Published online May 26, 2015. doi: 10.4331/wjbc.v6.i2.39
Peer-review started: January 28, 2015
First decision: March 6, 2015
Revised: March 18, 2015
Accepted: April 16, 2015
Article in press: April 20, 2015
Published online: May 26, 2015
Processing time: 114 Days and 4.6 Hours
Signaling within the tumor microenvironment has a critical role in cancer initiation and progression. Adipocytes, one of the major components of the breast microenvironment, have been shown to provide pro-tumorigenic signals that promote cancer cell proliferation and invasiveness in vitro and tumorigenicity in vivo. Adipocyte secreted factors such as leptin and interleukin-6 (IL-6) have a paracrine effect on breast cancer cells. In adipocyte-adjacent breast cancer cells, the leptin and IL-6 signaling pathways activate janus kinase 2/signal transducer and activator of transcription 5, promoting the epithelial-mesenchymal transition, and upregulating stemness regulators such as Notch, Wnt and the Sex determining region Y-box 2/octamer binding transcription factor 4/Nanog signaling axis. In this review we will summarize the major signaling pathways that regulate cancer stem cells in breast cancer and describe the effects that adipocyte secreted IL-6 and leptin have on breast cancer stem cell signaling. Finally we will introduce a new potential treatment paradigm of inhibiting the adipocyte-breast cancer cell signaling via targeting the IL-6 or leptin pathways.
Core tip: We discuss the relationship between adipocytes in the microenvironment and breast cancer cells. We emphasize the role of adipocyte-secreted leptin and interleukin-6 in inducing breast cancer cell epithelial-mesenchymal transition and activating stemness pathways. Finally we summarize possible microenvironmental therapeutic targets and the potential role of non-coding RNAs in adipocyte-breast cancer interactions.
- Citation: Wolfson B, Eades G, Zhou Q. Adipocyte activation of cancer stem cell signaling in breast cancer. World J Biol Chem 2015; 6(2): 39-47
- URL: https://www.wjgnet.com/1949-8454/full/v6/i2/39.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i2.39
Breast cancer occurs through the accumulation of genetic and epigenetic changes, activating oncogenes and silencing tumor suppressors in the mammary ductal or lobular tissues. Ductal carcinomas make up approximately 85% of diagnosed breast cancers, and are the focus of this review[1]. Ductal tumors progress linearly beginning as atypical hyperplasia that grow into ductal carcinoma in situ (DCIS) lesions which later acquire malignant phenotypes and become invasive ductal carcinomas[2]. The final stage of breast cancer involves dissemination of primary tumor cells and colonization of distant tissues by metastatic tumor cells.
Gene expression profiling has been used to identify molecular subtypes of breast cancer with different prognosis and treatment responses. Luminal A and B both express estrogen (ER) and progesterone receptors (PR), differing primarily in their proliferation kinetics. Luminal subtype breast cancers comprise the majority of tumors and are among the best prognosis, in part due to availability of antiestrogen therapies. Human epidermal growth receptor breast cancers overexpress or amplify HER2 and respond to targeted anti-HER2 therapies. Basal-like breast cancers are frequently triple-negative (ER-/PR-/HER2-), often harbor P53 mutations, and are aggressive with poor prognosis. A newly described molecular subtype, claudin-low breast cancers, also do not express ER and PR, but are identified through their characteristic lack of cell-cell adhesion molecules (claudins) and basal cytokeratins.
White adipose tissues account for approximately 80% of the volume of the adult breast, and are composed of a heterogeneous collection of cells including adipocytes, fibroblasts, capillaries, immune cells, and extracellular matrix. It was long believed the primary function of adipose tissue was energy storage; in fact stromal adipose is a complicated endocrine organ. Adipose tissues produce a wide variety of adipokines and signaling molecules that play numerous roles in breast tumor formation and progression[3]. This relationship is cemented by a well-established link between obesity and breast cancer. Obesity is a major risk factor for breast cancer development and patient survival, with a 33% increase of cancer mortality in obese patients[4]. The majority of the mammary microenvironment consists of adipocytes and adipocyte precursors. Mesenchymal stem cells differentiate into adipocytes through the two stages of adipogenesis, driven by transcription factors peroxisome proliferator-activated receptor γ and the C/EBP family. Initially mesenchymal stem cells commit to the adipocyte lineage forming preadipocytes, which become mature adipocytes through terminal differentiation[5]. Both preadipocytes[6] and mature adipocytes increase breast cancer growth, with marked effects on migration and the colony forming ability of breast cancer cells. Moreover, cancer associated adipocytes undergo phenotypic changes, forming a more supportive tumor niche[7]. Identifying the mechanisms of this relationship could lead to novel targets for prevention and treatment of breast cancer.
The standard of care for breast cancer is a combination of surgery, radiation and chemotherapy. Treatment success varies depending on molecular subtype of the tumor, and additional adjuvant and targeted therapies are available. While adjuvant hormonal therapies such as Tamoxifen are effective for ER+ patients, and targeted therapies such as the monoclonal antibody Trastuzumab are effective for HER2+ patients, there are no targeted treatments available for patients with basal-like or claudin-low breast cancer[8]. Additionally, drug resistance is a major factor in the treatment failure of all molecular subtypes. One suspected culprit of resistance is cancer stem cells. The cancer stem cell model describes an intratumoral subpopulation of cancer cells that have unregulated stem cell properties, primarily self-renewal and multipotent differentiation, which drive tumorigenesis and tumor heterogeneity[9]. First isolated from AML cell populations by using flow cytometry to sort cells based on the molecular markers CD34+ CD38-[10], cancer stem cells have been identified in breast cancer as the CD44+ CD24-/low ALDH1+ cell population[11,12]. Cancer stem cells are resistant to traditional cancer therapies due to their quiescence, DNA repair capabilities and overexpression of drug efflux pumps[13].
In part through the activation of cancer stem cell signaling, the tumor microenvironment plays a critical role in the development and progression of breast tumors. Targeting the microenvironment has the potential to inhibit cancer stem cells, preventing drug resistance and relapse across all molecular subtypes. This is an attractive therapeutic option due to the relative genetic stability and the reduced risk of resistance of the microenvironment[14]. Therapies targeting the microenvironment have been successful in multiple myeloma through targeting multiple myeloma cell-bone marrow interactions using bisphosphonates[15] and bortezomib[16]. Aromatase inhibitors, a recent success story in breast cancer, target post-menopausal estrogen produced by extragonadal aromatization in stromal cells as well as breast tissues and tumors[17].
In this review we will focus on the relationship between breast cancer cells and mature adipocytes, with emphasis on two of the best studied adipocyte secreted signaling molecules, leptin and interleukin-6 (IL-6). These molecules promote breast cancer progression through activation of the epithelial-mesenchymal transition and cancer stem cell signaling in breast cancer cells, and are potential novel microenvironmental targets.
A number of signaling pathways that have fundamental roles in the regulation of self-renewal and differentiation of adult and embryonic stem cells have been linked to breast cancer stem cells. Adipocyte secreted leptin and IL-6 can activate many of these pathways, dysregulating self-renewal and differentiation within breast cancer cells. Targeting these signaling pathways within the microenvironment may be an important method of targeting breast cancer stem cells. While numerous stemness pathways have been identified, we will focus on three of the best characterized: the Notch, Wnt and octamer binding transcription factor 4 (OCT-4)/ Sex determining region Y-box 2/Nanog pathways.
The Notch receptor is an important developmental mediator of self-renewal and regulator of cell fate decisions in many cell types, including within the mammary gland[18]. When ligand is bound, ADAM and γ-secretase proteases cleave the Notch receptor. The cleavage product is transported to the nucleus, where it activates gene transcription. In both basal like and ER+/PR+ breast cancer cell models Notch activates histone deacetylase SIRT2, which deacetylates and activates ALDH1A1, and increases mammosphere formation[19]. Inhibition of Notch signaling using a neutralizing antibody is sufficient to significantly reduce mammosphere formation of DCIS cells in vitro, indicating a crucial function in breast cancer stem cell signaling[20]. Constitutive Notch activation is a common feature of early stage breast cancer[20], and high levels of Notch are correlated with poor breast cancer prognosis[21].
The Wnt signaling pathway is crucial for embryonic development, and is involved in cell fate determination, proliferation and cell migration. When Wnt ligand is present, it binds the Frizzled receptor, allowing β-catenin to be transported to the nucleus and activate gene transcription[22]. In the absence of Wnt, β-catenin is targeted for proteasomal degradation. Wnt pathway target genes such as LEF1 and AXIN2 are upregulated in breast cancer cells, especially in breast cancer stem cell populations[23]. Wnt signaling is important for breast cancer stem cell self renewal, when treated with Wnt inhibitor DKK1, ER+ and ER- breast cancer cells had reduced mammosphere formation[23]. Unregulated activation of the Wnt pathway can occur via mutations in downstream Wnt target genes, β-catenin and overexpression of the Wnt ligand. Secretion of Wnt ligand from cells in the microenvironment has a paracrine effect on cells on the invasive edge of tumors, increasing their proliferative and invasive abilities[24].
Oct-4 is a member of the POU transcription factor family, and is critical for self-renewal of undifferentiated cells. It is normally only expressed in embryonic stem and embryonic germ cells, and is used as a marker for undifferentiated cells. Oct-4 is necessary to maintain stem cells in a pluripotent state[25]. Through interaction with HMG domain protein SOX2, Oct-4 activates transcription of several genes in pluripotent cells, including Fgf4, Utf1, Fbx15, and the genes encoding themselves, Sox2 and Pou5f. SOX2 and Oct-4 synergy also activates transcription of key pluripotent embryonic regulator Nanog[26], a homeodomain protein that maintains the primitive ectoderm in the embryo[27]. Nanog is expressed in cells that are able to form pluripotent stem cell lines, and plays a key role in inhibition of differentiation in these cells, as well as activation of self-renewal. Nanog also activates Oct-4 transcription, although it is not necessary for Oct-4 expression[28].
These key pluripotency transcription factors share numerous targets, and they are essential to the transcriptional pathways that regulate embryonic stem cell identity[29]. Therefore it is not surprising that all three are frequently activated in breast cancer stem cells[30-32]. Overexpression of Nanog enhances ER+/PR+ breast cancer cell ALDH1 expression and drug resistance[30], as well as invasiveness and mammosphere formation[33]. Nanog overexpression also increases tumor formation in vivo[33]. SOX2 is highly expressed in early stage breast tumors, and knockdown prevents mammosphere formation as well as delaying tumor formation in vivo[32]. OCT-4 overexpression in healthy primary breast tissue cultures generated cells capable of tumor initiation[34].
Through the epithelial-mesenchymal transition (EMT), epithelial cells lose cell polarity, cell-cell adhesion and undergo cytoskeletal reorganization gaining a motile, invasive phenotype. In healthy cells, EMT plays a critical role in development, embryogenesis and wound healing through the reorganization of tissues and germ layers[35]. The classic markers for EMT are loss of E-cadherin, a protein necessary for cell adhesion, and increase in N-cadherin. Additional mesenchymal proteins include smooth-muscle actin, vimentin and fibronectin. Change in expression of these markers is used to characterize EMT in vitro[36].
The tumor microenvironment produces EMT signaling molecules, promoting the mesenchymal phenotype necessary for cancer progression and metastasis. Cells in the inflamed microenvironment secrete transforming growth factor-β (TGF-β), stimulating Snail and Slug which transcriptionally repress E-cadherin[37]. Hypoxia in the microenvironment activates HIF-1, stimulating transcription of EMT activating protein Twist. In ER+ breast cancer cells, Twist is activated by locally produced IL-6 through signal transducer and activator of transcription 3 (STAT3) signaling[38]. EMT is also tightly regulated by microRNA signaling, most notably through repression of E-cadherin activators by the miR-200[39] and miR-34[40] families. miR-200 is significantly downregulated in breast cancer, a possible mechanism of EMT activation[41]. Loss of E-cadherin also increases the tumorigenicity of cancer cells, and is correlated with increased cancer grade[37].
Cancer stem cells and cells that have gone through EMT share many common characteristics. Breast cancer stem cells have protein expression consistent with EMT, decreased E-cadherin and increased N-cadherin and Slug expression[42]. Furthermore, non-tumorigenic immortalized human mammary cells that have undergone EMT are enriched for cancer stem cell markers such as CD44+/CD24- and signaling proteins SOX2, Nanog and octamer binding transcription factor 4 (OCT4)[42]. When mammary epithelial cells transformed through HER2 overexpression undergo EMT in vitro, the proportion of cancer stem cells is significantly increased. Human mammary epithelial cells transformed with a V12H-Ras oncogene overexpressing EMT proteins Twist or Snail were able to form tumors in vivo from significantly fewer cells than control transformed cells[42]. EMT is an important process for cancer progression and metastasis, generating invasive cells with a cancer stem cell-like phenotype, however the exact relationship between EMT and cancer stem remains to be determined.
Adipose tissue is an amalgamation of adipocytes, fibroblasts, stromal precursors and immune cells. Adipose signaling regulates fatty acid metabolism, homeostasis, and insulin signaling via adipocyte-secreted factors such as adiponectin and leptin. Adipose tissue has significant immune and inflammatory signaling functions involving adipokines such as IL-6 and tumor necrosis factor-α (TNF-α). While IL-6 and TNF-α are classically produced by the non-adipocyte members of the adipose tissue, it has been demonstrated that cancer associated adipocytes secrete IL-6, one of the primary cytokines involved in adipocyte-tumor cell interaction.
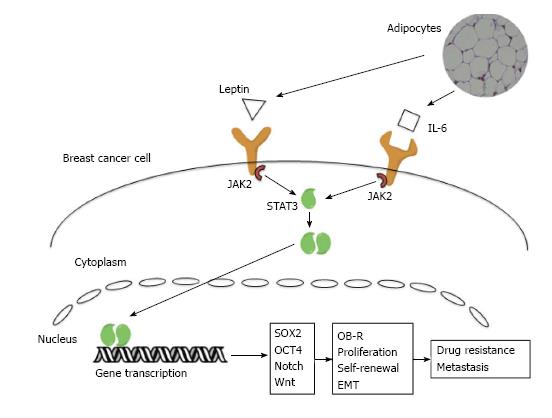
The stromal microenvironment plays a critical role in breast cancer formation and progression, however the pro-tumorigenic abilities of mature adipocytes have only been recognized in the past 10 years[7]. In order to isolate the effects of cytokines from those of adipocyte produced estrogen, it is important to demonstrate the effects in both ER+ and ER- model systems. Using conditioned media from adipocyte culture plates, both ER+ and ER- breast cancer cells had significant increases in proliferation and survival[43]. Crosstalk between adipocytes and breast cancer cells leads to change in phenotype of the adipocyte cells in addition to the changes seen in breast cancer cells. Mature adipocytes co-cultured with breast cancer cells exhibit delipidation, loss of terminal differentiation markers and overexpression of inflammatory cytokines and adipokines. The expression of these signaling molecules plays a critical role in the tumor supporting functions of adipocytes[7] (Figure 1). Two of the best-characterized signaling molecules in the breast cancer-adipocyte relationship are leptin and IL-6.
Leptin is a 16 kd protein encoded by the ob gene, first discovered in ob/ob mutant mice that exhibit an obese phenotype due to the lack of satiety. The classical function of leptin is appetite control. When fat stores reach a certain level, leptin is secreted, and activates leptin receptors (OB-R) in the brain to indicate satiety. While OB-R is found at highest concentrations in the hypothalamus, it has been identified in almost all tissues. Levels of circulating leptin directly correlate with total fat mass, as larger fat cells produce more. Leptin signaling is dysregulated in obese subjects, with up to seven times higher leptin secretion compared to lean[44].
Leptin receptor OB-R is a member of the class I cytokine receptor family. Different isoforms of the OB-R protein are able to activate several classical cytokine-signaling pathways, including the JAK/STAT, PI3K and MAPK pathways. Through these pathways leptin likely has significant, diverse effects on physiology and disease, many of which are not fully understood[45].
Leptin signaling is a significant factor in the adipocyte-tumor signaling relationship. OB-R mRNA expression is highly upregulated in patient tumors of all breast cancer subtypes, with little to no expression seen in normal epithelial tissue[46]. Leptin signaling enhances tumor formation, proliferation and invasion by activation of cancer stem cell signaling pathways Notch and Wnt, and by activating numerous oncogenic pathways, including HER2, AKT, STAT3 and NFkB. Both leptin and OB-R are significantly upregulated in breast cancer stem cells, which exhibit increased sensitivity to leptin signaling due to higher receptor expression[47]. In ER+/PR+ breast cancer cells, STAT3 activation via leptin signaling also increases expression of chaperone binding protein Hsp90, resulting in upregulation of the HER2 oncogene. shRNA knockdown of STAT3 inhibited leptin induced HER2 upregulation[48].
Silencing of OB-R using shRNA in triple negative breast cancer cells inhibited expression of Nanog, SOX2 and OCT4. These cells had reduced cell proliferation and mammosphere formation, indicative of a reduction in self-renewal. Additionally, OB-R silenced cells went through mesenchymal to epithelial transition, with increased E-Cadherin and decreased vimentin expression[49].
Through the use of two mouse models, leptin-deficient ob/ob mice and OB-R deficient db/db, the role of leptin in tumorigenesis was confirmed in vivo. Both models form early onset obesity with nearly identical characteristics, however ob/ob mice have no circulating leptin, whereas db/db have high levels of leptin. Using tumors resected from MMTV-WNT-1 transgenic mice, a single cell suspension was injected into db/db, ob/ob or wild type mice. Tumor growth was detected earlier in db/db compared to wild type, and tumor volume was up to 8 times that of the WT tumors. Ob/ob mice formed tumors at a significantly lower rate, with volumes similar in size to the wild type tumors. Through the use of a limiting dilution assay, the authors demonstrated that injection tumor cells from wild type mice resulted in 100% secondary tumor growth formation, whereas the same number of cells from leptin deficient tumors were unable to form secondary tumors[50]. This provides in vivo evidence of the necessity of leptin for breast cancer initiation and progression as well as implicating its role in breast cancer stem cell self-renewal. In triple-negative breast cancer cell cultures, OB-R activation increases levels of stem cell regulator Notch[21]. Inhibition of leptin signaling decrease expression of both Wnt and Notch in carcinogen induced mouse mammary tumors[51].
It is clear that leptin plays a significant role in activation of breast cancer stem cell signaling. Leptin activates several stemness pathways including the OCT-4/SOX2/Nanog axis, Notch signaling and Wnt/β-catenin signaling. Through these pathways leptin increases self-renewal, tumor initiation and ALDH1 expression, indicating an important role in adipocyte mediated pro-tumor signaling.
Adipose tissue is a significant source of IL-6, producing approximately one third of IL-6 found in the plasma. In healthy adipose tissue, non-adipocyte members produce the majority of adipose IL-6[44]. There are seven members of the IL-6 family; IL-6, oncostatin M (OSM), IL-11, leukemia inhibitory factor (LIF), cardiotrophin-like cytokine (CLC), ciliary neurotrophic factor (CNTF) and cardiotrophin-1 (CT-1). There are 3 plasma membrane receptors, gp130, LIFR and OSMR, which activate the JAK/STAT, MAPK and PI3K pathways. Through these pathways IL-6 cytokines activate genes involved in inflammation, differentiation, survival, apoptosis and proliferation[52]. Within the adipose tissue, IL-6 stimulates glucose uptake, and activates glucose and fatty acid oxidation as well as insulin release[53].
Serum level of IL-6 is a negative prognostic marker in breast cancer patients[51,54]. While adipocyte secretion of IL-6 is low, proximity with tumor cells upregulates IL-6 expression. As mature adipocytes are the most common cells in tumor stroma, the combined amount of adipocyte IL-6 may have a significant impact on tumor cells[7]. IL-6 stimulates invasion in both ER+ and ER- breast cancer cells, similar to the phenotype observed in adipocyte/breast cancer cell co-culture[7]. When ER+/ER-breast cancer cells were treated with adipocyte-conditioned media, addition of an IL-6 blocking antibody significantly inhibited the pro-invasive effect[7]. However, depletion of IL-6 does not completely eliminate the invasive effects, supporting the model that multiple secreted molecules are important in the adipocyte-breast cancer cell interaction[55].
IL-6 activates transcription of OCT4 though the janus kinase 2 (JAK2)/STAT3 pathway, inducing EMT[38]. In triple negative breast cancer cells, there is higher IL-6/JAK2/STAT3 pathway activity in CD44+CD24- cancer stem cells compared to the differentiated CD44+CD24+ population[56]. Addition of IL-6 to culture media increased the proportion of cancer stem cells in triple negative breast cancer cell lines as well as in primary cells isolated from triple negative tumors[57]. Both breast cancer stem cells and mesenchymal breast cancer cells secrete up to 1000-fold more IL-6 than non-stem epithelial breast cancer cells, indicating the presence of an autocrine positive feedback loop[58].
There is significant evidence that both adipocyte secreted IL-6 and leptin have pro-EMT activity, as well as promoting self-renewal and cancer stem cell signaling, however, these are just two of many signaling factors produced by adipocytes. Complete characterization of the adipocyte secretome in the breast cancer microenvironment is an important next step in the investigation of the adipocyte-breast cancer signaling relationship. Dirat et al[7] have demonstrated the phenotypic plasticity of adipocytes in culture with breast cancer cells, further description of the exact mechanisms and consequences of cancer-associated adipocytes will contribute significantly to our knowledge of the tumor microenvironment. While the breast cancer microenvironment is heterogeneous, adipocytes are the primary constituent. Blocking adipocyte-cancer cell interactions has the potential to inhibit cancer stem cell activity and prevent tumor initiation/progression.
Conditioned media from adipocytes treated with Genistein and Sulforaphane has been shown to inhibit mammosphere formation of breast cancer cells[59,60]. Furthermore, there is already a clinically available anti-IL-6 antibody, Tocilizumab. Developed as a treatment for inflammatory rheumatic diseases, Tocilizumab may be useful for inhibiting adipocyte/breast cancer cell IL-6 signaling[61]. There also is significant interest in targeting leptin signaling for treatment of breast cancer. Leptin-signaling inhibition has anti-tumor effects in both ER+, ER- and triple negative in vitro and in vivo models of breast cancer[62,63]. OB-R inhibitors, including leptin muteins[64], leptin peptide modulators[65], antibodies[66] and nanobodies[67] are under development, but have yet to enter clinical trials.
Future studies will reveal if other adipocyte-derived factors contribute to tumorigenesis. There are many adipocyte-secreted cytokines, with only a few currently investigated. It is likely that non-coding RNAs such as miRNAs and long non-coding RNAs (lncRNAs) are also involved in stromal-tumor cell signaling. Microvesicles such as exosomes mediate paracrine and endocrine transfer of miRNA and lncRNA, as well as proteins including TGF-β[68]. Targeting exosome mediated signaling may provide unique methods of inhibiting the adipocyte-breast cancer relationship.
miRNAs dysregulation is seen in almost all cancers[69] affecting nearly every hallmark of cancer[70]. Depending on the protein targeted, miRNAs act as either oncogenes or tumor suppressors, and miRNAs have a direct role in breast cancer stemness and progression[71,72]. Recently miRNAs with specific roles in adipogenesis and obesity have been identified[73]. There is differential miRNA expression in the adipocytes of obese mice compared to lean, including downregulation of miR-200 family microRNAs[74]. The miR-200 family inhibits epithelial mesenchymal transition through targeting of key EMT regulators such as ZEB1, SIP1[75] and SIRT1[41]. miR-200c expression is suppressed by IL-6, another potential mechanism for adipocyte-mediated increase in EMT signaling[76]. MiRNAs have important functions in both adipocytes and breast cancer cells, and may serve as signaling molecules or modify cancer cell interactions in the tumor microenvironment.
Current research on lncRNAs is rapidly changing the current paradigm of cell signaling. Initially found to have important regulatory roles during development and stem cell biology, lncRNAs may be involved in dysregulated signaling associated with transformation. lncRNA have diverse roles, and function during nearly all levels of gene expression. Differential lncRNAs expression may be used to predict patient outcomes or targeted to disrupt cell signaling[77]. Through profiling of preadipocyte and adipocyte transcriptomes, lncRNAs that control adipogenesis were identified[78]. Dissection of lncRNA’s specific roles in adipocytes and breast cancer cells may provide new avenues of treatment.
P- Reviewer: Cui YP, Freire-De-Lima CG, Zaravinos A S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982;42:4763-4770. [PubMed] |
| 2. | Volinia S, Galasso M, Sana ME, Wise TF, Palatini J, Huebner K, Croce CM. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proc Natl Acad Sci USA. 2012;109:3024-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Wang YY, Lehuédé C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 705] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 5. | Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1073] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 6. | Chamras H, Bagga D, Elstner E, Setoodeh K, Koeffler HP, Heber D. Preadipocytes stimulate breast cancer cell growth. Nutr Cancer. 1998;32:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 810] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 8. | Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 399] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 9. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6917] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 10. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4862] [Article Influence: 173.6] [Reference Citation Analysis (1)] |
| 11. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7730] [Article Influence: 351.4] [Reference Citation Analysis (0)] |
| 12. | Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3173] [Cited by in RCA: 3064] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 13. | Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2778] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 14. | Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4060] [Cited by in RCA: 5767] [Article Influence: 524.3] [Reference Citation Analysis (0)] |
| 15. | Guenther A, Gordon S, Tiemann M, Burger R, Bakker F, Green JR, Baum W, Roelofs AJ, Rogers MJ, Gramatzki M. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer. 2010;126:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Ozaki S, Tanaka O, Fujii S, Shigekiyo Y, Miki H, Choraku M, Kagawa K, Asano J, Takeuchi K, Kitazoe K. Therapy with bortezomib plus dexamethasone induces osteoblast activation in responsive patients with multiple myeloma. Int J Hematol. 2007;86:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Brodie A, Long B. Aromatase inhibition and inactivation. Clin Cancer Res. 2001;7:4343s-4349s; discussion 4411s-4412s. [PubMed] |
| 18. | Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605-R615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 553] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 19. | Zhao D, Mo Y, Li MT, Zou SW, Cheng ZL, Sun YP, Xiong Y, Guan KL, Lei QY. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J Clin Invest. 2014;124:5453-5465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007;3:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 22. | Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2765] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 23. | Lamb R, Ablett MP, Spence K, Landberg G, Sims AH, Clarke RB. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS One. 2013;8:e67811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 626] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 25. | Pan GJ, Chang ZY, Schöler HR, Pei D. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002;12:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731-24737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 843] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 27. | Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 499] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 28. | Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2459] [Cited by in RCA: 2388] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 29. | Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3597] [Cited by in RCA: 3378] [Article Influence: 168.9] [Reference Citation Analysis (0)] |
| 30. | Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, Repass J, Zaehres H, Shen JJ, Tang DG. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30:3833-3845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 31. | Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506-5511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1371] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 32. | Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R, Martin AG. Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene. 2012;31:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 33. | Lu X, Mazur SJ, Lin T, Appella E, Xu Y. The pluripotency factor nanog promotes breast cancer tumorigenesis and metastasis. Oncogene. 2014;33:2655-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Beltran AS, Rivenbark AG, Richardson BT, Yuan X, Quian H, Hunt JP, Zimmerman E, Graves LM, Blancafort P. Generation of tumor-initiating cells by exogenous delivery of OCT4 transcription factor. Breast Cancer Res. 2011;13:R94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Shook D, Keller R. Mechanisms, mechanics and function of epithelial–mesenchymal transitions in early development. Mech Dev. 2003;120:1351-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 420] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 36. | Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 37. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4877] [Cited by in RCA: 5118] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 38. | Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 578] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 39. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6234] [Article Influence: 566.7] [Reference Citation Analysis (0)] |
| 40. | Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Götz U, Hermeking H. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256-4271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 480] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 41. | Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992-26002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 42. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6972] [Cited by in RCA: 6810] [Article Influence: 400.6] [Reference Citation Analysis (0)] |
| 43. | Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C, Lisanti MP. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408-6423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 269] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1152] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 45. | Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 46. | Newman G, Gonzalez-Perez RR. Leptin-cytokine crosstalk in breast cancer. Mol Cell Endocrinol. 2014;382:570-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K. Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci USA. 2012;109:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Giordano C, Vizza D, Panza S, Barone I, Bonofiglio D, Lanzino M, Sisci D, De Amicis F, Fuqua SA, Catalano S. Leptin increases HER2 protein levels through a STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol Oncol. 2013;7:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Gillespie C, Quarshie A, Penichet M, Gonzalez-Perez RR. Potential Role of Leptin Signaling in DMBA induced Mammary Tumors by Non-Responsive C57BL/6J Mice Fed a High-Fat Diet. J Carcinogene Mutagene. 2012;3:132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2296] [Cited by in RCA: 2407] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 53. | Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 600] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 54. | Knüpfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007;102:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 55. | Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745-2755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 56. | Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R. The JAK2/STAT3 signaling pathway is required for growth of CD44 CD24 stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 751] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 57. | Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA. 2011;108:1397-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 512] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 58. | Yang L, Han S, Sun Y. An IL6-STAT3 loop mediates resistance to PI3K inhibitors by inducing epithelial-mesenchymal transition and cancer stem cell expansion in human breast cancer cells. Biochem Biophys Res Commun. 2014;453:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Montales MT, Rahal OM, Nakatani H, Matsuda T, Simmen RC. Repression of mammary adipogenesis by genistein limits mammosphere formation of human MCF-7 cells. J Endocrinol. 2013;218:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Li Q, Xia J, Yao Y, Gong DW, Shi H, Zhou Q. Sulforaphane inhibits mammary adipogenesis by targeting adipose mesenchymal stem cells. Breast Cancer Res Treat. 2013;141:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Alten R, Maleitzke T. Tocilizumab: a novel humanized anti-interleukin 6 (IL-6) receptor antibody for the treatment of patients with non-RA systemic, inflammatory rheumatic diseases. Ann Med. 2013;45:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Otvos L, Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, Nama K, Molino A, Piubello Q, Wade JD. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Verploegen SA, Plaetinck G, Devos R, Van der Heyden J, Guisez Y. A human leptin mutant induces weight gain in normal mice. FEBS Lett. 1997;405:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Otvos L, Terrasi M, Cascio S, Cassone M, Abbadessa G, De Pascali F, Scolaro L, Knappe D, Stawikowski M, Cudic P. Development of a pharmacologically improved peptide agonist of the leptin receptor. Biochim Biophys Acta. 2008;1783:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Fazeli M, Zarkesh-Esfahani H, Wu Z, Maamra M, Bidlingmaier M, Pockley AG, Watson P, Matarese G, Strasburger CJ, Ross RJ. Identification of a monoclonal antibody against the leptin receptor that acts as an antagonist and blocks human monocyte and T cell activation. J Immunol Methods. 2006;312:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Zabeau L, Verhee A, Catteeuw D, Faes L, Seeuws S, Decruy T, Elewaut D, Peelman F, Tavernier J. Selection of non-competitive leptin antagonists using a random nanobody-based approach. Biochem J. 2012;441:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Divoux A, Karastergiou K, Xie H, Guo W, Perera RJ, Fried SK, Smith SR. Identification of a novel lncRNA in gluteal adipose tissue and evidence for its positive effect on preadipocyte differentiation. Obesity (Silver Spring). 2014;22:1781-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5705] [Cited by in RCA: 6032] [Article Influence: 317.5] [Reference Citation Analysis (0)] |
| 70. | Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 344] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 71. | Liu S, Clouthier SG, Wicha MS. Role of microRNAs in the regulation of breast cancer stem cells. J Mammary Gland Biol Neoplasia. 2012;17:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Li Q, Yao Y, Eades G, Liu Z, Zhang Y, Zhou Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2014;33:2589-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 73. | Peng Y, Yu S, Li H, Xiang H, Peng J, Jiang S. MicroRNAs: emerging roles in adipogenesis and obesity. Cell Signal. 2014;26:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Chartoumpekis DV, Zaravinos A, Ziros PG, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, Habeos IG. Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS One. 2012;7:e34872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 75. | Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2843] [Cited by in RCA: 3084] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 76. | Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 77. | Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1470] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 78. | Sun L, Goff LA, Trapnell C, Alexander R, Lo KA, Hacisuleyman E, Sauvageau M, Tazon-Vega B, Kelley DR, Hendrickson DG. Long noncoding RNAs regulate adipogenesis. Proc Natl Acad Sci USA. 2013;110:3387-3392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |