Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.178
Peer-review started: June 2, 2015
First decision: July 27, 2015
Revised: October 12, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: February 26, 2016
Processing time: 272 Days and 18.4 Hours
AIM: To investigate the effect of high homocysteine (Hcy) levels on apolipoprotein E (apoE) expression and the signaling pathways involved in this gene regulation.
METHODS: Reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot were used to assess apoE expression in cells treated with various concentrations (50-500 μmol/L) of Hcy. Calcium phosphate-transient transfections were performed in HEK-293 and RAW 264.7 cells to evaluate the effect of Hcy on apoE regulatory elements [promoter and distal multienhancer 2 (ME2)]. To this aim, plasmids containing the proximal apoE promoter [(-500/+73)apoE construct] alone or in the presence of ME2 [ME2/(-500/+73)apoE construct] to drive the expression of the reporter luciferase gene were used. Co-transfection experiments were carried out to investigate the downstream effectors of Hcy-mediated regulation of apoE promoter by using specific inhibitors or a dominant negative form of IKβ. In other co-transfections, the luciferase reporter was under the control of synthetic promoters containing multiple specific binding sites for nuclear factor kappa B (NF-κB), activator protein-1 (AP-1) or nuclear factor of activated T cells (NFAT). Chromatin immunoprecipitation (ChIP) assay was accomplished to detect the binding of NF-κB p65 subunit to the apoE promoter in HEK-293 treated with 500 μmol/L Hcy. As control, cells were incubated with similar concentration of cysteine. NF-κB p65 proteins bound to DNA were immunoprecipitated with anti-p65 antibodies and DNA was identified by PCR using primers amplifying the region -100/+4 of the apoE gene.
RESULTS: RT-PCR revealed that high levels of Hcy (250-750 μmol/L) induced a 2-3 fold decrease in apoE mRNA levels in HEK-293 cells, while apoE gene expression was not significantly affected by treatment with lower concentrations of Hcy (100 μmol/L). Immunoblotting data provided additional evidence for the negative role of Hcy in apoE expression. Hcy decreased apoE promoter activity, in the presence or absence of ME2, in a dose dependent manner, in both RAW 264.7 and HEK-293 cells, as revealed by transient transfection experiments. The downstream effectors of the signaling pathways of Hcy were also investigated. The inhibitory effect of Hcy on the apoE promoter activity was counteracted by MAPK/ERK kinase 1/2 (MEK1/2) inhibitor U0126, suggesting that MEK1/2 is involved in the downregulation of apoE promoter activity by Hcy. Our data demonstrated that Hcy-induced inhibition of apoE took place through activation of NF-κB. Moreover, we demonstrated that Hcy activated a synthetic promoter containing three NF-κB binding sites, but did not affect promoters containing AP-1 or NFAT binding sites. ChIP experiments revealed that NF-κB p65 subunit is recruited to the apoE promoter following Hcy treatment of cells.
CONCLUSION: Hcy-induced stress negatively modulates apoE expression via MEK1/2 and NF-κB activation. The decreased apoE expression in peripheral tissues may aggravate atherosclerosis, neurodegenerative diseases and renal dysfunctions.
Core tip: This original manuscript investigates the effect of high homocysteine (Hcy) levels on apoE expression, and the signaling pathways involved in this gene regulation. Our novel findings show that high doses of Hcy decrease apolipoprotein E (apoE) expression. We revealed that this regulation involves nuclear factor kappa B activation, via MAPK/ERK kinase. The Hcy-mediated decrease of apoE expression in peripheral tissues may aggravate atherosclerosis, neurodegenerative diseases and renal dysfunctions. Thus, the current manuscript may be of interest for scientists working in the field of cardiovascular disease and related inflammatory disorders.
- Citation: Trusca VG, Mihai AD, Fuior EV, Fenyo IM, Gafencu AV. High levels of homocysteine downregulate apolipoprotein E expression via nuclear factor kappa B. World J Biol Chem 2016; 7(1): 178-187
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/178.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.178
Cardiovascular disease, the main cause of death in Western world, is contributed by both genetic and environmental risk factors. Among these, hyperhomocysteinemia (HHcy) has received extensive attention since it has been hypothesized to be an independent risk factor for atherosclerosis[1]. However, the first report of the association between genetically induced high plasma level of homocysteine (Hcy) and premature arterial lesions belongs to McCully et al[2]. Hcy is a key intermediate in the metabolic pathway of the sulfur-containing amino acids leading from exogenous essential methionine to cysteine. Whether a cause or a marker for vascular pathology, HHcy remains strongly associated with alterations such as endothelial dysfunction, smooth muscle cells proliferation, increased inflammation, plaque instability[3]. Many of these alterations act in a concerted fashion toward an atherogenic phenotype. Thus, HHcy induces upregulation of PDGF production by endothelial cells, which in turn promotes proliferation and migration of co-cultured vascular smooth muscle cells[4]. Hcy accelerates senescence and reduces the endothelial progenitor cells proliferation through telomerase inactivation and Akt dephosphorylation[5].
Overall, the effects of elevated Hcy are multifactorial, affecting both the blood vessel structure and the coagulation cascade. Not only the arterial vasculature is challenged by increased Hcy, but also the brain, the bone, the kidney, and blood cells. Neurodegenerative diseases such as Alzheimer’s[6] and Parkinson’s[7] are associated with HHcy. Elderly individuals with high plasma Hcy are susceptible to hip fractures, exhibiting reduced bone mineral density, alterations of collagen stability due to diminished cross-linking and impaired bone blood flow[8].
Hcy may exert its action through several mech-anisms, such as production of reactive oxygen species[9], which may also lead to decreased nitric oxide availability[10], endoplasmic reticulum stress[11], gene regulation[12-14], epigenetic changes such as altered DNA methylation patterns[15], altered protein modification (e.g., N-homocysteinylation of fibrinogen)[16].
Apolipoprotein E (apoE) is an anti-atherogenic 35 kDa glycoprotein associated with all classes of lipoproteins, and it is synthesized by the liver and different peripheral sources, such as macrophages, adipocytes, astrocytes, kidney cells[17]. ApoE plays an important role in lipid metabolism, atherogenesis, neurodegenerative disorders, inflammation, etc[17-19]. ApoE gene regulatory mechanisms discovered so far have been recently reviewed[20]. Nevertheless, no reports exist so far regarding the gene regulation of apoE under HHcy stress. It is only known from a recent report that at posttranslational level, HHcy exerts an inhibitory effect on in vivo and in vitro dimerization of apoE directly via the thiol group[21]. Herein, we investigated how increased Hcy levels affected apoE expression and the mechanisms involved in this modulation. The results obtained in the present study demonstrated that Hcy inhibits apoE expression and that this negative effect is mediated via nuclear factor kappa B (NF-κB) activation.
Homocysteine was from Sigma-Aldrich (St. Louis, MO, United States). DMEM and fetal calf serum were obtained from EuroClone (Milano, Italy). M-MLV reverse transcriptase, oligo(dT), GoTaq DNA polymerase, Luciferase Assay System, U0126, SP600125 and N-p-Tosyl-L-phenylalanine chloromethyl ketone (TPCK) inhibitors were obtained from Promega Corp. (Madison, WI). Primers and TRIzol reagent were from Invitrogen Life Technologies (Carlsbad, CA). Anti-apoE and anti-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, United States).
The construct (-500/+73)apoE-luc containing the apoE proximal promoter as well as the construct ME2/(-500/+73)apoE in which ME2 was cloned in front of the proximal apoE promoter in pGL3 basic vector were previously described[22]. The expression vectors for IKKβ, IKβ dominant negative (DN) and fusion heterodimer p65/p50, as well as the (NF-κB)3-luc construct containing three NF-κB binding sites, and the (AP-1)7-luc construct containing seven AP-1 binding sites, were kindly supplied by Prof. D. Kardassis (University of Crete Medical School, Greece).
RAW 264.7 and HEK-293 cells (both from ATCC) were cultured in DMEM supplemented with 10% fetal calf serum and antibiotics.
Total cellular RNA was extracted using TRIzol reagent according to the manufacturer’s instructions. After re-verse transcription, polymerase chain reaction (PCR) was performed using the following specific primers: Human apoE forward, 5´-CCAGCGGAGGTGAAGGAC; human apoE reverse, 5´-CGCTTCTGCAG GTCATCG; human β-actin forward 5´-CAACCGCGAGAAGATGACCC; human β-actin reverse, 5´-GGAAGGAAGGCTGGAAGAGTGC. The fragments obtained by Reverse Transcription-Polymerase chain reaction (RT-PCR) were of 584 bp for human apoE, and 458 bp for β-actin. The mRNA levels of β-actin were used to normalize the apoE gene expression.
RAW 264.7 and HEK-293 cells were transiently transfected by Ca3(PO4)2 co-precipitation method[23] with the above mentioned plasmids, containing promoters driving the luciferase reporter. Luciferase assay was performed using the Luciferase Assay Kit from Promega. Promoter activity was normalized to the activity of the co-transfected β-galactosidase, determined using o-nitrophenyl β-D-galactopyranoside as a substrate.
Cells were incubated with various concentrations of homocysteine for 24 h, and then cell lysates were subjected to SDS-PAGE on a 12% polyacrylamide gel and transferred onto nitrocellulose. The blots were probed with anti-apoE or anti-β-actin antibodies, followed by horseradish peroxidase-conjugated secondary antibodies. The protein bands were detected using Super Signal West Pico chemiluminescent substrate (Pierce).
Chromatin immunoprecipitation (ChIP) experiments were performed using chromatin prepared from HEK-293 cells treated with 500 μmol/L homocysteine or cysteine for 24 h. Briefly, the cells were fixed with p-formaldehyde and the chromatin was sheared by sonication; p65 proteins bound to DNA were immunoprecipitated with anti-p65 antibodies and DNA was identified by PCR using primers amplifying the region -100/+4 of the apoE gene with the following primers: Forward 5´-ACCTCGTGACTGGGGGCTG, and reverse 5´-GCTGGGGCTGAGTAGGAC. The PCR products were analyzed by agarose gel electrophoresis.
Results are means ± SD of triplicate experiments. One-way analysis of variance was performed using OriginPro 7.5. Differences with P < 0.05 were considered statistically significant.
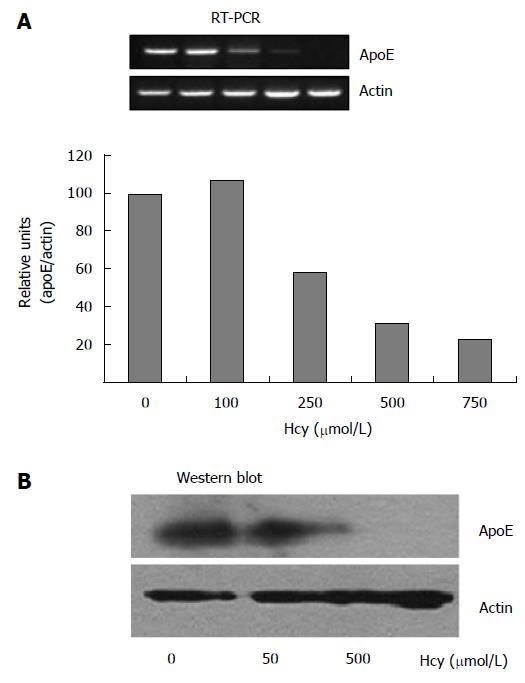
We investigated the modulation of apoE expression at mRNA and protein levels in HEK-293 cells, in response to various concentrations of homocysteine. The apoE gene expression in HEK-293 cells exposed to different concentrations of homocysteine (50-750 μmol/L) for 24 h was determined by RT-PCR using primers specific for apoE, and the results were normalized to actin expression. Our experiments showed that homocysteine concentrations of 250-750 μmol/L induced a 2-3 fold decrease of apoE mRNA levels in HEK-293 cells (Figure 1A), while lower concentrations of homocysteine (100 μmol/L) had no significant effect on apoE gene expression (P > 0.05) as compared to the control.
Next, we investigated the influence of Hcy treatment on apoE protein expression. HEK-293 cells were exposed to homocysteine (50-500 μmol/L) for 24 h and the endogenous levels of apoE protein were determined by immunoblotting using anti-apoE antibodies. Actin level was used as a control for the total protein load. Our data showed that apoE protein expression was not affected by 50 μmol/L Hcy (as compared to control cells), while it was dramatically reduced in cells exposed to 500 μmol/L Hcy (Figure 1B).
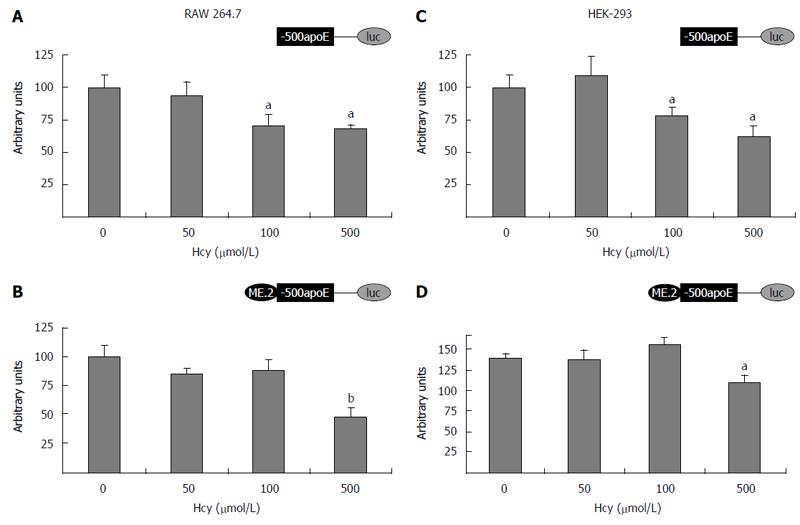
The ability of homocysteine to modulate the transcrip-tional activity of apoE promoter was investigated by transient transfection experiments in HEK-293 and RAW 264.7 cells. For this purpose, cells were transiently transfected with plasmids containing apoE proximal promoter [(-500/+73)apoE construct] or plasmids in which multienhancer ME2 was cloned in front of the proximal apoE promoter [ME2/(-500/+73)apoE construct]. After 18 h, the transfected cells were treated with various concentrations of homocysteine (50-500 μmol/L) and the next day, the reporter gene activity was determined by luciferase assay according to the manufacturer’s instructions (Promega). As presented in Figure 2, the treatment of cells with 500 μmol/L Hcy significantly decreased (P < 0.0025) the apoE promoter activity, in the presence (Figure 2B and D) or in the absence of ME2 (Figure 2A and C), in a dose dependent manner, in both RAW 264.7 (Figure 2A and B) and HEK-293 cells (Figure 2C and D). Treatment of RAW 264.7 or HEK-293 cells with low concentrations of Hcy (50 μmol/L) did not significantly affect (P > 0.05) the activity of apoE promoter either in the absence (Figure 2A and C) or in the presence of ME2 (Figure 2B and D).
Taken together, these experiments clearly indicated that high concentrations of homocysteine exerted a negative effect on apoE expression. Therefore, we next sought to investigate the mechanism underlying this inhibitory effect and to determine the signaling pathways possibly involved.
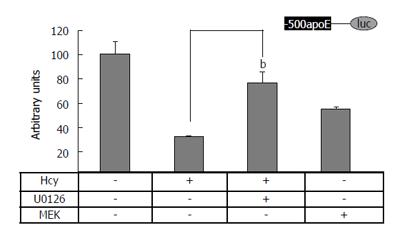
To test whether MAPK signaling pathway is involved in the Hcy-mediated downregulation of apoE promoter activity, HEK-293 cells were transiently transfected with the construct (-500/+73)apoE-luc in the presence or in the absence of MEK1 expression vectors or MEK inhibitor U0126 (10 μmol/L). The results of these experiments showed that MEK1 overexpression, as well as the treatment of cells with Hcy, significantly decreased (P < 0.05) the activity of apoE proximal promoter, as compared to the control (Figure 3, lane Hcy and lane MEK1). Notably, MEK inhibitor U0126 counteracted the inhibitory effect of Hcy on the apoE promoter activity (Figure 3, lane Hcy + U0126). Therefore, our data suggest that MEK1/2 is involved in Hcy-mediated downregulation of apoE promoter activity.
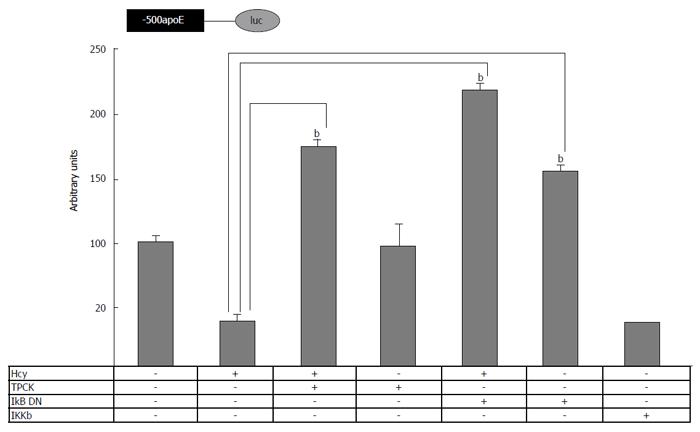
To determine whether Hcy modulates the apoE promoter activity through NF-κB signaling, cells transiently transfected with (-500/+73)apoE–luc construct were treated with Hcy simultaneously with NF-κB pathway inhibition. For this, either TPCK or overexpression of a DN form of IKβ was used. As shown in Figure 4, TPCK abrogated the inhibitory effect of Hcy on apoE promoter activity, similarly with the overexpression of IKβ DN. Moreover, the activity of apoE proximal promoter was significantly reduced (P < 0.05) by IKKβ overexpression, to a similar extent as Hcy treatment. Together, these data demonstrate the involvement of the NF-κB signaling pathway in the regulation of apoE promoter activity by homocysteine.
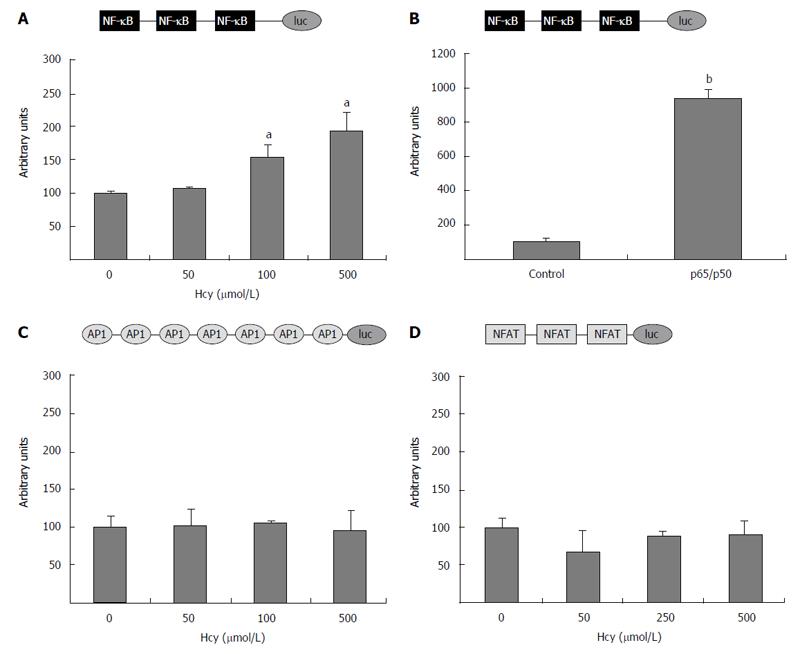
To evaluate the ability of homocysteine to activate NF-κB, HEK-293 cells were transiently transfected with (NF-κB)3-luc construct containing three NF-κB binding sites. The transfected cells were treated with various concentrations of homocysteine (50-500 μmol/L) for 24 h and then, the reporter gene activity was determined. Our data showed that cell treatment with high concentrations of homocysteine (250 and 500 μmol/L) significantly increased (P < 0.05) the activity of the promoter containing the three NF-κB binding sites, while low concentrations of homocysteine (50 μmol/L) did not considerably affect (P > 0.05) its activity (Figure 5A). As a positive control for NF-κB activity, a synthetic fusion p65/p50 heterodimer was co-transfected. As expected, the activity of the (NF-κB)3-luc construct was greatly enhanced by p65/p50 overexpression (Figure 5B).
We subsequently tested whether homocysteine is able to modulate the activity of AP-1 complex or NFAT transcription factor. To this aim, HEK-293 cells were transiently transfected with (AP-1)7-luc construct (containing seven AP-1 binding sites) or (NFAT)3-luc construct (having three NFAT binding sites), and exposed to different Hcy concentrations (50-500 μmol/L). None of the Hcy concentrations tested affected the activity of AP-1 or NFAT constructs (P > 0.05).
Taken together, these results demonstrate that homocysteine downregulates the apoE promoter via NF-κB activation; however, AP-1 or NFAT transcription factors do not mediate Hcy effect on apoE transcription.
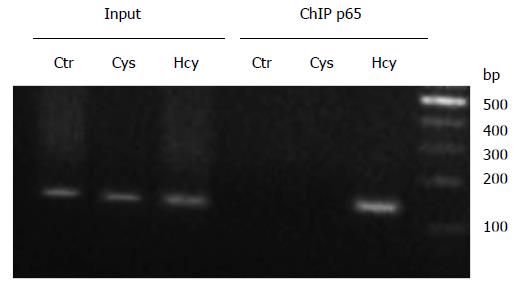
Considering our findings that revealed the involvement of NF-κB signaling pathway in Hcy-mediated modulation of apoE promoter activity, we hypothesized that this downregulatory effect is exerted through NF-κB binding to the apoE promoter. Our previous data demonstrated that NF-κB p50 subunit binds to the apoE promoter[24,25]. Therefore, we tested whether NF-κB p65 subunit binds to the apoE promoter using the ChIP assay performed in HEK-293 cells treated with 500 μmol/L Hcy or cysteine (Cys). Chromatin was immunoprecipitated with anti-p65 antibodies and analyzed by PCR using primers to amplify the region -100/+4 of apoE gene. As shown in Figure 6, the results of ChIP experiments indicated that Hcy induced the recruitment of NF-κB p65 subunit to apoE promoter (Figure 6, lane “p65” Hcy). No binding of p65 proteins was observed to the apoE promoter following Cys treatment or in untreated cells (Figure 6, lanes “p65” Cys and Ctr, respectively). PCR using the input as template and primers for the apoE promoter resulted in the expected bands presented in Figure 6 (lanes “Input” Ctr, Cys and Hcy, respectively).
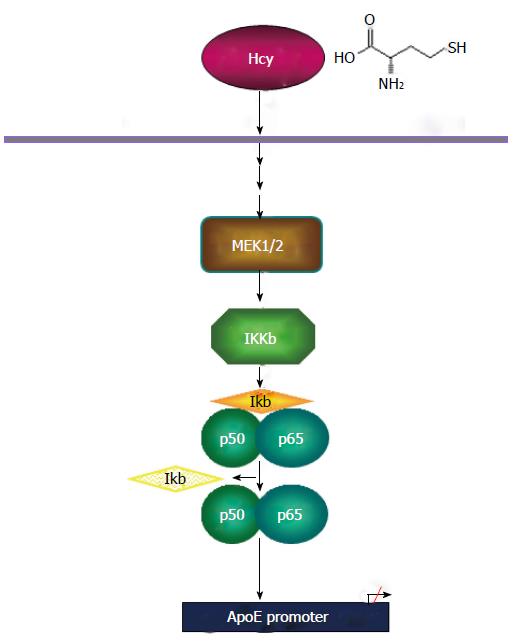
In summary, the results of our study clearly demonstrate that homocysteine downregulates apoE expression via MEK1/2 mediated activation of NF-κB. In addition, our results indicate that the activation of AP-1 or NFAT transcription factors is not involved in Hcy inhibition of apoE. Based on these findings, we propose a mechanism of homocysteine-mediated inhibition of apoE gene transcription, schematically illustrated in Figure 7.
Homocysteine (Hcy), a nonprotein amino acid resulted upon methionine demethylation is found in plasma in concentrations ranging from 3 to 15 μmol/L. In mild and intermediate HHCy, Hcy plasma levels are found in the domain of 16-100 μmol/L, while in severe HHCy, Hcy values are higher than 100 μmol/L. HHCy is an independent risk factor for cardio- and cerebro-vascular diseases, including atherosclerosis, as recently reviewed in[26], but has also implications in different disorders such as hepatic steatosis[27] and neurological disorders[28]. In addition, it was demonstrated that HHCy may aggravate kidney dysfunction[29] and is associated with impaired renal function in male patients with gout[30].
Our data demonstrate that high levels of Hcy decrease apoE expression in human embryonic kidney (HEK-293) cells (Figure 1A and B). The absence or the decreased apoE levels in different peripheral tissues leads to different pathologies. Kidney biopsies of apoE-deficient mice revealed increased mesangial cell proliferation and matrix formation, key features of the pathogenesis of renal diseases independently of hyperlipidemia[31,32]. In the atherosclerotic plaque, the macrophage-derived apoE plays important roles in the cholesterol efflux, in addition to its antioxidant and anti-inflammatory functions as reviewed in[33]. Thus, the decreased apoE expression in macrophages leads to exacerbation of atherosclerosis.
Our results show that Hcy represses the endogenous apoE gene in a dose dependent manner. A significant effect on apoE expression was noticed only for Hcy doses higher than 100 μmol/L. This is relevant considering that these concentrations are similar to the severe HHCy. These doses were in the same range with those used by other authors in experiments showing the Hcy-mediated modulation of heme oxygenase-1 expression in hepatocytes[34], matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells[35] or vascular smooth muscle cells proliferation[36].
To be able to exert its downstream regulatory effects, Hcy is transported into the cells as shown for primary vascular endothelial cells[37] and is transcytosed through the endothelium to reach the adjacent tissues and cells[38].
Homocysteine acts via NMDA receptors and renal NMDA and Group 1 metabotropic glutamate receptors have been associated with HHcy-induced glomerulosclerosis[39]. A question arises regarding the mechanism through which HCy can exert its effects in a cell line like HEK 293, which is apparently void of NMDA receptors and has been used as such to functionally characterize the activity of various receptor subtypes overexpressed in this cell line[40]. However, Hcy can exert its effects not only directly by receptor-mediated cell targeting, but also by indirect actions, as through binding to serum proteins, mainly albumin. Up to 70% of the total HCy is in its protein-bound form and there is a strong correlation between albuminemia and total HCy content[41]. Furthermore, homocysteine was found to be among the strongest protein–bound uremic toxins, which leads to reduced removal upon conventional hemodialysis and thus poor prognosis in atherosclerosis-associated renal complications. Thus, an indirect action could explain the high, non-physiological concentrations at which effects were noticed in our experiments, as well as in reports of other groups. Additionally, it was shown that Hcy could act as a ligand[42,43] or a competitive inhibitor[44,45] for various amino acid transport systems in both laboratory animals and humans, not only in renal cells, but also in other cell types. Therefore, despite the lack of NMDA receptors, it could be envisioned that Hcy may exert its effects in HEK-293 cells by certain non-receptor, indirect actions.
We studied the regulatory mechanisms of Hcy-induced apoE modulation. For this, we used plasmids containing the luciferase gene under apoE regulatory elements, the proximal promoter and the distal enhancer (multienhancer 2), in transient transfection experiments. The data showed that Hcy-mediated regulation involved the repression of the promoter activity (Figure 2). This Hcy-induced downregulation was observed also for other apolipoproteins such as apoAI[12]. The synergic decrease of the anti-atherogenic apolipoproteins apoAI and apoE is significant for atherosclerosis aggravation under hyperhomocysteinemic stress. The involvement of MEK1/2 in Hcy induced apoE regulation was also demonstrated (Figure 3). Similarly, MEK1/2 was shown to be involved in Hcy-induced regulation of MMP-9 in microvascular endothelial cells[46].
At the level of transcription factors, many down-stream targets for Hcy were revealed to be implicated in the gene modulation of various proteins: PPARα for apoAI[12], CREB for Herp protein[47], Nrf2 for heme oxygenase-1[13]. Our study demonstrated by transient transfections and ChIP assay, that NF-κB is a downstream target of Hcy, involved in apoE gene regulation (Figures 4 and 6, respectively). In addition, our data showed that NF-κB binding sites included in a synthetic promoter are activated by Hcy (Figure 5A). Recently, the research group of Wang demonstrated the induction in monocytes of inflammatory factors by Hcy, among which NF-κB[48]. Unexpectedly, Hcy did not activate AP-1 (Figure 5C), through MEK1/2, as we detected for LPS-induced gene modulation[24]. However, our findings are in agreement with the data concerning the involvement of NF-κB, but not AP-1, as downstream effectors of high doses of homocysteine[49]. Moreover, we also showed that NFAT pathway is not implicated in Hcy signaling (Figure 5D) in contrast with other Hcy-induced proatherogenic events modulated by this transcription factor known for its participation in pathological cardiac remodeling and vascular lesion formation[50,51].
In conclusion, we report that high level of homocys-teine, through MEK1/2, activates NF-κB transcription factors that act on the apoE promoter mediating the repression of apoE gene expression. The importance of these findings is given by the fact that the negative effect of high Hcy concentrations on apoE expression in peripheral tissues may aggravate atherosclerosis, neurodegenerative diseases and renal dysfunctions.
Homocysteine (Hcy) is an independent risk factor for the cardiovascular disease and associated pathologies such as neurodegenerative diseases and renal failure. Apolipoprotein E (apoE) is an anti-atherogenic apolipoprotein synthesized by the liver and different peripheral sources, such as macrophages, adipocytes, astrocytes, kidney cells, with an important role in lipid metabolism, atherogenesis, and inflammation. ApoE deficient mice are susceptible to atherosclerosis. Data about Hcy effects on apoE modulation are sparse, and it would be of interest to establish a possible connection between the two participants with antagonistic behavior in atherogenesis.
Previous work from our and others’ laboratories, demonstrated the down-regulation of apoE in macrophages under inflammatory conditions, via activation of the transcription factor nuclear factor kappa B (NF-κB), leading to a decreased cholesterol efflux in the atherosclerotic lesion and a consequent aggravation of the atherosclerotic phenotype.
So far, the only report connecting Hcy to apoE showed that at the posttranslational level, hyperhomocysteinemia (HHCy) exerted an inhibitory effect on in vivo and in vitro dimerization of apoE directly via the thiol group, but no data were available on the transcriptional regulation of apoE under Hcy stress. Herein, the authors investigated the effect of increased Hcy levels on apoE expression and the mechanisms involved in this modulation. The results obtained in the present study demonstrate that Hcy inhibited apoE expression and that this negative effect is mediated via the activation of the pro-inflammatory transcription factor NF-κB.
The authors reported that high levels of homocysteine repress apoE gene expression, via MEK/NF-κB signaling pathway that act on apoE promoter in HEK-293 cells. This downregulation of apoE expression in peripheral tissues may be one way through high Hcy concentrations aggravate atherosclerosis, neurodegenerative diseases and renal dysfunctions and provides a link between atherogenesis, inflammation and Hcy stress.
HHCy, characterized by an abnormally high level of plasma Hcy, is a risk factor for cardiovascular diseases.
In this manuscript, the authors investigated how HHcy downregulates ApoE, thus potentially contributing to the development of atherosclerosis. This is an interesting study where the experiments are reasonably well planned and executed. They collected data showing Hcy regulation of the apoE promoter and expression using cell culture model. They observed that high Hcy dose downregulated ApoE mRNA and protein in cultured RAW 264.7 and HEK293 cells and further found that activity of the ApoE proximal promoter (-500/+73)-luciferase reporter is regulated by Hcy in transient transfected cell lines. Their data potentially provide insight of Hcy related atherosclerosis and thus contribute to our understanding of atherosclerosis development.
P- Reviewer: Ahmad N, Bai G S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Israelsson B, Brattström LE, Hultberg BL. Homocysteine and myocardial infarction. Atherosclerosis. 1988;71:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111-128. [PubMed] |
| 3. | Guilland JC, Favier A, Potier de Courcy G, Galan P, Hercberg S. [Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease?. 1. Basic data]. Pathol Biol (Paris). 2003;51:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Zhang D, Chen Y, Xie X, Liu J, Wang Q, Kong W, Zhu Y. Homocysteine activates vascular smooth muscle cells by DNA demethylation of platelet-derived growth factor in endothelial cells. J Mol Cell Cardiol. 2012;53:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Zhu JH, Chen JZ, Wang XX, Xie XD, Sun J, Zhang FR. Homocysteine accelerates senescence and reduces proliferation of endothelial progenitor cells. J Mol Cell Cardiol. 2006;40:648-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Shen L, Ji HF. Associations between Homocysteine, Folic Acid, Vitamin B12 and Alzheimer’s Disease: Insights from Meta-Analyses. J Alzheimers Dis. 2015;46:777-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Zhang L, Yan J, Xu Y, Long L, Zhu C, Chen X, Jiang Y, Yang L, Bian L, Wang Q. The combination of homocysteine and C-reactive protein predicts the outcomes of Chinese patients with Parkinson’s disease and vascular parkinsonism. PLoS One. 2011;6:e19333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Fratoni V, Brandi ML. B vitamins, homocysteine and bone health. Nutrients. 2015;7:2176-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9:1941-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Weiss N, Heydrick SJ, Postea O, Keller C, Keaney JF, Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state--impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41:1455-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Wu S, Gao X, Yang S, Meng M, Yang X, Ge B. The role of endoplasmic reticulum stress in endothelial dysfunction induced by homocysteine thiolactone. Fundam Clin Pharmacol. 2015;29:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Liao D, Yang X, Wang H. Hyperhomocysteinemia and high-density lipoprotein metabolism in cardiovascular disease. Clin Chem Lab Med. 2007;45:1652-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Mani M, Golmohammadi T, Khaghani S, Zamani Z, Azadmanesh K, Meshkani R, Pasalar P. Homocysteine induces heme oxygenase-1 expression via transcription factor Nrf2 activation in HepG2 cell. Iran Biomed J. 2013;17:93-100. [PubMed] |
| 14. | Wang G, Siow YL, O K. Homocysteine induces monocyte chemoattractant protein-1 expression by activating NF-kappaB in THP-1 macrophages. Am J Physiol Heart Circ Physiol. 2001;280:H2840-H2847. [PubMed] |
| 15. | Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135:5-8. [PubMed] |
| 16. | Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J Physiol Pharmacol. 2008;59 Suppl 9:155-167. [PubMed] |
| 17. | Zannis VI, Kan HY, Kritis A, Zanni EE, Kardassis D. Transcriptional regulatory mechanisms of the human apolipoprotein genes in vitro and in vivo. Curr Opin Lipidol. 2001;12:181-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 372] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Van Eck M, Herijgers N, Yates J, Pearce NJ, Hoogerbrugge PM, Groot PH, Van Berkel TJ. Bone marrow transplantation in apolipoprotein E-deficient mice. Effect of ApoE gene dosage on serum lipid concentrations, (beta)VLDL catabolism, and atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:3117-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Kardassis D, Gafencu A, Zannis VI, Davalos A. Regulation of HDL genes: transcriptional, posttranscriptional, and posttranslational. Handb Exp Pharmacol. 2015;224:113-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Minagawa H, Watanabe A, Akatsu H, Adachi K, Ohtsuka C, Terayama Y, Hosono T, Takahashi S, Wakita H, Jung CG. Homocysteine, another risk factor for Alzheimer disease, impairs apolipoprotein E3 function. J Biol Chem. 2010;285:38382-38388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Trusca VG, Fuior EV, Florea IC, Kardassis D, Simionescu M, Gafencu AV. Macrophage-specific up-regulation of apolipoprotein E gene expression by STAT1 is achieved via long range genomic interactions. J Biol Chem. 2011;286:13891-13904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068-6074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Gafencu AV, Robciuc MR, Fuior E, Zannis VI, Kardassis D, Simionescu M. Inflammatory signaling pathways regulating ApoE gene expression in macrophages. J Biol Chem. 2007;282:21776-21785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Stavri S, Trusca VG, Simionescu M, Gafencu AV. Metformin reduces the endotoxin-induced down-regulation of apolipoprotein E gene expression in macrophages. Biochem Biophys Res Commun. 2015;461:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 670] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 27. | Robert K, Nehmé J, Bourdon E, Pivert G, Friguet B, Delcayre C, Delabar JM, Janel N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005;128:1405-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014;10:281-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | Cheung GT, Siow YL, O K. Homocysteine stimulates monocyte chemoattractant protein-1 expression in mesangial cells via NF-kappaB activation. Can J Physiol Pharmacol. 2008;86:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Choi ST, Kim JS, Song JS. Elevated serum homocysteine levels were not correlated with serum uric acid levels, but with decreased renal function in gouty patients. J Korean Med Sci. 2014;29:788-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Chen G, Paka L, Kako Y, Singhal P, Duan W, Pillarisetti S. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J Biol Chem. 2001;276:49142-49147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Wen M, Segerer S, Dantas M, Brown PA, Hudkins KL, Goodpaster T, Kirk E, LeBoeuf RC, Alpers CE. Renal injury in apolipoprotein E-deficient mice. Lab Invest. 2002;82:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Davignon J. Apolipoprotein E and atherosclerosis: beyond lipid effect. Arterioscler Thromb Vasc Biol. 2005;25:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Luo X, Xiao L, Yang H, Zhang R, Jiang M, Ni J, Lei T, Wang N. Homocysteine downregulates gene expression of heme oxygenase-1 in hepatocytes. Nutr Metab (Lond). 2014;11:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Guo H, Lee JD, Uzui H, Yue H, Wang P, Toyoda K, Geshi T, Ueda T. Effects of heparin on the production of homocysteine-induced extracellular matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells. Can J Cardiol. 2007;23:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Zou T, Yang W, Hou Z, Yang J. Homocysteine enhances cell proliferation in vascular smooth muscle cells: role of p38 MAPK and p47phox. Acta Biochim Biophys Sin (Shanghai). 2010;42:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Büdy B, O’Neill R, DiBello PM, Sengupta S, Jacobsen DW. Homocysteine transport by human aortic endothelial cells: identification and properties of import systems. Arch Biochem Biophys. 2006;446:119-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Jiang X, Yang F, Brailoiu E, Jakubowski H, Dun NJ, Schafer AI, Yang X, Durante W, Wang H. Differential regulation of homocysteine transport in vascular endothelial and smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1976-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Dryer SE. Glutamate receptors in the kidney. Nephrol Dial Transplant. 2015;30:1630-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Hayes D, Wiessner M, Rauen T, McBean GJ. Transport of L-[14C]cystine and L-[14C]cysteine by subtypes of high affinity glutamate transporters over-expressed in HEK cells. Neurochem Int. 2005;46:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Suliman ME, Stenvinkel P, Bárány P, Heimbürger O, Anderstam B, Lindholm B. Hyperhomocysteinemia and its relationship to cardiovascular disease in ESRD: influence of hypoalbuminemia, malnutrition, inflammation, and diabetes mellitus. Am J Kidney Dis. 2003;41:S89-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Zalups RK, Ahmad S. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol. 2004;15:2023-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Wang Y, Zalups RK, Barfuss DW. Potential mechanisms involved in the absorptive transport of cadmium in isolated perfused rabbit renal proximal tubules. Toxicol Lett. 2010;193:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Brunini TM, Yaqoob MM, Novaes Malagris LE, Ellory JC, Mann GE, Mendes Ribeiro AC. Increased nitric oxide synthesis in uraemic platelets is dependent on L-arginine transport via system y(+)L. Pflugers Arch. 2003;445:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Kimmich GA, Randles J, Wilson J. Na(+)-coupled alanine transport in LLC-PK1 cells. Am J Physiol. 1994;267:C1119-C1129. [PubMed] |
| 46. | Moshal KS, Sen U, Tyagi N, Henderson B, Steed M, Ovechkin AV, Tyagi SC. Regulation of homocysteine-induced MMP-9 by ERK1/2 pathway. Am J Physiol Cell Physiol. 2006;290:C883-C891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Lenz B, Bleich S, Beutler S, Schlierf B, Schwager K, Reulbach U, Kornhuber J, Bönsch D. Homocysteine regulates expression of Herp by DNA methylation involving the AARE and CREB binding sites. Exp Cell Res. 2006;312:4049-4055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Meng S, Ciment S, Jan M, Tran T, Pham H, Cueto R, Yang XF, Wang H. Homocysteine induces inflammatory transcriptional signaling in monocytes. Front Biosci (Landmark Ed). 2013;18:685-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Desai A, Lankford HA, Warren JS. Homocysteine augments cytokine-induced chemokine expression in human vascular smooth muscle cells: implications for atherogenesis. Inflammation. 2001;25:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Suzuki YJ, Shi SS, Blumberg JB. Modulation of angiotensin II signaling for GATA4 activation by homocysteine. Antioxid Redox Signal. 1999;1:233-238. [PubMed] |
| 51. | Zetterqvist AV, Berglund LM, Blanco F, Garcia-Vaz E, Wigren M, Dunér P, Andersson AM, To F, Spegel P, Nilsson J. Inhibition of nuclear factor of activated T-cells (NFAT) suppresses accelerated atherosclerosis in diabetic mice. PLoS One. 2014;8:e65020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |