Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.64
Peer-review started: June 11, 2015
First decision: August 25, 2015
Revised: September 22, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: February 26, 2016
Processing time: 261 Days and 18.7 Hours
Cell-cell and cell-matrix signaling and communication between adhesion sites involve mechanisms which are required for cellular functions during normal development and homeostasis; however these cellular functions and mechanisms are often deregulated in cancer. Aberrant signaling at cell-cell and cell-matrix adhesion sites often involves downstream mediators including Rho GTPases and tyrosine kinases. This review discusses these molecules as putative mediators of cellular crosstalk between cell-cell and cell-matrix adhesion sites, in addition to their attractiveness as therapeutic targets in cancer. Interestingly, inter-junctional crosstalk mechanisms are frequently typified by the way in which bacterial and viral pathogens opportunistically infect or intoxicate mammalian cells. This review therefore also discusses the concept of learning from pathogen-host interaction studies to better understand coordinated communication between cell-cell and cell-matrix adhesion sites, in addition to highlighting the potential therapeutic usefulness of exploiting pathogens or their products to tap into inter-junctional crosstalk. Taken together, we feel that increased knowledge around mechanisms of cell-cell and cell-matrix adhesion site crosstalk and consequently a greater understanding of their therapeutic targeting offers a unique opportunity to contribute to the emerging molecular revolution in cancer biology.
Core tip: Deregulation of cell-cell and cell-matrix signaling makes well-established contributions to key elements of cancer initiation and progression. In this review we discuss mechanisms of crosstalk between these spatially-distinct adhesion sites in epithelial cells, with a view to understanding their therapeutic targeting in epithelial tumors (carcinomas). A particular focus is placed upon Rho GTPases and tyrosine kinases as mediators of inter-junctional crosstalk, in addition to the concept of opportunistic pathogenic infection as a paradigm for inter-junctional crosstalk. Overall, this review posits that a greater understanding of cell-cell and cell-matrix adhesion crosstalk will drive novel aspects of cancer drug discovery.
- Citation: Smith YE, Vellanki SH, Hopkins AM. Dynamic interplay between adhesion surfaces in carcinomas: Cell-cell and cell-matrix crosstalk. World J Biol Chem 2016; 7(1): 64-77
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/64.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.64
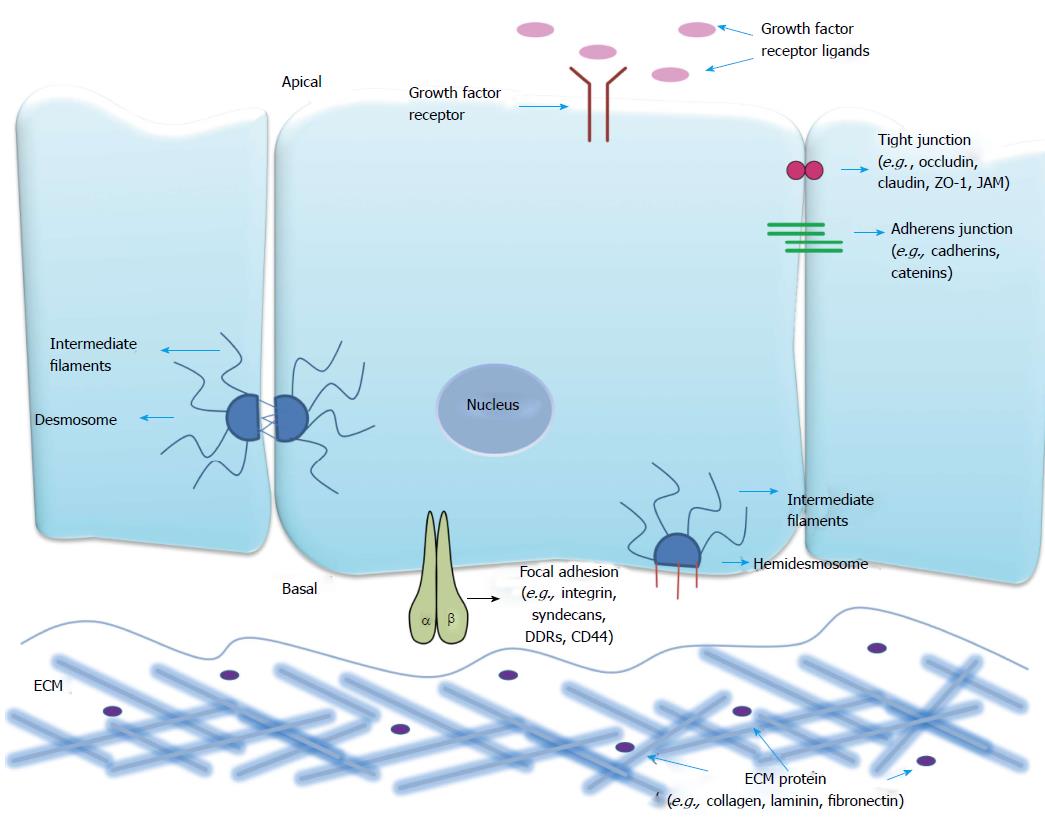
The semi-permeable plasma membrane acts as a barrier between interior and external environments of the cell. The plasma membrane forms specialised domains with distinct protein and lipid compositions crucial for many biological processes like cell adhesion, signaling, directed migration, cell division, cell fate determination and barrier function. These specialised domains contain concentration gradients of proteins differentiating the cell surface into basal, lateral and apical domains; facing respectively the extracellular matrix (ECM), adjoining cells and the outside environment (schematically represented in Figure 1).
Cell-cell junctions form part of the lateral surface domain which segregates apical and basal plasma membrane constituents[1], and act as a physical barrier in regulating substance flow across the tissues. Throughout the developmental process of higher organisms, formation and remodeling of cell-cell junctions occurs repeatedly[2]. The main cell-cell anchorage systems are tight junctions (TJs), adherens junctions (AJs) and desmosomes, which are distinguished from one another structurally and based on molecular composition.
TJs are seen in various cell types like epithelial, vascular endothelial, mesothelial, schwann, oligodendrocytes and sertoli[3]. In mammalian cells TJs appear at the apical end of the lateral intercellular membrane, whereas in invertebrate-like insect epithelial cells their counterpart septate junctions are located in the basal domains. TJs also demarcate the boundary between the apical and basolateral membrane domains of a cell and serve as a semipermeable barrier to the transport of ions, solutes and water[3]. TJs are multi-protein complexes that can be subdivided into integral membrane and cytoplasmic plaque proteins. Junctional adhesion molecules (JAMs), occludin, claudins, tricellulin and marvelD3 are integral proteins that mediate cell-cell adhesion[4], while ZO-1, -2, -3 and cingulin are cytoplasmic proteins that connect membrane proteins to the cytoskeleton[5]. Occludin, tricellulin and marvelD3 contain a Marvel (MAL-related proteins for vesicle trafficking and membrane link) domain. Claudin, occludin, tricellulin and marvelD3 are tetra-spanning proteins with both N and C termini in the cytosol, and which possess two extracellular loops. JAMs belong to the immunoglobulin (Ig) superfamily and consist of two extracellular Ig domains, a single transmembrane domain and a C-terminal cytoplasmic domain containing a PDZ-interacting domain. Alterations in epithelial TJs have been reported to contribute to a variety of pathologies including inflammation and cancer, yet a complete mechanistic understanding of these phenomena remains elusive. For a more comprehensive review of this topic, the reader is directed to the following reviews[6,7].
AJs are complexes that form the initial link between neighbouring cells in a developing tissue barrier, and thereby make important contributions to embryogenesis and tissue homeostasis[8]. The core structural components of AJs are Nectin and Cadherin family cell adhesion molecules, which are linked to the actin cytoskeleton through their binding proteins afadin and catenins[9,10]. Cadherin extracellular domains form calcium-dependent homophilic interactions between neighboring cells[11], while their cytoplasmic domains interact with three distinct known catenins (α, β, γ)[10]. Nectins (1-4) are calcium-independent adhesion proteins with three IgG like loops in their extracellular domains and a cytoplasmic domain containing a C-terminal PDZ binding motif[10,12].
Desmosomes are intercellular junctions that link intermediate filaments (IFs) to sites of intercellular adhesion, facilitating the formation of supracellular scaffolding that distributes mechanical forces throughout a tissue[13]. They are observed particularly in tissue types that experience mechanical stress, such as the intestinal mucosa, gallbladder, uterus and oviduct, liver, pancreas, stomach, salivary and thyroid glands and the epithelial cells of the nephron, but are most abundant in the skin and myocardium[14,15]. The components of desmosomes come from three gene families: Desmosomal cadherins (desmogleins and desmocollins), the armadillo family of nuclear and junctional proteins (plakoglobin and plakophilin 1-3) and finally the plakins (desmoplakin, plectin, envoplakin, periplakin)[16]. The cytoplasmic tails of desmosomal cadherins provide binding platforms for the armadillo family members. The plakin family members in turn link the stress-bearing IF cytoskeleton to specialized regions of the plasma membrane[13]. This interaction of IFs with desmosomes propagates the tensile strength imparted by the IF cytoskeleton across entire tissues, and is essential for tissue integrity[17].
Interactions between cells and the ECM are important in the overall architecture and maintenance of tissue integrity[18]. During development, these interactions play key roles in tissue morphogenesis, cellular polarisation and epithelial cell attachment[19,20]. Cellular interactions with the ECM rely upon interactions between ECM proteins, focal adhesion receptors and signaling molecules, including those of the cytoskeleton, to form multifaceted adhesion complexes. We refer interested readers to a recent review which discusses these complexes in further detail[21].
A plethora of cell-matrix proteins exist and are dynamically regulated depending on the tissue location, organisation, structure and components of the tissue microenvironment. This in turn dynamically regulates cellular responses[18]. Communication between cells and the ECM is mediated by transmembrane focal adhesion receptors including syndecans, discoidin domain receptors (DDRs), CD44 and integrins.
Syndecans are heparin sulfate proteoglycans that span the plasma membrane. Syndecans have been shown to play a role in multiple cellular processes including migration, differentiation and organisation of the ECM and cytoskeleton, features which are attributed to the presence of glycosaminoglycan chains in the extracellular domain[22].
DDRs are members of the receptor tyrosine kinase (RTK) family with collagen-specific ligands[23]. Collagen binds to DDR causing tyrosine phosphorylation and consequent activation of downstream signaling pathways. While the specific mechanisms of action of DDRs are still being elucidated, DDRs have been identified as important receptors in regulating cell polarity, adhesion, migration and invasion[24]. In addition, DDRs have been suggested as negative regulators of integrin signaling[25].
CD44 is a glycoprotein and member of the hyaluronate family of receptors. It mainly binds the ECM protein hyaluronan but can also bind other ECM proteins including laminin, collagen and fibronectin[26]. CD44 is known to regulate cellular growth, differentiation, survival, migration and adhesion through different mechanisms including direct ligand binding, formation of co-receptors with RTKs and binding to ERM (ezrin, radixin and moesin) proteins thereby forming a link between the membrane and actin cytoskeleton[27].
Integrins are heterodimeric transmembrane receptors composed of α and β subunits that are considered the main matrix adhesion receptors mediating intracellular, cytoskeletal and ECM interactions[28]. Integrins can signal bidirectionally across the membrane, whereby intracellular signals can cause extracellular changes (“inside-out” signaling) and extracellular signaling can cause intracellular changes (“outside-in” signaling)[29]. Intracellular signaling via integrins is mediated through different signaling mediators including focal adhesion kinase (FAK), mitogen-activated protein kinases (MAPK) and members of the Rho-GTPase family[30]. Extracellular ligands for integrins include collagens, laminins or RGD-containing proteins[31]. Integrins regulate numerous important cellular features including migration, proliferation, survival and differentiation[32].
ECM remodelling is an important feature influencing cell to cell communication, a process which is regulated by degradation and modification of components of the ECM. Matrix metalloproteinases, ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) and serine proteinases play a major role in this process to maintain homeostasis within the ECM[33].
Signaling crosstalk is an interaction between two or more independently-initiated signaling pathways, the outcomes of which include the amplification or attenuation of the signal. In the context of crosstalk between cell-cell and cell-matrix adhesive signaling, it is intriguing to note that both sites contain not only structural proteins but also signaling effectors which fit the bill as potential mediators of crosstalk. Notably, a number of non-RTKs are localized to cell-cell and cell matrix adhesions and act as key players regulating crosstalk in the context of adhesive networks[34-36]. Furthermore, tyrosine kinase signaling is one of the most frequently deregulated signaling pathways in solid tumors, and has been well established to contribute to the initiation and progression of tumors[37].
Non-RTK proteins in conjunction with cell-cell and cell-matrix adhesion are crucial effectors of a broad range of stimuli, and mediate activation of signal transduction events regulating cell survival, morphology and migration of malignant and non-malignant cells. One of the key molecules in this respect is the non-RTK Src, activation of which has dual functions in regulating formation of cell-cell and cell-matrix adhesions. Src is recruited and activated upon E-cadherin ligation and this provides a positive feedback loop that signals through Phosphoinositide-3 Kinase (PI-3 kinase) to promote the stability of cell-cell contacts[36]. However, constitutively active Src disrupts cell-cell contacts and alters cell morphology[38], and its presence at integrin-matrix adhesions leads to peripheral accumulation of activated myosin, leading to disruption of cell-cell junctions[39]. Recent studies have focused on Src-dependent regulation of E-cadherin and other tumor progression-related events such as epithelial to mesenchymal transition (EMT) with the development of metastasis. In pancreatic adenocarcinoma, c-Src (Src) is frequently activated and controls tumor progression[40,41]. The amount of E-cadherin protein in a cell plays an important role in tumor progression to invasive, metastatic carcinoma[41,42]. It was shown that Src downregulates E-cadherin and induces EMT, favoring metastasis in pancreatic adenocarcinoma[41]. In breast carcinomas, complete loss of E-cadherin may be an important step in the formation of lobular carcinoma in situ, as a precursor of invasive lobular breast cancer[43]. In colon cancer, Src-induced deregulation of E-cadherin has been shown to require integrin signaling[44]. Another study showed how the ECM environment controls tumor growth[45]. Specifically, in pancreatic cancer cell lines with high metastatic potential, E-cadherin expression levels were normal and cells possessed functional E-cadherin/catenin complexes. When the cells were cultured on type I or III collagen, E-cadherin gene expression was repressed and E-cadherin and catenin protein concentrations were reduced. In contrast, growth on fibronectin or collagen IV had no effect on the level of E-cadherin. Overexpression of activated c-Src in pancreatic cancer cells mimicked collagen-induced E-cadherin down-regulation and altered cell migration[45]. In non-transformed mammary epithelial cells PI-3 Kinase-Rac1-c-JNK signaling has been associated with collagen I -induced cell scattering, up-regulation of N-Cadherin and consequent EMT[46]. It has been reported that other members of the Src kinase family mediate crosstalk between cell-cell and cell-matrix junctions, including the proto-oncogene c-Yes which is known to regulate epithelial tight junction formation by phosphorylation of a key tyrosine residue in occludin[47].
Further illustrating the potential importance of Src signaling in cell-cell to cell-matrix site crosstalk, the Src substrate FAK, itself a non-RTK, has also been shown to act as a downstream effector of integrin adhesion and signaling. By virtue of its binding to intracellular regions of β-integrin subunits, FAK plays a pivotal role as a signal integrator downstream of cell-ECM interactions and other receptor and non-RTKs[48]. Furthermore Src-FAK signaling promotes E-cadherin internalization during cancer progression, thus facilitating EMT and enhanced tumor cell motility[49]. In other cell types, as is the case with KM12C colon cancer cells that have retained E-cadherin, FAK can also have a negative influence on cadherin-mediated intercellular adhesion[44].
The non-RTKs Fes/Fer also regulate cell adhesion and cytoskeletal reorganization through the modification of AJs[50]. Fer is involved in communication between cell-cell and cell-matrix junctions by shuttling between N-cadherin-containing AJs and β1-integrin focal adhesion (FA) complexes[51]; and can be activated by either cadherin or integrins[34,52,53]. Fer phosphorylates cadherin-associated cortactin and this process is involved in mediating intercellular adhesion strength[54]. In breast cancer Fer has been speculated to promote metastasis by regulating α6 and β1 integrin-dependent cell adhesion[55].
Given the fact that a number of non-RTKs mediate crosstalk between cell-cell and cell-matrix adhesion sites, any deregulation in the signaling network could have profound impacts on cell-cell and cell-matrix adhesion favoring malignancies.
Several growth factor RTKs have also been reported to regulate cell-cell and cell-matrix adhesion both in cancer and normal cells. Among those, Ephrin receptors have been implicated in crosstalk with β-integrins in prostate cancer cells to strengthen adhesion to collagen type I and preferentially metastasize to collagen I-enriched bone tissue[56]. During breast cancer development, cancer cells metastasize to distant organs and interact with ECM and stroma to survive and remain dormant[57]. Another RTK which has been linked to adhesive crosstalk is the fibroblast growth factor (FGF) receptor. Specifically, estrogen-dependent breast cancer cells have been shown to be partially re-differentiated by FGF-2, which initiates upregulation of integrin α5β1 lost during malignant transformation[58]. Dual signaling by FGFR through PI3K and independently through integrin α5β1 induces activation of FAK and membrane localization and activation of the small GTPase RhoA and GRAF, a GTPase-regulator associated with FAK. This results in inactivation of RhoA, cortical rearrangement of F-actin and consequent switching of cancer cells to a dormant phenotype that promotes cancer cell survival[59]. In normal cells RhoA is essential for the assembly and function of epithelial cell-cell junctions whereas overexpression of RhoA in rat hepatoma cells induces an invasive phenotype[60]. Apart from this, FGFR signaling promotes cell adhesion in pancreatic cancer cells. N-CAM stimulates β1-integrin-mediated cell-matrix adhesion by activating FGFR signaling and could be a potential mechanism for preventing dissemination of tumor cells[61]. Activation of another growth factor receptor, that for EGF, prevents disruption of TJs in colon cancer cells. Specifically MAPK has been shown to interacts with occludin and mediates EGF-induced prevention of TJ disruption following hydrogen peroxide exposure[62]. Finally, the IGF1 receptor forms a ternary complex with E-cadherin and αv-integrins at cell-cell contacts in cultured colon cancer cells and human normal colonic mucosa. IGF1 binding to this receptor disrupts the complex and causes relocalisation of αv-integrins to focal contacts in conjunction with an increase in cell migration[63].
RTKs are known to play key roles in regulating several biological processes, and crosstalk with cell adhesion molecules is important in the context of normal development. However a large body of evidence from different laboratories suggests that such cooperation between cell-cell, cell-matrix and RTK signaling regulates cellular behaviors that are important for cancer progression.
Rho GTPases (guanosine triphosphatases) comprise a family of proteins within the Ras-like small G protein family, and include the molecules Rho, Rac, and Cdc42[64]. Rho GTPases play an important role in maintaining cell structure and polarity through their critical influence on dynamics of the actin cytoskeleton, thereby regulating cellular processes including cell adhesion and coordinating cell-cell and cell-matrix adhesion complexes[65]. Increased expression of Rho GTPases has been linked to many different cancer types including breast, lung, colorectal, testicular and prostate cancer[66-70]. Aberrant signaling of Rho GTPases has been attributed to promoting features of tumorigenesis including migration, invasion, cell survival, angiogenesis and metastasis[71]. As Rho GTPase molecules play an important role in regulating actin dynamics, cell adhesion and signaling processes, both at the cell-cell and cell-matrix level, it poses the question whether Rho GTPases mediate crosstalk between cell-cell specific and cell-matrix specific proteins and pathways, with particular reference to their involvement in cancer.
Rho GTPases are active when bound to GTP and inactive when bound to GDP, and a range of regulatory factors that dynamically influence the exchange kinetics of such reactions can themselves be signal control points or drug targets in cancer. These include the families of guanine nucleotide exchange factors which promote increased binding of GTP and therefore increase Rho GTPase activity[72]. In contrast, GTPase activating proteins inactivate GTP signaling by inducing GTP hydrolysis[72,73]. The switch between membranous active GTP-bound GTPases and cytosolic inactive GDP-bound GTPases is regulated by guanine dissociation inhibitors or GDI-like proteins[73].
Previous studies in our laboratory have shown a link in expression between the TJ protein JAM-A and the focal adhesion molecule β1 integrin in breast cancer tissues[74]. Further investigations to tease out the potential molecular crosstalk between JAM-A and β1 integrin identified the Rho GTPase Rap 1 as an important intermediary in controlling breast cancer cell migration[75]. Attenuation or knockdown of JAM-A in MCF7 breast cancer cells reduced the expression of β1 integrin along with αv and α5 integrins, resulting in reduced cell migration. The data presented in this study suggests a mechanism whereby activation of Rap1 by JAM-A occurs indirectly though association of JAM-A with the downstream signaling molecules Afadin (AF-6) and PDZ-GEF2, the guanine exchange factor[75]. Notably this mechanism was previously identified in SKCO-15 colonic epithelial cells, whereby loss of JAM-A, Afadin or PDZ-GEF2 resulted in decreased Rap1 and β1 integrin expression in addition to decreased cellular migration[76].
Another mechanism through which Rho GTPases could potentially influence crosstalk between cell-cell and cell-matrix junctions is via coincident inputs from structural proteins located at both sites. One such example is the cytoskeletal protein vinculin, which is recruited to (and localises at) both cell-cell and cell-matrix junctions[77] with a higher affinity for cell-matrix than cell-cell adhesion sites[78]. At the cell-cell junction, vinculin stabilises E-cadherin through binding with β-catenin[79] and strengthens actin binding by binding with α-catenin[80]. At the cell-matrix junction vinculin plays an important role in mediating cell signaling between cytoskeletal and ECM proteins via its role in focal adhesion formation[81]. Activation of vinculin at the cell-matrix junction requires binding to several proteins including talin and paxillin[81,82] with interaction of active vinculin and paxillin being dependent on Rac1 activation[83]. Additionally, active vinculin is important with respect to cell-matrix adhesion via stabilising adhesion complexes and promoting integrin clustering[84]. Vinculin has also been implicated in facilitating membrane protrusion via extension of lamellipodia and cell spreading, following binding and recruitment of Actin-related protein (Arp) 2/3 to sites of integrin clustering[85]. Of interest, the formation of the vinculin/Arp 2/3 complex was found to be regulated by PIP2 (phosphatidylinositol-4,5-bisphosphate) and Rac1[85]. While research to date has mainly investigated the role of vinculin in the cell-matrix context, further investigation is warranted to investigate if Rac1 can directly regulate vinculin functionality at the cell-cell junction, thereby offering a potential common pathway through which vinculin can elicit its functions and mediate crosstalk between adhesion sites.
MAGI-1 (MAGUK with inverted domain 1) is a scaffolding protein containing a PDZ domain which has been shown to regulate structural and functional features at the cell-cell junction and to increase cell adhesion to matrix proteins[86,87]. Interestingly, MAGI-1 is required for vascular endothelial-cadherin-dependent Rap-1 activation, which, upon activation facilitates relocation of vinculin from the cell-ECM location to the cell-cell location thereby regulating AJ formation[88]. MAGI-1 is known to stabilise E-cadherin and β-catenin localisation at the AJ, which in turn increases actin stress fiber and focal adhesion formation. MAGI-1 has been implicated as a putative negative regulator of metastasis in a colon cancer model, whereby overexpression of MAGI-1 was shown to increase integrin-mediated cell adhesion to matrix proteins and outside-in signaling via FAK, ERK 1/2 and Akt, and to inhibit anchorage-independent growth, invasion and migration in addition to Wnt signaling[87]. This study proposes a mechanism whereby inhibition of cyclooxygenase-2, which is overexpressed in a number of cancers (including colon), upregulates MAGI-1 which in turn inhibits Wnt/β-catenin signaling by decreasing free β-catenin[87]. As such MAGI-1 identifies itself as an important regulator of cell-cell and cell-matrix functions and signaling.
Nectins have been identified as important molecules capable of co-operatively communicating between cell-cell and cell-matrix junctions via their role in integrin-cadherin crosstalk. Nectins are Ig-like cell adhesion molecules which regulate the formation of cell-cell junctions, including TJs and AJs[12]. Nectins have been shown to regulate cellular features including cell polarity, proliferation, movement and survival[89], the deregulation of which is implicated in cancer. Nectins can physically interact with the αvβ3 integrin at cell-cell adhesion sites, leading to activation of Src kinase which is required for the formation of cadherin-based AJs[90]. Src is also activated by nectins following nectin-nectin interactions at cell-cell contact sites, which induces activation of the GTPase Rap1, resulting in downstream activation of the other GTPases Cdc42 and Rac[90]. Cdc42 activation results in filopodia formation, while Rac activation leads to lamellipodia formation[91], important structures for cell migration and movement[92]. This identifies a potential mechanism whereby activation of Rap1 by nectin-based cell contact can influence actin dynamics at the cell-matrix interface, which in turn regulate functional behaviours associated with migration. Additionally, interactions between integrins and Rho GTPases can elicit pro-migratory functions, as integrins are known to activate the Rho GTPases Rac and Cdc42[93] with Cdc42 being shown to drive trans-endothelial migration of cancer cells by enhancing the expression of β1 integrin[94], an important process in metastasis. The versatility of Rho family GTPases in driving diverse signaling events clearly illustrates their potential to influence mammalian physiology and pathophysiology. However an intriguing aside is that mammalian Rho GTPase signaling is also commonly exploited for non-mammalian advantage, specifically in invasion or intoxication strategies of multiple bacterial pathogens. Interested readers are referred to an excellent recent review on this topic[95], while the next section of this manuscript will instead concentrate on the concept that pathogenic interactions with mammalian cells represent a paradigm of successful crosstalk between cell-cell and cell-matrix adhesion sites.
By virtue of their strategic bridging location between the apical and basolateral membrane domains of epithelial cell surfaces, it has been well-established that TJ proteins are attractive targets for pathogens seeking to infect cells or to modify cellular functions towards a survival advantage. Among those TJ components known to be directly exploited as viral pathogen receptors or co-receptors are JAM-A (reovirus[96], rotavirus[97]) and CAR (adenovirus 5, group B coxsackieviruses[98]), but many non-viral pathogens are also recognised to use TJ disruption as a broader primary mechanism to open up access to basolaterally-located attachment receptors (e.g., Yersinia[99]) or to orchestrate signaling events that drive disease [e.g., Helicobacter pylori (H. pylori)[100]]. An emerging literature is revealing that pathogens are masters of inducing crosstalk between epithelial cell-cell and cell-matrix adhesive interfaces to facilitate their own goals, of which various examples will be discussed herein.
In the case of viral infection of epithelial barriers, cell entry is a key requirement, and abundant examples illustrate cooperativity between cell-cell and cell-matrix sites to facilitate an attachment-invasion sequence. JAM-A has been described to act as an attachment receptor for the Reoviridae family member rotavirus via its VP4 spike protein[97], in conjunction with integrins acting as post-attachment receptors for the same non-enveloped virus[101]. Furthermore gene silencing screens have identified a potential role for not just JAM-A but also claudins-6, -9 and -14 in mediating rotaviral infection of cells[102]; which fits with the observation that a component of the VP4 spike (VP8) has sequence homology to the extracellular loops of claudin and can diminish TJ gate and fence function[103]. Compromising the paracellular barrier in this fashion would confer a distinct advantage upon the ability of the rotavirus to access basolaterally-located integrin co-receptors; which it could then bind via opportunistic expression of integrin-recognition tripeptide sequences in its outer capsid[104]. Accordingly, multiple viruses including the human cytomegalovirus[105] have been demonstrated to possess classic integrin binding sequences.
Another Reoviridae family member which has been shown to orchestrate crosstalk between cell-cell and cell-matrix sites to facilitate infection (and thereby survival) is the reovirus. Specifically, reovirus uses JAM-A as its cellular attachment receptor[96], whereupon β1-integrin is required for viral internalization via interactions with RGD/KGE sequences on the λ2 reovirus protein[106]. It is interesting to note that that the reovirus attachment protein sigma1 can, via a higher affinity interaction, substitute for one molecule of JAM-A in the homo-dimerisation event which characterises the adhesive function of JAM-A[107]. This could have implications for the integrity of the epithelial barrier which would, in turn, modify pathogen access to the normally restricted sub-epithelial compartment.
The final example of virus-induced adhesive crosstalk which will be discussed here is that of coxsackie and adenoviruses. A protein member of the Ig superfamily which is structurally related to JAM-A, namely coxsackie and adenovirus receptor (CAR), has been identified as the attachment receptor for both adenovirus-5 (Ad5) and group B coxsackieviruses[98]. However it has been demonstrated that post-attachment internalization of adenovirus depends upon interactions between virally-expressed RGD sequences and alpha-V integrins[108]. Accordingly, CAR overexpression can modify the activation status of integrins via activation of p44/42 MAP kinase, which concomitantly enhances cell-matrix adhesion and Ad5 infection[109]. Intriguingly, a feedback loop whereby Ad5 infection also promotes CAR dimerization and further MAPK-dependent viral infection[109] supports the assumption that infection dynamics are critically regulated by crosstalk between cell-cell and cell-matrix sites. Given that both CAR and integrin expression levels are sensitive to modulation by certain cytokines[110], it is likely that the specific environmental milieu of a tissue can influence not only adhesive crosstalk but consequently the likelihood of infection.
Mechanisms of infection differ somewhat in the context of bacteria, which, unlike viruses, are not entirely dependent on cellular invasion for their propagation. However a recurring theme is that bacteria often disrupt cell-cell junctional barriers to access basolaterally-located receptors, facilitating injection of bacterial proteins into host cells. One such example is Yersinia pseudotuberculosis, which has been shown to attach to a pool of β1-integrin that co-localizes with ZO-1 at TJ of renal epithelial cells[111]. The seemingly incongruous spatial overlap of a cell-cell and cell-matrix protein may, in this case, be explained by disruptions in F-actin and the redistribution of actin-affiliated cell-cell adhesion proteins following cellular penetration of the cytotoxic Yersinia outer membrane protein YopE[111]. Alternatively an environment of acute inflammation could promote access to basolaterally-located β1-integrin, as it has been demonstrated that colonic epithelial cells become sensitized to Y. pseudotuberculosis infection following the induction of micro-discontinuities in sites where neutrophils have trans-migrated across the epithelial barrier[99]. A pro-inflammatory cytokine balance could also perturb cell-cell adhesion and barrier function in a manner that permits pathogen access to basolateral receptors, as it has been demonstrated that IFNγ causes internalization of the tight junction protein occludin via macropinocytosis[112]. Interestingly, selective macropinocytosis of occludin has been illustrated downstream of Coxsackievirus B binding to CAR at TJ, with a block on viral entry in the absence of either occludin or a functional macropinocytotic pathway[113]. The nature of occludin as a common target for some bacterial and viral infections is further evidenced by a report that the E. coli-derived bacterial toxin cytotoxic necrotizing factor-1 (CNF-1) induces occludin and ZO-1 internalization via an uptake pathway involving caveolar lipid rafts[114]. In light of the crosstalk mediators alluded to in previous paragraphs, it is no coincidence that CNF-1 simultaneously activates Rho, Rac and Cdc42 in conjunction with impairment of epithelial barrier function and putative activation of focal adhesion assembly[114].
The final example of bacteria inducing inter-adhesion site crosstalk which will be dealt with in this review is that of H. pylori, the causative organism of peptic ulcer disease and itself a Group I carcinogen. The H. pylori effector protein CagA has been shown to disrupt TJ integrity by way of forming a complex with JAM-A and ZO-1[100]. Furthermore another gene in the cag pathogenicity island, CagL, reportedly binds to αV-β1-integrin via an RGD sequence, simultaneously activating FAK and Src activation whilst facilitating CagA entry into cells[115]. Taken together, such examples of cooperativity between cell-cell and cell-matrix adhesion sites make it intriguing to speculate that the crosstalk mechanisms which pathogens have evolved could be exploited for therapeutic advantage in not only infection but also cancer contexts.
Tyrosine kinase signaling is frequently deregulated in both solid and haematological tumors, and has been solidly linked to tumor initiation and progression. Both receptor and non-RTKs could mediate crosstalk signals between cell-cell and cell-matrix adhesion sites. In cancer cells RTKs are often constitutively active, and drug-based targeting of RTKs will only be successful if the targeted RTK is a major regulator of cancer cell survival. The most commonly deregulated RTKs include EGFR, HER2, MET, VEGFR, PDGFR, FGFR and IGF-1R[116]. Accordingly several monoclonal antibodies and small-molecule inhibitors have been developed to target these receptors. Since EGF receptor family members are frequently overexpressed in breast cancer patients[117,118], monoclonal antibodies like Trastuzumab (Herceptin), pertuzumab (Omitrag) and small-molecule inhibitors like lapatinib, gefitinib, erlotinib, AEE788 and CI-1033 have formed prototype targeted therapies[119]. For more detailed information on EGF receptor family members and their inhibitors, interested readers are directed to the following review article[120]. Further monoclonal antibody (pertuzumab) and small-molecule inhibitors (AEE788 and CI-1033) have been approved for clinical testing in various cancers like ovarian, prostate, and non-small cell lung cancer (NSCLC) whereas small molecule inhibitors (gefitinib and erlotinib) have been approved for treating NSCLC.
Another attractive option for targeted therapy in cancer has been the non-RTK Src, whose expression is known to be elevated in several tumors and has been implicated in disease progression, invasion and metastasis. Among the non-RTKs Src has been one of the most widely studied, encompassing its family members Lyn, Fyn, Lck, Hck, Fgr, Blk, Yrk, and Yes. To date several Src inhibitors such as dasatinib, SKI-606 (Bosutinib), and AZD0530 have been developed and are undergoing clinical trials in breast cancer patients[121-126]. Dasatinib is an effective inhibitor of several kinases including Bcr-abl, Src family kinases, c-KIT and PDGFR-β5[127], with promising preclinical results indicating its ability to inhibit the growth of breast luminal and basal tumors independently of ER or HER2 status[128,129]. Analogously, SKI-606 inhibits several Src family kinases and Bcr-Abl and suppresses migration and invasion of breast cancer cells[130]. AZD0530 (Saracatinib) is a more selective inhibitor of Src and Bcr-Abl in gastric and prostate cancer[131,132], and in breast cancer (when combined with estrogen blockers) has been shown to inhibit proliferation of cancer cells in vitro and in vivo[133].
Since both receptor and non-RTKs are frequently overexpressed in tumors and play key roles in signaling crosstalk, this section has highlighted the logic of targeting their signaling for downregulation as a mechanism of stalling cancer progression. However it must always be remembered that cancer is a complex disease resulting from deregulation of several signaling pathways, whether concurrently or sequentially, therefore long-term therapeutic strategies must always consider targeting multiple signaling pathways for the most effective outcome.
Activation of Rho GTPases and their interactions with multiple different molecules can initiate many signaling pathways and downstream effects, including cancer-related behaviours such as migration, proliferation, cytoskeletal changes and apoptosis[134]. As such, Rho GTPases present themselves as attractive therapeutic targets, and many endeavors have been made to target these molecules in cancer, with promising preclinical results so far[134]. A Rac-specific small molecule inhibitor, NSC23766, has been found to inhibit features of invasion and anchorage-independent growth in human prostate cancer PC-3 cells[135]. Additionally, this compound was also found to inhibit breast cancer cell growth via cell-dependent mechanisms, either through cell cycle arrest or apoptosis[136]. Since then, several derivatives of this Rho GTPase inhibitor have been developed including AZA1 and AZA197.
A Cdc42 and Rac1 GTPase inhibitor, AZA1, a modified derivative of NSC23766, has been shown to inhibit migration and proliferation in addition to promoting apoptosis in prostate cancer cell lines in vitro[137]. This study also demonstrated increased survival and a reduction in tumor growth in a 22Rv1 androgen-independent xenograft model of prostate cancer[137]. In addition, another modified derivative of NSC23766 and a Cdc42 inhibitor, AZA197, has been shown to inhibit proliferation, migration and invasion in addition to exhibiting pro-apoptotic functionality in human colon cancer cell lines in vitro[138]. In this study, AZA197 also inhibited proliferation and promoted apoptosis while decreasing tumor growth and increasing survival in a human colon cancer xenograft mouse model[138].
Additionally, two Rac specific inhibitors, EHT 1864 and EHop-016, have shown anti-tumorigenic properties in breast cancer. EHT 1864, a Rac-specific inhibitor, was shown to inhibit estrogen-mediated breast cancer cell proliferation and to decrease growth of tamoxifen-resistant breast cancer cells[139] thereby identifying potential subtypes of breast cancer which this Rho GTPase inhibitor may preferentially target. In another study, EHT 1864 was also shown to have anti-proliferative, anti-invasive and pro-apoptotic effects in breast cancer cells[140]. The small molecule Rac inhibitor, EHop-016, has also shown promising preclinical results in targeting Rac activity in breast cancer. In one study, treatment with EHop-016 resulted in reduced tumor growth, inhibition of metastasis and angiogenesis in addition to inhibiting proliferation and increasing apoptosis in MDA-MB-435 breast cancer cells[141].
Rho GTPases have been shown to play a role in cell signaling and functions critical to tumor formation and metastasis. Interestingly, Rho GTPases, as discussed in this review, have been shown to interact with and activate molecules present at both the cell-cell and/or cell-matrix locations and may provide mediators of crosstalk between these cellular locations. As demonstrated in different cancer models, inhibition of Rho GTPases suggests a novel and potentially beneficial approach in cancer treatment and in the prevention of metastasis. It is interesting to speculate that targeting specific Rho GTPases which function at both the cell-cell and cell-matrix junctions may indeed offer enhanced inhibitory effects and present potential advantages over single site-directed inhibitors.
We have already illustrated that enzymes (such as kinases and GTPases) offer huge potential as cancer drug targets by virtue of their capacity to modify cell signaling, including that between cell-cell and cell-matrix junctions. However there is also an emerging literature on the concept that pathogens which bind to (or interfere with) cell-surface adhesion receptors could be used as tumor cytolytic agents, drug delivery systems or even as immunomodulators in various cancers. The simplest argument is in the use of pathogens or their derivatives as cytolytic agents. This has been well-evidenced by the interaction of Clostridium perfringens enterotoxin (CPE) with its cellular receptors, the tight junction proteins claudin-3 or -4, having a cytolytic effect on claudin-overexpressing breast cancer cells[142] and also brain metastases of breast primary tumors[143]. Therapeutic potential of using CPE has also been suggested for ovarian[144,145], endometrial[146] and pancreatic[147] tumors. Similarly, the JAM-A-binding pathogen reovirus has been mooted as a potential therapy for certain brain cancers, although the mechanisms of cytolysis may yet be JAM-independent[148]. Furthermore targeting the CAR (CAR; a TJ-enriched Ig superfamily member related to JAM-A) with conditionally-replicating adenoviruses has been discussed in melanoma treatment[149]. Cytolysis aside, using pathogens or modified derivatives to deliver transgenes[150] or cytotoxic molecules (akin to the “magic bullet” concept with monoclonal antibodies) is also an interesting concept. Although not without conceptual problems, some selectivity might be conferred by the careful selection of cancers in which the aforementioned pathogen receptors are known to be overexpressed. Finally the concept of immunomodulation via controlled pathogen attack of human cells also offers promise - from the very early observations of tumor regression in patients with certain Streptococcal infections (essentially a vaccination strategy involving the so-called Coley’s toxin)[151] to the more recent report of differential sensitivity to infection of reoviruses expressing the co-stimulatory molecule B7-1[150]).
While a complete description of such phenomena is beyond the scope of this review, it is nonetheless intriguing to speculate that future developments in the arena of using pathogens or pathogenic derivatives to modulate cell-cell to cell-matrix adhesion site crosstalk (amongst other signaling mechanisms) may prove valuable in cancer therapeutics.
This review has endeavored to highlight pathways and signaling mechanisms of crosstalk between cell-cell and cell-matrix adhesion sites, with particular reference to the relevance of these concepts in cancer biology. Interestingly, successful crosstalk between spatially-distinct adhesion sites has been exemplified by the mechanisms which several pathogens use to invade or intoxicate epithelial barriers; and it is likely that much could be learned from such models in designing drugs to combat dysfunctional signaling crosstalk events associated with cancer progression. Some of the key putative mediators driving crosstalk between cell-cell and cell-matrix adhesion sites include Rho GTPases and kinases, hence these were concentrated upon in this review. Since Rho GTPases and kinases concurrently play a central role in tumorigenesis, we highlighted some current pharmacological strategies to target these molecules. We also alluded to the intriguing possibility of using modified bacterial or viral components as therapeutic tools to tap into crosstalk mechanisms which they have evolved mastery of. Taken together, we propose that rational strategies targeting specific regulators of cell-cell and cell-matrix adhesion may have far-reaching therapeutic potential in attenuating the signaling events associated with cancer initiation or progression.
P- Reviewer: Bonanno L, Wang JY S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73-98. [PubMed] |
| 2. | Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:a002998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 573] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 5. | González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 593] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 6. | Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677-3682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Sawada N. Tight junction-related human diseases. Pathol Int. 2013;63:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 708] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 9. | Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15:397-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 438] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 10. | Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 376] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 12. | Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 445] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 13. | Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 14. | Brooke MA, Nitoiu D, Kelsell DP. Cell-cell connectivity: desmosomes and disease. J Pathol. 2012;226:158-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778:572-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 282] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Nekrasova O, Green KJ. Desmosome assembly and dynamics. Trends Cell Biol. 2013;23:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3:pii: a005033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 399] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 19. | Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183-1198. [PubMed] |
| 20. | Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 823] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 21. | Humphries JD, Paul NR, Humphries MJ, Morgan MR. Emerging properties of adhesion complexes: what are they and what do they do? Trends Cell Biol. 2015;25:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Choi Y, Chung H, Jung H, Couchman JR, Oh ES. Syndecans as cell surface receptors: Unique structure equates with functional diversity. Matrix Biol. 2011;30:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18:1108-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 269] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 25. | Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, Mahasenan KV, Mobashery S, Huang P, Agarwal G. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013;288:7430-7437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 503] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1645] [Cited by in RCA: 1806] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 28. | Humphries MJ, Travis MA, Clark K, Mould AP. Mechanisms of integration of cells and extracellular matrices by integrins. Biochem Soc Trans. 2004;32:822-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Wegener KL, Campbell ID. Transmembrane and cytoplasmic domains in integrin activation and protein-protein interactions (review). Mol Membr Biol. 2008;25:376-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339:481-488. [PubMed] |
| 31. | Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 32. | Legate KR, Wickström SA, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 572] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 33. | Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:pii: a005058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1541] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 34. | Weber GF, Bjerke MA, DeSimone DW. Integrins and cadherins join forces to form adhesive networks. J Cell Sci. 2011;124:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 35. | Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, Yap AS. E-cadherin adhesion activates c-Src signaling at cell-cell contacts. Mol Biol Cell. 2007;18:3214-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Jones RJ, Brunton VG, Frame MC. Adhesion-linked kinases in cancer; emphasis on src, focal adhesion kinase and PI 3-kinase. Eur J Cancer. 2000;36:1595-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 719] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 39. | Avizienyte E, Fincham VJ, Brunton VG, Frame MC. Src SH3/2 domain-mediated peripheral accumulation of Src and phospho-myosin is linked to deregulation of E-cadherin and the epithelial-mesenchymal transition. Mol Biol Cell. 2004;15:2794-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, Gallick GE. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Nagathihalli NS, Merchant NB. Src-mediated regulation of E-cadherin and EMT in pancreatic cancer. Front Biosci (Landmark Ed). 2012;17:2059-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920-6929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 602] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 43. | Berx G, Van Roy F. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 2001;3:289-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 293] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Menke A, Philippi C, Vogelmann R, Seidel B, Lutz MP, Adler G, Wedlich D. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res. 2001;61:3508-3517. [PubMed] |
| 46. | Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745-11753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Chen YH, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell. 2002;13:1227-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta. 2004;1692:121-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 50. | Murray MJ, Davidson CM, Hayward NM, Brand AH. The Fes/Fer non-receptor tyrosine kinase cooperates with Src42A to regulate dorsal closure in Drosophila. Development. 2006;133:3063-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Arregui C, Pathre P, Lilien J, Balsamo J. The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. J Cell Biol. 2000;149:1263-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | El Sayegh TY, Arora PD, Fan L, Laschinger CA, Greer PA, McCulloch CA, Kapus A. Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol Biol Cell. 2005;16:5514-5527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol Cell Biol. 2007;27:6140-6152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | El Sayegh TY, Arora PD, Laschinger CA, Lee W, Morrison C, Overall CM, Kapus A, McCulloch CA. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J Cell Sci. 2004;117:5117-5131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Ivanova IA, Vermeulen JF, Ercan C, Houthuijzen JM, Saig FA, Vlug EJ, van der Wall E, van Diest PJ, Vooijs M, Derksen PW. FER kinase promotes breast cancer metastasis by regulating α6- and β1-integrin-dependent cell adhesion and anoikis resistance. Oncogene. 2013;32:5582-5592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Yu M, Wang J, Muller DJ, Helenius J. In PC3 prostate cancer cells ephrin receptors crosstalk to β1-integrins to strengthen adhesion to collagen type I. Sci Rep. 2015;5:8206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Zhang XH, Giuliano M, Trivedi MV, Schiff R, Osborne CK. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res. 2013;19:6389-6397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 58. | Korah R, Boots M, Wieder R. Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 2004;64:4514-4522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Barrios J, Wieder R. Dual FGF-2 and intergrin alpha5beta1 signaling mediate GRAF-induced RhoA inactivation in a model of breast cancer dormancy. Cancer Microenviron. 2009;2:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 1999;59:2004-2010. [PubMed] |
| 61. | Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumor-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001;3:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 62. | Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J. 2006;393:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 63. | Canonici A, Steelant W, Rigot V, Khomitch-Baud A, Boutaghou-Cherid H, Bruyneel E, Van Roy F, Garrouste F, Pommier G, André F. Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. Int J Cancer. 2008;122:572-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 413] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 65. | Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4808] [Cited by in RCA: 4798] [Article Influence: 177.7] [Reference Citation Analysis (0)] |
| 66. | Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumors: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 67. | Zhou C, Licciulli S, Avila JL, Cho M, Troutman S, Jiang P, Kossenkov AV, Showe LC, Liu Q, Vachani A. The Rac1 splice form Rac1b promotes K-ras-induced lung tumorigenesis. Oncogene. 2013;32:903-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Zhou J, Yang J, Li K, Mo P, Feng B, Wang X, Nie Y, Fan D. RhoE is associated with relapse and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2013;20:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, Yoshida K. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10:4799-4805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 70. | Engers R, Ziegler S, Mueller M, Walter A, Willers R, Gabbert HE. Prognostic relevance of increased Rac GTPase expression in prostate carcinomas. Endocr Relat Cancer. 2007;14:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019. [PubMed] |
| 72. | Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1264] [Cited by in RCA: 1457] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 73. | Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 927] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 74. | McSherry EA, McGee SF, Jirstrom K, Doyle EM, Brennan DJ, Landberg G, Dervan PA, Hopkins AM, Gallagher WM. JAM-A expression positively correlates with poor prognosis in breast cancer patients. Int J Cancer. 2009;125:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | McSherry EA, Brennan K, Hudson L, Hill AD, Hopkins AM. Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Res. 2011;13:R31. [PubMed] |
| 76. | Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Peng X, Nelson ES, Maiers JL, DeMali KA. New insights into vinculin function and regulation. Int Rev Cell Mol Biol. 2011;287:191-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 78. | Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW, Liddington RC. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 79. | Peng X, Cuff LE, Lawton CD, DeMali KA. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J Cell Sci. 2010;123:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 80. | Weiss EE, Kroemker M, Rüdiger AH, Jockusch BM, Rüdiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141:755-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 211] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 81. | Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 664] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 82. | Turner CE, Glenney JR, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 546] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 83. | Deakin NO, Ballestrem C, Turner CE. Paxillin and Hic-5 interaction with vinculin is differentially regulated by Rac1 and RhoA. PLoS One. 2012;7:e37990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Carisey A, Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur J Cell Biol. 2011;90:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 85. | DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 86. | Laura RP, Ross S, Koeppen H, Lasky LA. MAGI-1: a widely expressed, alternatively spliced tight junction protein. Exp Cell Res. 2002;275:155-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Zaric J, Joseph JM, Tercier S, Sengstag T, Ponsonnet L, Delorenzi M, Rüegg C. Identification of MAGI1 as a tumor-suppressor protein induced by cyclooxygenase-2 inhibitors in colorectal cancer cells. Oncogene. 2012;31:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Ogita H, Rikitake Y, Miyoshi J, Takai Y. Cell adhesion molecules nectins and associating proteins: Implications for physiology and pathology. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:621-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Sakamoto Y, Ogita H, Hirota T, Kawakatsu T, Fukuyama T, Yasumi M, Kanzaki N, Ozaki M, Takai Y. Interaction of integrin alpha(v)beta3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J Biol Chem. 2006;281:19631-19644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3378] [Cited by in RCA: 3426] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 92. | Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3010] [Cited by in RCA: 2948] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 93. | Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 515] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 94. | Reymond N, Im JH, Garg R, Vega FM, Borda d’Agua B, Riou P, Cox S, Valderrama F, Muschel RJ, Ridley AJ. Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J Cell Biol. 2012;199:653-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 95. | Lemichez E, Aktories K. Hijacking of Rho GTPases during bacterial infection. Exp Cell Res. 2013;319:2329-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 499] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 97. | Torres-Flores JM, Silva-Ayala D, Espinoza MA, López S, Arias CF. The tight junction protein JAM-A functions as coreceptor for rotavirus entry into MA104 cells. Virology. 2015;475:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 98. | Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2385] [Cited by in RCA: 2316] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 99. | McCormick BA, Nusrat A, Parkos CA, D’Andrea L, Hofman PM, Carnes D, Liang TW, Madara JL. Unmasking of intestinal epithelial lateral membrane beta1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect Immun. 1997;65:1414-1421. [PubMed] |
| 100. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 583] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 101. | Baker M, Prasad BV. Rotavirus cell entry. Curr Top Microbiol Immunol. 2010;343:121-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Silva-Ayala D, López T, Gutiérrez M, Perrimon N, López S, Arias CF. Genome-wide RNAi screen reveals a role for the ESCRT complex in rotavirus cell entry. Proc Natl Acad Sci USA. 2013;110:10270-10275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Nava P, López S, Arias CF, Islas S, González-Mariscal L. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J Cell Sci. 2004;117:5509-5519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 104. | Graham KL, Fleming FE, Halasz P, Hewish MJ, Nagesha HS, Holmes IH, Takada Y, Coulson BS. Rotaviruses interact with alpha4beta7 and alpha4beta1 integrins by binding the same integrin domains as natural ligands. J Gen Virol. 2005;86:3397-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA. 2004;101:15470-15475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 106. | Maginnis MS, Forrest JC, Kopecky-Bromberg SA, Dickeson SK, Santoro SA, Zutter MM, Nemerow GR, Bergelson JM, Dermody TS. Beta1 integrin mediates internalization of mammalian reovirus. J Virol. 2006;80:2760-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 107. | Kirchner E, Guglielmi KM, Strauss HM, Dermody TS, Stehle T. Structure of reovirus sigma1 in complex with its receptor junctional adhesion molecule-A. PLoS Pathog. 2008;4:e1000235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 108. | Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1749] [Cited by in RCA: 1689] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 109. | Farmer C, Morton PE, Snippe M, Santis G, Parsons M. Coxsackie adenovirus receptor (CAR) regulates integrin function through activation of p44/42 MAPK. Exp Cell Res. 2009;315:2637-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Brüning A, Runnebaum IB. CAR is a cell-cell adhesion protein in human cancer cells and is expressionally modulated by dexamethasone, TNFalpha, and TGFbeta. Gene Ther. 2003;10:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 111. | Tafazoli F, Holmström A, Forsberg A, Magnusson KE. Apically exposed, tight junction-associated beta1-integrins allow binding and YopE-mediated perturbation of epithelial barriers by wild-type Yersinia bacteria. Infect Immun. 2000;68:5335-5343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 113. | Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 114. | Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 115. | Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, Berger J, Sewald N, König W. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 508] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 116. | Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011;34:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 117. | Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 118. | Stern DF. Tyrosine kinase signalling in breast cancer: ErbB family receptor tyrosine kinases. Breast Cancer Res. 2000;2:176-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2397] [Cited by in RCA: 2482] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 120. | Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat. 2012;136:331-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 522] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 121. | Finn RS. Targeting Src in breast cancer. Ann Oncol. 2008;19:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 122. | Isakoff SJ, Wang D, Campone M, Calles A, Leip E, Turnbull K, Bardy-Bouxin N, Duvillié L, Calvo E. Bosutinib plus capecitabine for selected advanced solid tumors: results of a phase 1 dose-escalation study. Br J Cancer. 2014;111:2058-2066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 123. | Moy B, Neven P, Lebrun F, Bellet M, Xu B, Sarosiek T, Chow L, Goss P, Zacharchuk C, Leip E. Bosutinib in combination with the aromatase inhibitor letrozole: a phase II trial in postmenopausal women evaluating first-line endocrine therapy in locally advanced or metastatic hormone receptor-positive/HER2-negative breast cancer. Oncologist. 2014;19:348-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 124. | Scher KS, Somlo G. Dasatinib: a novel therapy for breast cancer? Expert Opin Investig Drugs. 2013;22:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Karim SA, Creedon H, Patel H, Carragher NO, Morton JP, Muller WJ, Evans TR, Gusterson B, Sansom OJ, Brunton VG. Dasatinib inhibits mammary tumor development in a genetically engineered mouse model. J Pathol. 2013;230:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Gucalp A, Sparano JA, Caravelli J, Santamauro J, Patil S, Abbruzzi A, Pellegrino C, Bromberg J, Dang C, Theodoulou M. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin Breast Cancer. 2011;11:306-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 127. | Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658-6661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 1010] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 128. | Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/”triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 129. | Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 130. | Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther. 2008;7:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 131. | Nam HJ, Im SA, Oh DY, Elvin P, Kim HP, Yoon YK, Min A, Song SH, Han SW, Kim TY. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. 2013;12:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 132. | Yang JC, Bai L, Yap S, Gao AC, Kung HJ, Evans CP. Effect of the specific Src family kinase inhibitor saracatinib on osteolytic lesions using the PC-3 bone model. Mol Cancer Ther. 2010;9:1629-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Chen Y, Alvarez EA, Azzam D, Wander SA, Guggisberg N, Jordà M, Ju Z, Hennessy BT, Slingerland JM. Combined Src and ER blockade impairs human breast cancer proliferation in vitro and in vivo. Breast Cancer Res Treat. 2011;128:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 134. | Prudnikova TY, Rawat SJ, Chernoff J. Molecular pathways: targeting the kinase effectors of RHO-family GTPases. Clin Cancer Res. 2015;21:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 135. | Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 2006;406:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 136. | Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, Zhang B. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 137. | Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS One. 2013;8:e74924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Zins K, Gunawardhana S, Lucas T, Abraham D, Aharinejad S. Targeting Cdc42 with the small molecule drug AZA197 suppresses primary colon cancer growth and prolongs survival in a preclinical mouse xenograft model by downregulation of PAK1 activity. J Transl Med. 2013;11:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 139. | Rosenblatt AE, Garcia MI, Lyons L, Xie Y, Maiorino C, Désiré L, Slingerland J, Burnstein KL. Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor levels and is a novel therapeutic strategy in breast cancer. Endocr Relat Cancer. 2011;18:207-219. [PubMed] |
| 140. | Katz E, Sims AH, Sproul D, Caldwell H, Dixon MJ, Meehan RR, Harrison DJ. Targeting of Rac GTPases blocks the spread of intact human breast cancer. Oncotarget. 2012;3:608-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 141. | Castillo-Pichardo L, Humphries-Bickley T, De La Parra C, Forestier-Roman I, Martinez-Ferrer M, Hernandez E, Vlaar C, Ferrer-Acosta Y, Washington AV, Cubano LA. The Rac Inhibitor EHop-016 Inhibits Mammary Tumor Growth and Metastasis in a Nude Mouse Model. Transl Oncol. 2014;7:546-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 142. | Kominsky SL, Vali M, Korz D, Gabig TG, Weitzman SA, Argani P, Sukumar S. Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin 3 and 4. Am J Pathol. 2004;164:1627-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 143. | Kominsky SL, Tyler B, Sosnowski J, Brady K, Doucet M, Nell D, Smedley JG, McClane B, Brem H, Sukumar S. Clostridium perfringens enterotoxin as a novel-targeted therapeutic for brain metastasis. Cancer Res. 2007;67:7977-7982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 144. | Santin AD, Cané S, Bellone S, Palmieri M, Siegel ER, Thomas M, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Treatment of chemotherapy-resistant human ovarian cancer xenografts in C.B-17/SCID mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer Res. 2005;65:4334-4342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 145. | Casagrande F, Cocco E, Bellone S, Richter CE, Bellone M, Todeschini P, Siegel E, Varughese J, Arin-Silasi D, Azodi M. Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer. 2011;117:5519-5528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 146. | Santin AD, Bellone S, Marizzoni M, Palmieri M, Siegel ER, McKenney JK, Hennings L, Comper F, Bandiera E, Pecorelli S. Overexpression of claudin-3 and claudin-4 receptors in uterine serous papillary carcinoma: novel targets for a type-specific therapy using Clostridium perfringens enterotoxin (CPE). Cancer. 2007;109:1312-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 147. | Michl P, Buchholz M, Rolke M, Kunsch S, Löhr M, McClane B, Tsukita S, Leder G, Adler G, Gress TM. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 148. | van den Hengel SK, Balvers RK, Dautzenberg IJ, van den Wollenberg DJ, Kloezeman JJ, Lamfers ML, Sillivis-Smit PA, Hoeben RC. Heterogeneous reovirus susceptibility in human glioblastoma stem-like cell cultures. Cancer Gene Ther. 2013;20:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 149. | Schmitz M, Graf C, Gut T, Sirena D, Peter I, Dummer R, Greber UF, Hemmi S. Melanoma cultures show different susceptibility towards E1A-, E1B-19 kDa- and fiber-modified replication-competent adenoviruses. Gene Ther. 2006;13:893-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Hemmi S, Geertsen R, Mezzacasa A, Peter I, Dummer R. The presence of human coxsackievirus and adenovirus receptor is associated with efficient adenovirus-mediated transgene expression in human melanoma cell cultures. Hum Gene Ther. 1998;9:2363-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 160] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 151. | Maletzki C, Klier U, Obst W, Kreikemeyer B, Linnebacher M. Reevaluating the concept of treating experimental tumors with a mixed bacterial vaccine: Coley’s Toxin. Clin Dev Immunol. 2012;2012:230625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |