and restrict the spread of repressive histones during early embryogenesis; and the other loops subdivide the Scr regulatory region into independent domains of enhancer accessibility. The enhancer-blocking activity of these CBE elements varies greatly in strength and tissue distribution. Further, tandem pairing of SF1 and SF2 facilitate the bypass of distal enhancers in transgenic flies, providing a mechanism for endogenous enhancers to circumvent genomic interruptions resulting from chromosomal rearrangement. This study demonstrates how a network of chromatin boundaries, centrally organized by SF1, can remodel the 3D genome to facilitate gene regulation during development.
Core tip: Genomic organization in higher eukaryotes needs to fulfill at least three distinct functions: Gene compaction, gene insulation and gene regulation. Chromatin loops appear to be a common structural unit that serves all these functions. A recent study has characterized a series of chromatin boundary elements (CBEs) in the Drosophila Hox cluster. Selective and dynamic interactions between these CBEs tether chromatin loops that not only insulate neighboring genes, but also organize enhancer traffic to regulate gene expression during development.
- Citation: Ma Z, Li M, Roy S, Liu KJ, Romine ML, Lane DC, Patel SK, Cai HN. Chromatin boundary elements organize genomic architecture and developmental gene regulation in Drosophila Hox clusters. World J Biol Chem 2016; 7(3): 223-230
- URL: https://www.wjgnet.com/1949-8454/full/v7/i3/223.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i3.223
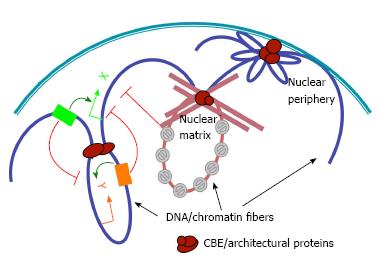
Transcriptional regulation plays a pivotal role in controlling gene activity during development, physiological responses and diseases. The current paradigm of eukaryotic transcriptional regulation emphasizes the assembly of activator complexes at distal regulatory DNA elements called enhancers[1-4]. Studies have shown that communication between these enhancers and their target promoters also constitutes a critical and highly regulated step towards transcription activation. Although the mechanisms of such communication are not fully understood, studies have shown that distal regulatory elements looping to gene promoters can trigger transcriptional activation or repression[5-7]. Mounting evidence suggests that configuration of chromatin fibers in the three-dimensional (3D) space can profoundly affect the access of regulatory sequences to genes during cell differentiation (Figure 1)[6,8-12].
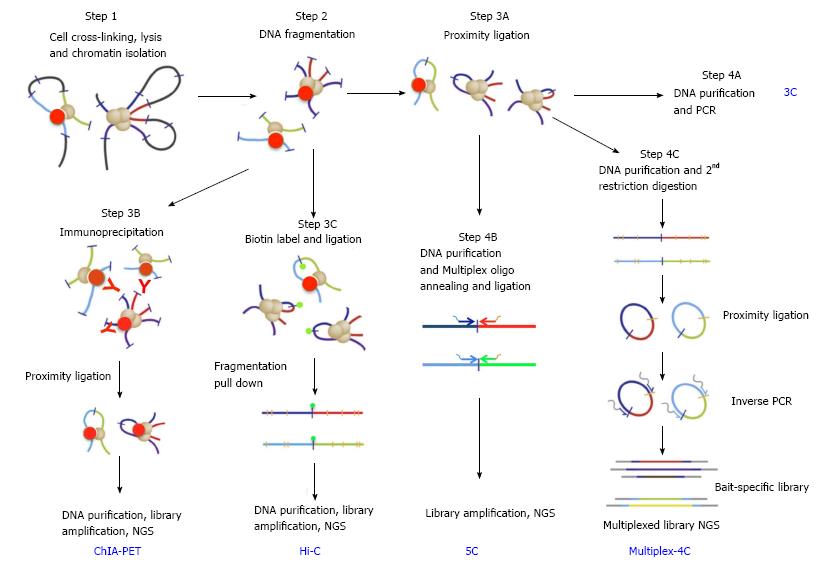
The mechanisms that regulate chromatin loop formation are poorly understood. Evidence converges on a type of specialized regulatory DNA called chromatin boundary elements (CBEs), also known as insulators. These elements were originally identified as DNA sequences that separate neighboring genomic domains[13,14]. They also interrupt enhancer-promoter communications without affecting the activities of these elements per se[9,15-20]. Boundary-like elements have been found from yeast to humans, often between divergently expressed genes. This is consistent with their functional role in maintaining independent domains of gene regulation. The best-characterized CBEs include the vertebrate beta-globin insulator, the Drosophila Gypsy insulator, and the boundaries from the Drosophila Hox loci[21-27]. The initial clue that CBEs may function by pairing with each other and tether chromatin loops came when their enhancer-blocking activity was found to depend on the position, orientation and arrangement of these elements[28-37]. In addition, the loop domains tethered by CBEs in the Drosophila Hox complexes, including Fab-7, Fab-8 and SF1, have also been shown to defined domains of distinct histone modifications that correlate with local gene activity[27,38,39]. Importantly, CBEs can play multi-faceted roles in gene regulation by either blocking or promoting enhancer-promoter interactions, depending on the topology and configuration of these loops[27,36,40-42]. The recent advent of genome-wide chromosomal-capture technology (3C, 4C, 5C and Hi-C, Figure 2) and protein association (ChIP) methods has provided powerful tools for assessing the spatial organization of the chromatin fibers and genomic conformation in vivo[8,43-51]. Recent reports indicate that CBE sequences, such as CTCF binding sites, correlate well with borders of Topological Associating Domains (TADs), which are megabase-sized units of genomic interaction domains[8,10,36,48,51-53]. These findings suggest that the interactions among CBEs/insulators may tether long-range chromatin loops that underlie much of the genomic architecture.
Diverse DNA sequences and protein factors have been associated with CBE function and genomic architecture. The most-conserved and best-characterized CBE/architectural protein is CTCF, a zinc-finger DNA-binding protein[18,19,48,50,54-56]. It mediates enhancer-blocking activity and underpins long-range chromatin loops in both Drosophila and vertebrates. Cohesin and condensin complexes, in conjunction with other CBE factors, are also well-conserved machinery that mediates long-range chromatin interactions from yeast to humans[50,57-59]. Additional classes of CBE complexes have been identified in Drosophila, anchored by the SuHw, BEAF, GAGA and ZW5 DNA-binding factors, respectively (for recent reviews see[60]). The Drosophila centrosomal protein CP190 associates with these complexes and contributes to their boundary and architectural function[59-63]. In addition, gene promoters, retrotransposons, and house keeping genes have also been shown to associate with CBE or architectural functions[48,64-66].
Maps of global genomic interactions (Hi-C) have been generated from numerous mammalian and Drosophila cell lines. These maps indicate that the TAD organization is a pervasive feature of the interphase nuclei[8,10,47,48,60]. Further, megabase-sized TAD domains appear to be relatively stable across different cell lineages, even conserved across species[48]. Consistent with this, constitutive and robust binding of CBE/architectural proteins collocalizes with TAD borders[51]. In contrast, dynamic reconfiguration of chromatin loops at sub-TAD (inter-TAD) scale appears to underlie much of the regulatory interactions[5,6,8,67-69]. Such dynamic reorganization may be critical for establishing distinct gene activity and nuclear organizations that are cell fate specific[60,68,70]. A recent work on the Drosophila Hox gene regulation revealed that transient and cell-specific chromatin loops coincide with domains of enhancer and promoter access, as well as domains of repressive histone modifications[27].
An important question that now arises is how to distinguish the static structural roles of chromatin loops in genomic architecture from the regulated chromatin loops that are tissue- and developmental stage-specific. It is unclear whether the two types of architecture utilize different cis- and trans-acting components. Significantly, most of CBE/architectural proteins identified so far are ubiquitous and constitutively expressed, raising questions about their roles in regulation.
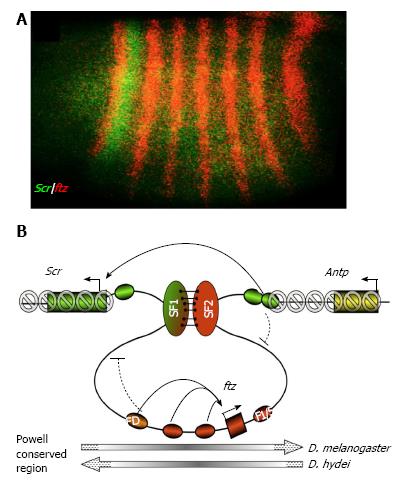
A recent manuscript by Li et al[27] provided evidence that developmentally regulated chromatin loop domains are involved in gene regulation in the Drosophila Hox cluster. Hox/HOM genes control animal segment identity along the anterior-posterior body axis. The complexes represent the most conserved gene family, not only in gene function, gene regulation, but also in gene organization. Indeed, the striking “collinear” relationship between the order of Hox genes on the chromosome and the order of their expression domains along the body axis has often been cited as the defining feature of the animal kingdom. In a previous publication, the group has reported the identification of SF1, a CBE located within the Drosophila Hox cluster between Sex comb reduced (Scr) and its neighbor fushi tarazu (ftz, Figure 3A[26,27,71]). SF1 was shown to contain a strong enhancer-blocking activity in the early Drosophila embryos. It also exhibits a strong activity in protecting the transgenic miniwhite reporter against the influences of neighboring chromatin in late development. In the current study, the authors used the 3C technique to probe for points of contact that SF1 makes in the surrounding genomic regions. The search had lead to the identification of several novel CBEs, located downstream of ftz[27]. In particular, SF1 pairs transiently but strongly with an SF2 CBE to enclose ftz in a chromatin loop in the 4-8 h embryos (Figure 3B)[27]. This ftz loop coincides with a domain of chromatin depleted in repressive histone marks including H3K27Me3 and H3K9Me3 (grey circles, Figure 3B). The loop also corresponds to a domain of restricted access to the ftz promoter by the surrounding Hox enhancers (curved arrows, Figure 3B). In the 12-16h embryos, SF1 dissociates from SF2, resulting in a spread of repressive marks into the ftz domain and a dramatic increase in interference to the ftz promoter by the neighboring regulatory sequences[27,72]. These results suggest that the loop tethered by SF1 and SF2 plays a key role in insulating both Scr and ftz regulation. Importantly, pairing of SF1 and SF2 in tandem allows a distal enhancer to “bypass” the block by both CBEs, providing a mechanism for the Scr enhancers located downstream of ftz to “leap” over ftz, and communicate with the Scr promoter (green ovals, Figure 3B[45,73-75]). Besides the SF1-SF2 pairing, SF1 also anchors other chromatin loops, which may subdivide and facilitate the Scr early and late regulatory elements[27]. Furthermore, evidence was provided that the activities of some of these novel CBEs are tissue restricted (for details see[27]). These results suggest that attributes of chromatin conformation can be regulated in a tissue-, stage- and gene-specific fashion to direct transcriptional outcome. Interestingly, the SF1-SF2 interval represent an evolutionarily conserved genomic block (Powell Conserved Region) that houses the entire ftz gene and is in an inverted orientation in several Drosophila species (Grey arrows, bottom of Figure 3B[76]). These observations suggest that chromatin loops may insulate the communications between genes and their regulatory sequences from the chromosome rearrangements, resulting in intermingling and interdependence of the genomic “modules” during evolution.
The above study has provided strong evidence that CBE-tethered chromatin loops can direct enhancer traffic during development. However, several questions remain to be addressed. First, in order to demonstrate the in vivo function of SF1 and SF2-tethered chromatin loops in ftz and Scr regulation, it is critical to examine the chromatin configuration and ftz and Scr expression patterns in mutant animals where SF1 or SF2 is deleted. In particular, since the chromatin loop tethered by SF1 and SF2 was hypothesized to restrict enhancer access to the ftz promoter and impede the spread of silent chromatin into the active ftz domain, one would expect to see increased capture of the ftz promoter by the neighboring Scr enhancers, and a higher level of repressive histone marks within the ftz region in mutant animals. Consequently, reduced or ectopic expression of the ftz gene may be observed. Similarly, the SF1-SF2 loop was postulated to facilitate the Scr distal enhancers to their promoter. Deletion of SF1 or SF2 would disrupt the loop configuration and the Scr enhancer-promoter interactions, leading to changes in the Scr expression.
In the above study the authors also noted that the SF1-SF2 chromatin loop coincides with the inverted ftz region in several Drosophila species. Furthermore, several genomic rearrangements in the Antennapedia Hox complex besides the ftz domain also coincide with potential CBE-tethered chromatin loops. The authors hypothesized that chromatin loops tethered by boundary elements may promote genomic crossover and at the same time insulate any potential deleterious impacts of these rearrangements to the surrounding genes. The hypothesis can be tested from several angles. Demonstration of corresponding loop domains in these Drosophila species, validation of boundary activity that flank the loop domains, and general correlation between genomic rearrangements and CBE-tethered chromatin loops would go a long way proving this hypothesis.
Despite recent advances in our understanding of chromatin structure and genomic organization, how key features of our genetic material re regulate development, physiological and pathological responses remain poorly understood. Below are three major gaps in our understanding of the mechanisms and function of genomic architecture.
Although conformation capture technologies have provided a critical means for assessing the genomic interactome and architecture, the methods have so far been largely applied to cultured cell lines. This is due to the need of quantities of homogeneous cell population for using the mapping technology. These studies can provide important information into aspects of nuclear and genomic organization. However, the maps do not faithfully reflect the characteristics and dynamics of animal cells and tissues during development. On the other hand, maps have been generated from mixed tissues, such as those from whole embryos. Those are not ideal either, as they reflect an average of the interactions from many cell types, and may suffer from low signal to noise ratio[77]. Future studies using cells from authentic animal models with highly defined tissue and developmental identity would be essential. Purification methods that can be applied to diverse cell and tissue types, and the use of single cell technologies in mapping genomic interactions should provide critical insights into the organization and function of genomic organization and nuclear architecture in a more physiologically relevant setting.
Another gap in our knowledge is in the causal relationship between chromatin configuration and gene regulation. Much of the current studies correlate global TAD structures with domains of active or silent chromatin. Numerous studies have also correlated chromatin loop organization with local enhancer-promoter interactions during gene activation[27,78,79]. However, to demonstrate that these changes in chromosomal configuration play a primary role in regulating enhancer-promoter interactions or in organizing chromatin domains, rather than simply correlating with these events, it would be essential to examine these interactions and domains in mutants where the corresponding genomic configurations are disrupted. In addition, future studies should also aim to provide a more quantitative description how changes in chromatin configuration instruct or permit gene regulation events.
The protein factors that control developmental stage- and tissue-specific chromatin loops are poorly characterized. In particular, majority of the currently known CBE/architectural proteins are constitutively and broadly expressed in animal tissues. Therefore, regulated chromatin conformation may require novel factors that are more restricted in their temporal or spatial distribution. Recent studies have suggested that such factors may associate with chromatin and with each other weakly, and loop within a shorter range[27,78,79]. As a result, their association and function may only be revealed from ChIP experiments and conformation captures of higher reading depth, and possibly from purified or enriched source tissues. Combinations of molecular genetic and biochemical approaches may be required to identify these proteins.
The human genome is highly organized in the three dimensional space. CBEs represent an important mechanism that organizes such complex architecture. Functionally, these elements separate neighboring genes to ensure their independent regulation. Boundary elements also tether chromatin loops to promote communications between distant genetic elements. As such, chromatin boundary activity is essential for proper genomic function including transcriptional regulation. Despite such critical roles, how CBE activities and chromatin loop formation are regulated, especially in a developmental context, is still poorly documented and poorly understood. Current gaps in our knowledge are in their roles in authentic animal models, in their instructing rather permitting genomic interactions during transcriptional regulation, and in their cis and trans- components that can mediate tissue- and stage-specific genomic architecture. Future studies that combine single cell technology, live imaging and other genomic, cell biological and biochemical approaches should mend these gaps and further elucidate this novel mechanism that control our genomic output.
Manuscript source: Invited manuscript
Specialty type: Biochemistry and molecular biology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bai G, Chui YL, Gunther T, Lawen A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 825] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 2. | Escalante CR, Yie J, Thanos D, Aggarwal AK. Structure of IRF-1 with bound DNA reveals determinants of interferon regulation. Nature. 1998;391:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 318] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Merika M, Williams AJ, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol Cell. 1998;1:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 362] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell. 2000;103:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 588] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 5. | Kondo T, Isono K, Kondo K, Endo TA, Itohara S, Vidal M, Koseki H. Polycomb potentiates meis2 activation in midbrain by mediating interaction of the promoter with a tissue-specific enhancer. Dev Cell. 2014;28:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Kim A, Dean A. Chromatin loop formation in the β-globin locus and its role in globin gene transcription. Mol Cells. 2012;34:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Mora A, Sandve GK, Gabrielsen OS, Eskeland R. In the loop: promoter-enhancer interactions and bioinformatics. Brief Bioinform. 2015; Nov 19; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Phillips-Cremins JE, Sauria ME, Sanyal A, Gerasimova TI, Lajoie BR, Bell JS, Ong CT, Hookway TA, Guo C, Sun Y. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 918] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 9. | Bondarenko VA, Jiang YI, Studitsky VM. Rationally designed insulator-like elements can block enhancer action in vitro. EMBO J. 2003;22:4728-4737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Li L, Lyu X, Hou C, Takenaka N, Nguyen HQ, Ong CT, Cubeñas-Potts C, Hu M, Lei EP, Bosco G. Widespread rearrangement of 3D chromatin organization underlies polycomb-mediated stress-induced silencing. Mol Cell. 2015;58:216-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 11. | Branco MR, Pombo A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 2006;4:e138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 12. | Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev. 2007;17:400-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 13. | Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 477] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Chung JH, Whiteley M, Felsenfeld G. A 5’ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 692] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Geyer PK, Corces VG. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes Dev. 1992;6:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 324] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Holdridge C, Dorsett D. Repression of hsp70 heat shock gene transcription by the suppressor of hairy-wing protein of Drosophila melanogaster. Mol Cell Biol. 1991;11:1894-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Cai H, Levine M. Modulation of enhancer-promoter interactions by insulators in the Drosophila embryo. Nature. 1995;376:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1269] [Cited by in RCA: 1284] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 19. | Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1125] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 20. | Ameres SL, Drueppel L, Pfleiderer K, Schmidt A, Hillen W, Berens C. Inducible DNA-loop formation blocks transcriptional activation by an SV40 enhancer. EMBO J. 2005;24:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Galloni M, Gyurkovics H, Schedl P, Karch F. The bluetail transposon: evidence for independent cis-regulatory domains and domain boundaries in the bithorax complex. EMBO J. 1993;12:1087-1097. [PubMed] |
| 22. | Karch F, Galloni M, Sipos L, Gausz J, Gyurkovics H, Schedl P. Mcp and Fab-7: molecular analysis of putative boundaries of cis-regulatory domains in the bithorax complex of Drosophila melanogaster. Nucleic Acids Res. 1994;22:3138-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Hagstrom K, Muller M, Schedl P. Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev. 1996;10:3202-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Barges S, Mihaly J, Galloni M, Hagstrom K, Müller M, Shanower G, Schedl P, Gyurkovics H, Karch F. The Fab-8 boundary defines the distal limit of the bithorax complex iab-7 domain and insulates iab-7 from initiation elements and a PRE in the adjacent iab-8 domain. Development. 2000;127:779-790. [PubMed] |
| 25. | Zhou J, Ashe H, Burks C, Levine M. Characterization of the transvection mediating region of the abdominal-B locus in Drosophila. Development. 1999;126:3057-3065. [PubMed] |
| 26. | Belozerov VE, Majumder P, Shen P, Cai HN. A novel boundary element may facilitate independent gene regulation in the Antennapedia complex of Drosophila. EMBO J. 2003;22:3113-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Li M, Ma Z, Liu JK, Roy S, Patel SK, Lane DC, Cai HN. An Organizational Hub of Developmentally Regulated Chromatin Loops in the Drosophila Antennapedia Complex. Mol Cell Biol. 2015;35:4018-4029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Cai HN, Zhang Z, Adams JR, Shen P. Genomic context modulates insulator activity through promoter competition. Development. 2001;128:4339-4347. [PubMed] |
| 29. | Cai HN, Shen P. Effects of cis arrangement of chromatin insulators on enhancer-blocking activity. Science. 2001;291:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Muravyova E, Golovnin A, Gracheva E, Parshikov A, Belenkaya T, Pirrotta V, Georgiev P. Loss of insulator activity by paired Su(Hw) chromatin insulators. Science. 2001;291:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Yannaki E, Tubb J, Aker M, Stamatoyannopoulos G, Emery DW. Topological constraints governing the use of the chicken HS4 chromatin insulator in oncoretrovirus vectors. Mol Ther. 2002;5:589-598. [PubMed] |
| 32. | van Steensel B, Delrow J, Bussemaker HJ. Genomewide analysis of Drosophila GAGA factor target genes reveals context-dependent DNA binding. Proc Natl Acad Sci USA. 2003;100:2580-2585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Roy S, Ernst J, Kharchenko PV, Kheradpour P, Negre N, Eaton ML, Landolin JM, Bristow CA, Ma L, Lin MF. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science. 2010;330:1787-1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1047] [Cited by in RCA: 959] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 34. | Smith ST, Wickramasinghe P, Olson A, Loukinov D, Lin L, Deng J, Xiong Y, Rux J, Sachidanandam R, Sun H. Genome wide ChIP-chip analyses reveal important roles for CTCF in Drosophila genome organization. Dev Biol. 2009;328:518-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Nakayama T, Nishioka K, Dong YX, Shimojima T, Hirose S. Drosophila GAGA factor directs histone H3.3 replacement that prevents the heterochromatin spreading. Genes Dev. 2007;21:552-561. [PubMed] |
| 36. | Guo Y, Xu Q, Canzio D, Shou J, Li J, Gorkin DU, Jung I, Wu H, Zhai Y, Tang Y. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell. 2015;162:900-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 688] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 37. | Lin SG, Guo C, Su A, Zhang Y, Alt FW. CTCF-binding elements 1 and 2 in the Igh intergenic control region cooperatively regulate V(D)J recombination. Proc Natl Acad Sci USA. 2015;112:1815-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Cléard F, Moshkin Y, Karch F, Maeda RK. Probing long-distance regulatory interactions in the Drosophila melanogaster bithorax complex using Dam identification. Nat Genet. 2006;38:931-935. [PubMed] |
| 39. | Bowman SK, Deaton AM, Domingues H, Wang PI, Sadreyev RI, Kingston RE, Bender W. H3K27 modifications define segmental regulatory domains in the Drosophila bithorax complex. Elife. 2014;3:e02833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Melnikova L, Juge F, Gruzdeva N, Mazur A, Cavalli G, Georgiev P. Interaction between the GAGA factor and Mod(mdg4) proteins promotes insulator bypass in Drosophila. Proc Natl Acad Sci USA. 2004;101:14806-14811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Gruzdeva N, Kyrchanova O, Parshikov A, Kullyev A, Georgiev P. The Mcp element from the bithorax complex contains an insulator that is capable of pairwise interactions and can facilitate enhancer-promoter communication. Mol Cell Biol. 2005;25:3682-3689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Kyrchanova O, Toshchakov S, Parshikov A, Georgiev P. Study of the functional interaction between Mcp insulators from the Drosophila bithorax complex: effects of insulator pairing on enhancer-promoter communication. Mol Cell Biol. 2007;27:3035-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2775] [Cited by in RCA: 2636] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 44. | Dostie J, Richmond TA, Arnaout RA, Selzer RR, Lee WL, Honan TA, Rubio ED, Krumm A, Lamb J, Nusbaum C. Chromosome Conformation Capture Carbon Copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006;16:1299-1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1030] [Cited by in RCA: 863] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 45. | Belton JM, McCord RP, Gibcus JH, Naumova N, Zhan Y, Dekker J. Hi-C: a comprehensive technique to capture the conformation of genomes. Methods. 2012;58:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 796] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 46. | Stadhouders R, Kolovos P, Brouwer R, Zuin J, van den Heuvel A, Kockx C, Palstra RJ, Wendt KS, Grosveld F, van Ijcken W. Multiplexed chromosome conformation capture sequencing for rapid genome-scale high-resolution detection of long-range chromatin interactions. Nat Protoc. 2013;8:509-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5731] [Cited by in RCA: 6015] [Article Influence: 375.9] [Reference Citation Analysis (0)] |
| 48. | Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4690] [Cited by in RCA: 4834] [Article Influence: 371.8] [Reference Citation Analysis (0)] |
| 49. | Gurudatta BV, Yang J, Van Bortle K, Donlin-Asp PG, Corces VG. Dynamic changes in the genomic localization of DNA replication-related element binding factor during the cell cycle. Cell Cycle. 2013;12:1605-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Mizuguchi T, Fudenberg G, Mehta S, Belton JM, Taneja N, Folco HD, FitzGerald P, Dekker J, Mirny L, Barrowman J. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe. Nature. 2014;516:432-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 51. | Van Bortle K, Nichols MH, Li L, Ong CT, Takenaka N, Qin ZS, Corces VG. Insulator function and topological domain border strength scale with architectural protein occupancy. Genome Biol. 2014;15:R82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 52. | Botta M, Haider S, Leung IX, Lio P, Mozziconacci J. Intra- and inter-chromosomal interactions correlate with CTCF binding genome wide. Mol Syst Biol. 2010;6:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Narendra V, Rocha PP, An D, Raviram R, Skok JA, Mazzoni EO, Reinberg D. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science. 2015;347:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 413] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 54. | Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 845] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 55. | Moon H, Filippova G, Loukinov D, Pugacheva E, Chen Q, Smith ST, Munhall A, Grewe B, Bartkuhn M, Arnold R. CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator. EMBO Rep. 2005;6:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006;20:2349-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 560] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 57. | Rollins RA, Korom M, Aulner N, Martens A, Dorsett D. Drosophila nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol Cell Biol. 2004;24:3100-3111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, Fisher AG, Merkenschlager M. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 59. | Gómez-Díaz E, Corces VG. Architectural proteins: regulators of 3D genome organization in cell fate. Trends Cell Biol. 2014;24:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Bushey AM, Ramos E, Corces VG. Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev. 2009;23:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 61. | Pai CY, Lei EP, Ghosh D, Corces VG. The centrosomal protein CP190 is a component of the gypsy chromatin insulator. Mol Cell. 2004;16:737-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 62. | Mohan M, Bartkuhn M, Herold M, Philippen A, Heinl N, Bardenhagen I, Leers J, White RA, Renkawitz-Pohl R, Saumweber H. The Drosophila insulator proteins CTCF and CP190 link enhancer blocking to body patterning. EMBO J. 2007;26:4203-4214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Gerasimova TI, Lei EP, Bushey AM, Corces VG. Coordinated control of dCTCF and gypsy chromatin insulators in Drosophila. Mol Cell. 2007;28:761-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Ohtsuki S, Levine M. GAGA mediates the enhancer blocking activity of the eve promoter in the Drosophila embryo. Genes Dev. 1998;12:3325-3330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Noma K, Cam HP, Maraia RJ, Grewal SI. A role for TFIIIC transcription factor complex in genome organization. Cell. 2006;125:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 66. | Van Bortle K, Ramos E, Takenaka N, Yang J, Wahi JE, Corces VG. Drosophila CTCF tandemly aligns with other insulator proteins at the borders of H3K27me3 domains. Genome Res. 2012;22:2176-2187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Berlivet S, Paquette D, Dumouchel A, Langlais D, Dostie J, Kmita M. Clustering of tissue-specific sub-TADs accompanies the regulation of HoxA genes in developing limbs. PLoS Genet. 2013;9:e1004018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 68. | Wu W, Morrissey CS, Keller CA, Mishra T, Pimkin M, Blobel GA, Weiss MJ, Hardison RC. Dynamic shifts in occupancy by TAL1 are guided by GATA factors and drive large-scale reprogramming of gene expression during hematopoiesis. Genome Res. 2014;24:1945-1962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Joshi O, Wang SY, Kuznetsova T, Atlasi Y, Peng T, Fabre PJ, Habibi E, Shaik J, Saeed S, Handoko L. Dynamic Reorganization of Extremely Long-Range Promoter-Promoter Interactions between Two States of Pluripotency. Cell Stem Cell. 2015;17:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 70. | Ulianov SV, Khrameeva EE, Gavrilov AA, Flyamer IM, Kos P, Mikhaleva EA, Penin AA, Logacheva MD, Imakaev MV, Chertovich A. Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res. 2016;26:70-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 71. | Li M, Belozerov VE, Cai HN. Modulation of chromatin boundary activities by nucleosome-remodeling activities in Drosophila melanogaster. Mol Cell Biol. 2010;30:1067-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, MacAlpine DM. Unlocking the secrets of the genome. Nature. 2009;459:927-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 709] [Cited by in RCA: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 73. | Calhoun VC, Levine M. Long-range enhancer-promoter interactions in the Scr-Antp interval of the Drosophila Antennapedia complex. Proc Natl Acad Sci USA. 2003;100:9878-9883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Gindhart JG, King AN, Kaufman TC. Characterization of the cis-regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics. 1995;139:781-795. [PubMed] |
| 75. | Gorman MJ, Kaufman TC. Genetic analysis of embryonic cis-acting regulatory elements of the Drosophila homeotic gene sex combs reduced. Genetics. 1995;140:557-572. [PubMed] |
| 76. | Maier D, Sperlich D, Powell JR. Conservation and change of the developmentally crucial fushi tarazu gene in Drosophila. J Mol Evol. 1993;36:315-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, Parrinello H, Tanay A, Cavalli G. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148:458-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1473] [Cited by in RCA: 1431] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 78. | Carter D, Chakalova L, Osborne CS, Dai YF, Fraser P. Long-range chromatin regulatory interactions in vivo. Nat Genet. 2002;32:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 496] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 79. | Lanzuolo C, Roure V, Dekker J, Bantignies F, Orlando V. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat Cell Biol. 2007;9:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |