Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.169
Revised: January 20, 2014
Accepted: April 3, 2014
Published online: May 26, 2014
Processing time: 231 Days and 1.9 Hours
Activated protein C (APC) is a physiological anticoagulant, derived from its precursor protein C (PC). Independent of its anticoagulation, APC possesses strong anti-inflammatory, anti-apoptotic and barrier protective properties which appear to be protective in a number of disorders including chronic wound healing. The epidermis is the outermost skin layer and provides the first line of defence against the external environment. Keratinocytes are the most predominant cells in the epidermis and play a critical role in maintaining epidermal barrier function. PC/APC and its receptor, endothelial protein C receptor (EPCR), once thought to be restricted to the endothelium, are abundantly expressed by skin epidermal keratinocytes. These cells respond to APC by upregulating proliferation, migration and matrix metalloproteinase-2 activity and inhibiting apoptosis/inflammation leading to a wound healing phenotype. APC also increases barrier function of keratinocyte monolayers by promoting the expression of tight junction proteins and re-distributing them to cell-cell contacts. These cytoprotective properties of APC are mediated through EPCR, protease-activated receptors, epidermal growth factor receptor or Tie2. Future preventive and therapeutic uses of APC in skin disorders associated with disruption of barrier function and inflammation look promising. This review will focus on APC’s function in skin epidermis/keratinocytes and its therapeutical potential in skin inflammatory conditions.
Core tip: The anti-inflammatory, barrier stabilisation and anti-apoptotic properties of APC make it an appealing treatment for skin conditions associated with inflammation, barrier disruption and keratinocyte dysfunction.
- Citation: McKelvey K, Jackson CJ, Xue M. Activated protein C: A regulator of human skin epidermal keratinocyte function. World J Biol Chem 2014; 5(2): 169-179
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/169.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.169
Protein C (PC) is a vitamin-K dependent glycoprotein that circulates in blood plasma in its zymogenic and activated forms [activated PC (APC)]. PC/APC was first characterised for its role in blood coagulation, but has a range of cytoprotective functions including anti-inflammation, anti-apoptosis and barrier stabilisation. Although originally thought to be synthesised almost exclusively by the liver and vascular endothelial cells, PC/APC has been found to be synthesised by skin epidermal keratinocytes. Keratinocytes are the major cell type in the skin epidermis, the most outer layer of human skin that provides a semi-impermeable barrier against injury from the external environment, including ultraviolet radiation, heat, water loss and infectious pathogens. On keratinocytes, PC/APC promotes cell proliferation, survival, migration, and the barrier function. This review will focus on the actions of APC on skin epidermis/keratinocytes and its therapeutical potential in the treatment of skin inflammatory conditions.
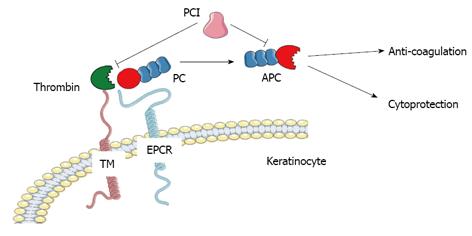
The PC pathway plays a key role in the regulation of blood coagulation. As a vitamin K-dependent zymogen, PC is activated to APC when thrombin binds to thrombomodulin and cleaves the activation peptide (Figure 1). This conversion is augmented by its specific receptor, endothelial cell protein C receptor (EPCR)[1]. In human plasma APC is present at relatively low levels approximation 40 pmol/L and has a short physiological half-life of approximation 20 min compared to PC at 70 nmol/L and approximation 10 h[2,3]. Thrombin is the only endogenous activator of PC. The importance of APC as an anticoagulant is reflected by findings that deficiencies in PC result in severe familial disorders of thrombosis[4]. Replenishment of PC in patients with systemic or local hypercoagulation can reverse the abnormality.
Independent of its effect on anti-coagulation, APC possesses strong anti-inflammatory and anti-apoptotic properties, as well as enhancing endothelial and epithelial barrier integrity (Figure 1).
Inhibiting inflammation: The anti-inflammatory effects of APC are associated with a decrease in pro-inflammatory cytokines and a reduction in leukocyte recruitment. APC inhibits neutrophil, monocyte and lymphocyte chemotaxis[5] and directly suppresses expression and activation of nuclear factor (NF)-κB[6]; a pathway that controls the expression of a wide range of inflammatory genes including tumour necrosis factor (TNF)-α and cell adhesion molecules. Acute inflammation is exacerbated in mice genetically predisposed to a severe PC deficiency[7]. In vitro, APC suppresses the activation of NF-κB and production of TNF-α, upregulates matrix metalloproteinase (MMP)-2 activity yet inhibits MMP-9 in rheumatoid synovial fibroblasts and monocytes[8]. In addition to the degradation of extracellular matrix, these MMPs can regulate inflammation by processing cytokines/chemokines with MMP-9 having stimulatory and MMP-2 having inhibitory effects on inflammation both in vitro and in vivo[9-11].
Promoting cell proliferation and inhibiting cell apoptosis: APC promotes cell proliferation in cultured human umbilical vein endothelial cells[12], smooth muscle cells[13], keratinocytes[14], neural stem and progenitor cells[15,16], neuroblasts[17], osteoblasts[18] and ovine tenocytes[19]. Consistent with the stimulatory effects on cell growth, APC displays strong anti-apoptotic properties in keratinocytes, endothelial cells and podocytes[14,20-22]. APC-dependent anti-apoptotic activity shows improved survival in human and various animal models of sepsis[23-28]. APC inhibits spontaneous monocyte apoptosis leading to increased lifespan and phagocytosis in vivo[29] and protects murine cortical neurons from N-methyl-D-aspartate and staurosporine excitotoxicity-induced apoptosis[30].
Stabilising endothelial and epithelial barrier: Endothelial cells normally form a dynamically regulated stable barrier at the blood-tissue interface. Breakdown of this barrier is a key pathogenic factor in inflammatory disorders. APC enhances endothelial barrier integrity by stabilising the cytoskeleton and reducing endothelial permeability[20,31-33]. Recently, APC has been shown to promote epithelial barrier function in human skin epidermal keratinocytes[34] and mouse intestine[35].
APC’s signalling pathway: Many of the anti-inflammatory properties of APC are mediated through EPCR, which itself is anti-inflammatory[36]. APC bound to EPCR can activate protease-activated receptor (PAR)-1 and promote the anti-inflammatory actions of APC[37]. Cytoprotective effects of APC are also mediated by the other PAR receptors. Akin to PAR-1, APC can bind to PAR-2 and activate the Akt signaling pathway to promote keratinocyte proliferation[37]. Independent of EPCR, APC can inhibit podocyte apoptosis by activating PAR-3[38]. APC-mediated arrest of lymphocyte chemotaxis is dependent on epidermal growth factor receptor (EGFR)[39]. In addition, EGFR transactivation by APC/EPCR/PAR-1 supports cell motility and invasiveness of endothelial cells and breast cancer cells[40]. APC utilises the angiopoietin/Tie2 axis to promote endothelial barrier function[33]. In addition other receptors such as integrins[41] and apolipoprotein E receptor-2[42] also mediate the effects of APC.
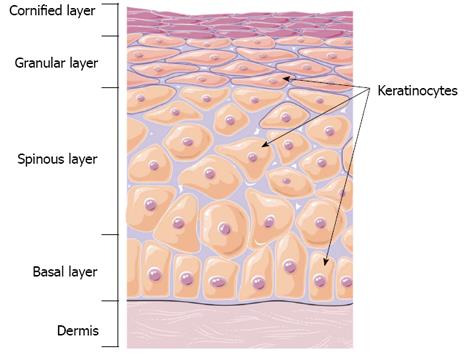
The skin forms an effective barrier between the human body and outside environment and protects the body from mechanical trauma, pathogens, radiation, dehydration, and dangerous temperature fluctuations[43]. Skin consists of two main layers, the outermost epidermis layer and the underlying dermis (Figure 2). The epidermis is a stratified epithelium composed of proliferating basal and differentiated suprabasal keratinocytes. The dermisprovides the epidermis with mechanical support and nutrients. The barrier function of skin is provided by the epidermis. Defective epidermal barrier is responsible for many inflammatory and blistering skin disorders[43,44].
Keratinocytes are the most abundant cell type in the epidermis and are responsible for maintaining structure and homeostasis of the epidermal barrier. The epidermal barrier is generated by a sophisticated differentiation program[44] comprising stratified epithelium composed of basal, spinous, granular, and cornified layers (Figure 2)[45]. The basal layer consists of proliferating keratinocytes, that maintain the epidermis and post-mitotic basal keratinocytes which migrate out of the basal layer. This migration marks the start of epidermal differentiation that ends with the formation of the cornified layer, where keratinocytes end their lives and are sloughed off. The epidermis has complete self-renewal capacity with an estimated turnover time of approximately 40 d in humans[46].
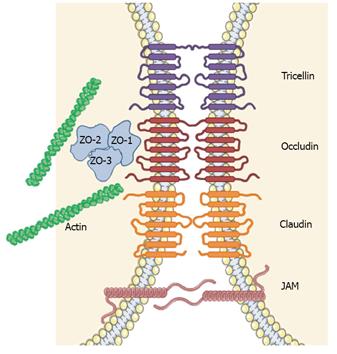
The physical barrier of the epidermis is localised primarily in the upper layers of the epidermis (granular and cornified layers). The barrier properties of nucleated keratinocytes in the granular layer are largely dependent on the function and integrity of the tight junctions [involving the proteins tricellin, occludin, claudins and junctional adhesion molecule (JAM)] and their corresponding intracellular proteins, such as zona occludin (ZO)-1[44], which seal the intercellular space between neighbouring keratinocytes and control the pathway of molecules and liquid (Figure 3)[46].
Deregulation of these junction proteins perturbs this barrier[43] and is characteristic of many inflammatory skin diseases[47,48]. Psoriatic skin, characterised by small scaly plaques, has an over-expression of occludin and ZO-1, while claudin-1 and 3 are down-regulated[49,50]. Keratinocyte cytoskeletal elements are also important for maintaining the epidermal barrier. Among the genetic mutations in atopic dermatitis is the filaggrin gene (FLG)[51,52], which encodes a protein in the corneal epidermal layer and aids terminal differentiation of keratinocytes, water retention and barrier stabilisation[53]. Loss or mutation of this gene contribute to the red, dry, itchy skin that is hallmark of this condition.
In addition, keratinocytes provide an immunological barrier in response to injury or infection. Keratinocytes are a potent source of cytokines and chemokines[54]; freshly isolated and cultured keratinocytes express toll-like receptors[55] and inflammasomes[56]. This allows keratinocytes to elicit innate immune responses to microbial components when the epidermal barrier is breached, particularly through secretion of interleukin (IL)-1β and activation of leukocytes.
Upon activation, keratinocytes express a plethora of cytokines, chemokines and accessory molecules, which can transmit both positive and negative signals to cells of the innate and adaptive immune system. Dysregulation of the immune response of keratinocytes is implicated in the pathogenesis of chronic inflammatory skin diseases.
Keratinocytes in the epidermis express all the components of the PC/APC pathway, including EPCR[57], thrombomodulin[58], thrombin and PC inhibitor[59], PAR-1, EGFR[60], and Tie2[34] which can regulate the activation of PC to APC and mediate the functions of APC on keratinocytes in skin epidermis.
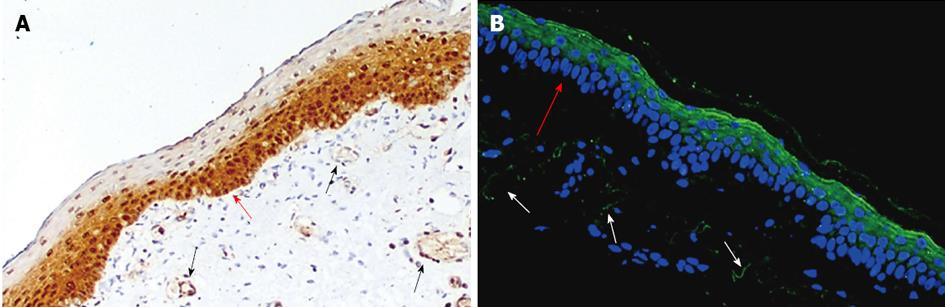
PC/APC: Since its discovery in 1960[61], PC has been characterised as the vitamin-K dependent protein precursor for the anticoagulant APC[62]. Thought to be exclusively synthesised by the liver and vascular endothelial cells, recent evidence shows that keratinocytes can also synthesise PC[60]. Cultured keratinocytes express PC mRNA and protein, and APC activity is presented on these cells[60]. In neonatal foreskin, PC is strongly expressed in the basal and suprabasal layers of the epidermis, with weaker expression in the outer cornified layer[60]. In the adult skin, however, the PC/APC is strongly stained in the upper layer of epidermis (Figure 4)
Thrombin: Thrombin is the only endogenous activator of PC. Keratinocytes express mRNA for the thrombin precursor, pro-thrombin[63]. Pro-thrombin and thrombin are expressed at low levels in normal epidermis, with thrombin markedly upregulated in scar tissue[63]. Thrombin activity is regulated by keratinocyte thrombomodulin at sites of cutaneous injury[64].
Thrombomodulin: Upon binding to thrombomodulin on surface of vascular endothelial cells, thrombin cleaves PC at the activation peptide between Arg211 and Leu212 and converts it to APC. Cultured human keratinocytes constitutively express thrombomodulin on their cell surface[58,64]. In normal epidermis thrombomodulin is present in spinous layer and on the outer root sheath of hair follicles[58,64].
PC inhibitor: PC inhibitor is a non-specific serpin that inhibits a variety of serine proteases, including PC and thrombin[65]. This inhibitor can inhibit the activation of PC to APC by inactivating thrombin and/or preventing thrombin binding to thrombomodulin[66,67]. It can also inactivate APC. PC inhibitor mRNA and protein is constitutively expressed by immortalised human keratinocytes (HaCaT) and epidermoid carcinoma cells (A431) in culture[59]. Normal skin from the trunk of adults show strong staining for PC inhibitor antigen throughout the epidermal layers[59].
In summary, epidermal keratinocytes express all aspects of the PC system to not only activate PC to APC, but regulate this activation process and APC activity (Figure 4).
EPCR: EPCR is a type I transmembrane protein which exhibits significant homology with the major histocompatibility class 1/CD1 family of proteins. EPCR is the main receptor to regulate the function of PC/APC. Although first described as being restricted to the endothelium, EPCR is abundantly expressed by cultured human keratinocytes and is strongly expressed in the basal and suprabasal layers of the epidermis of neonatal foreskin[57].
EPCR has similar affinity for both PC and APC[1]. After binding to EPCR, APC cleaves PAR-1 to promote its cytoprotective functions in keratinocytes[57]. In addition PAR-1, EGFR and Tie2 are shown to mediate keratinocyte proliferation, migration and barrier stabilisation. In addition, EPCR enhances the rate of PC/APC activation by thrombin/thrombomodulin 3-4 fold[68]. Inhibition of EPCR reduces the level of circulating APC by more than 80% following thrombin infusion[69].
PAR-1: PARs are a family of G-protein coupled receptors which utilise G-protein and non-G-protein signaling pathways to mediate their cellular responses[70]. They are expressed by a wide range of cell types in the skin, including keratinocytes[57]. PARs are activated by a range of proteases through cleavage of an activation peptide. The most common endogenous activator is thrombin which activates PAR-1, PAR-3 and PAR-4, but not PAR-2. Other serine proteases including trypsin, mast cell tryptase and factor Xa activate PAR-2. In keratinocytes, PAR-1 mediates APC’s induction of cell proliferation, anti-inflammatory and barrier protective effects[34,57].
Cytoprotective effects of APC are also mediated by the other PAR receptors. APC can bind to PAR-2[37] and activate the Akt signaling pathway to promote keratinocyte proliferation[71]. Though only PAR-2 activity appears to be required for APC-mediated wound healing in a murine model[71].
EGFR: EGFR is a crucial receptor for autocrine growth of healthy epidermis. Its activation suppresses terminal differentiation, promotes cell proliferation and survival, and regulates cell migration during epidermal morphogenesis and wound healing[72]. Following tissue injury, EGFR is upregulated to promote re-epithelialisation of the wound by encouraging keratinocyte proliferation and migration. EGFR regulates cell adhesion, extracellular matrix degrading enzymes, and cell migration to contribute to the migratory and invasive potential of keratinocytes[72]. In human skin, EGFR and EPCR are expressed in the basal and suprabasal layers of the epidermis, consistent with the localisation of PC/APC[60]. Expression of EGFR by keratinocytes appears to be synchronised with the PC pathway. APC treatment increases EGFR expression while silencing of PC decreases EGFR levels[60].
Tie2: Tie2 is a protein-tyrosine kinase receptor expressed by endothelial and epithelial cells. Its major ligands are angiopoeitin 1 and 2 which bind with similar affinity[73,74]. Both Tie2 and its activated form phosphorylated (P)-Tie2 are present on neonatal foreskin and adult skin keratinocytes[34]. However, adult skin keratinocytes show less intensive staining for Tie-2 and P-Tie2 when compared with neonatal foreskin keratinocytes. Foreskin epidermis exhibits faint staining of Tie2 but strong staining for P-Tie2, which is mainly located in the uppermost layers of the epidermis (Figure 4). Similarly, P-Tie2 is expressed by normal adult skin epidermis, although the staining intensity is considerably lower than neonatal foreskin.
APC promotes proliferation and inhibits apoptosis in keratinocytes: APC promotes cell proliferation in cultured human skin keratinocytes[14]. The replicative capacity of keratinocytes is mediated by EGFR, and acts to inhibit terminal differentiation and apoptosis. APC increases keratinocyte proliferation, while gene silencing of PC increases apoptosis in keratinocytes 3-fold[60]. Proliferation is mediated by APC’s regulation of mitogen activated protein (MAP) kinase activity[12,14-16,18]. This family of highly conserved serine/threonine protein kinases enhances DNA synthesis, and regulates cell survival/apoptosis and differentiation[13]. In human skin keratinocytes, PC/APC-induced proliferation is mediated by EPCR, PAR-2, EGFR, activation of ERK1/2 and PI3K/Src/Akt signalling and suppression of p38[34,60,71].
Consistent with the stimulatory effects on cell growth, APC displays strong anti-apoptotic properties. APC prevents apoptosis of keratinocytes[14]. The molecular mechanism of APC’s ability to protect cells from apoptosis is multi-faceted. APC regulates caspase activation, DNA degradation and the induction of anti-apoptotic mediators[25-28]. PC regulates the activation of apoptosis marker caspase-3, of which the inactive form is expressed in a wide range of tissues, including the epidermis[75]. In normal oral epithelium, cleaved caspase-3 distinguishes apoptotic keratinocytes from cells that are terminally differentiated[76]. Recent findings indicate that caspase-14, not caspase-3, is activated during normal keratinocyte differentiation[77]. Therefore caspase-3 activation appears to be restricted to keratinocytes undergoing apoptosis, and is increased by blocking PC by siRNA consistent with a role for PC in preventing keratinocyte apoptosis[60].
While additional anti-apoptotic pathways for APC have not yet been demonstrated in keratinocytes, in hypoxic retinal epithelia and photoreceptor cells APC reduces caspase-8 and 9[78]; decreases p21 and p53 proteins in murine model of sepsis-induced apoptosis[79]; and prevents glucose-induced apoptosis in endothelial cells and podocytes by reducing Bax induction and Bcl-2 suppression[21].
APC promotes migration of keratinocytes: Keratinocyte migration is a crucial step in stratification of the epidermis to form a protective barrier, and during re-epithelialisation of a wound site. EGF is a chemotactic factor for keratinocytes, as shown by phagokinetic track analysis[80]. In human skin, EGFR localises with PC/APC and EPCR in the basal and suprabasal layers of the epidermis[60]. Recombinant human (rh) APC treatment of keratinocytes increases EGFR activation and keratinocyte migration[57,60]. APC promotes keratinocyte migration at concentrations 5 µg/mL but had an inhibitory effect at 20 µg/mL[14]. At 5 µg/mL APC, the migration of keratinocytes was equivalent to that induced by 50 ng/mL EGF[14]. Gene silencing of PC inhibits EGFR expression and reduces keratinocyte migration by 20% using an in vitro scratch wounding assay[60].
MMP secretion appears to be are required for keratinocyte migration, as blockade of MMP’s using GM6001, a broad spectrum MMP inhibitor, eliminated cell migration in a dose-dependent manner and delayed in vitro wound healing[60]. Full-thickness rat excisional skin wound healing model, a single topical application of rhAPC enhances wound healing compared to saline by stimulating re-epithelialisation[71,81]. This is also observed in human skin wound healing. In humans, topical application of 200 µg/mL rhAPC to chronic wounds of varying aetiology reduced wound area by 52%-95% over 16 wk[82]. A follow-up study of venous and diabetic ulcers treated with 400 µg/mL rhAPC showed a significant reduction in wound area and volume compared to baseline at 20 wk[83].
APC reduces inflammation of keratinocytes: APC regulates the expression of serine protease MMP-2. MMPs degrade tissue components and are commonly associated with skin inflammatory conditions[84]. In cultured human keratinocytes, APC enhances MMP-2 activity[14] which has anti-inflammatory properties[11,85] and plays a vital role in the tissue repair process by remodelling the extracellular matrix[86]. In contrast, MMP-9, which exhibits pro-inflammatory actions[11,87-89], is suppressed by APC[8,90].
Other indirect effects APC may have on suppressing cytokine production and activation is via inhibition of NF-κB subunits p50 and p52[28]. APC inhibits calcium- and lipopolysaccharide-stimulated activation of NF-κB in keratinocytes[14]. The NF-κΒ pathway is important for the expression of a wide variety of inflammatory genes including TNF-α and cell adhesion molecules, intercellular adhesion molecule-1, vascular cell adhesion molecule-1and E-selectin.
APC promotes barrier function of keratinocyte monolayers: The barrier protective effect of APC is relevant to skin epidermal keratinocytes[34]. Keratinocytes play a critical role in maintaining epidermal barrier function via tight junctions[43,91,92]. Dysregulation of tight junction proteins such as occludins, claudins and JAMs perturbs this barrier[43,91] and contributes to many skin inflammatory conditions[93].
APC enhances the barrier function of cultured human keratinocyte monolayers in a dose-dependent manner by up-regulating tight junction protein and re-distributing them to cell-cell contacts via regulation of Tie2 and subsequent activation of Akt[34]. In response to APC treatment, Tie2 is activated within 30 min on keratinocyte monolayers, and relocates to cell-cell contacts where it impedes barrier permeability[34]. Expression of ZO-1, claudin-1 and vascular endothelial cadherin are subsequently increased. Interestingly, APC does not activate Tie2 through its major ligand, angiopoeitin-1, but binds directly to EPCR, cleaves PAR-1, and transactivates EGFR, then Tie2 which activates PI3K/Akt signalling to increase stabilisation of the keratinocyte barrier[34].
The skin, the body’s largest organ, provides an epidermal barrier to protect the body from external insults, maintain temperature and control evaporation. Breaches of this barrier are common events. However, the inability to restore this barrier function can result in health problems, including inflammatory skin diseases, which are very common and have high morbidity. This group of diseases includes: acne, which affects 50% of teenagers (5% have severe acne); rosacea which affects 10% of the adult population; atopic dermatitis which affects up to 20% population; psoriasis which affects 2%-3% population[94,95]; chronic wounds which affect < 1% population and the devastating, often fatal, toxic epidermal necrolysis[96,97]. These diseases can be controlled to a certain extent, but no cure exists and they have high morbidity[98,99].
Management of most skin inflammatory conditions involves the use of emollients, phototherapy, topical corticosteroids, antibiotics, retinoids, immunomodulators (tacrolimus, pimecrolimus), or systemic treatments (ciclosporin, azathioprine). While targeted immunosuppressive drugs have been developed, including TNF-α inhibitors, antibodies and receptor blockers, in most studies they do not show improved outcome and their use for skin inflammatory conditions remains controversial[97]. For other conditions such as Stevens-Johnson syndrome and toxic epidermal necrolysis, to date no treatment has been identified to be capable of halting the progression of skin detachment[96].
APC is emerging as a critical regulator of keratinocyte and epidermal function. APC protects the epidermis by promoting keratinocyte proliferation, survival, reducing inflammation and maintaining barrier function. These keratinocyte cytoprotective functions are dependent on APC’s interaction with EPCR, PARs, EGFR and Tie2.
Topical administration of rhAPC has shown promising results in the field of skin wound healing. Single or multiple topical applications of rhAPC to excisional wound sites reduced oedema and leukocyte infiltration, in addition to promoting angiogenesis and re-epithelialisation of wounds in rat models of skin wound healing[71,81]. These same APC-mediated benefits have been demonstrated in humans chronic wounds of venous and diabetic origin[82,83], as well as recalcitrant orthopaedic wounds[100].
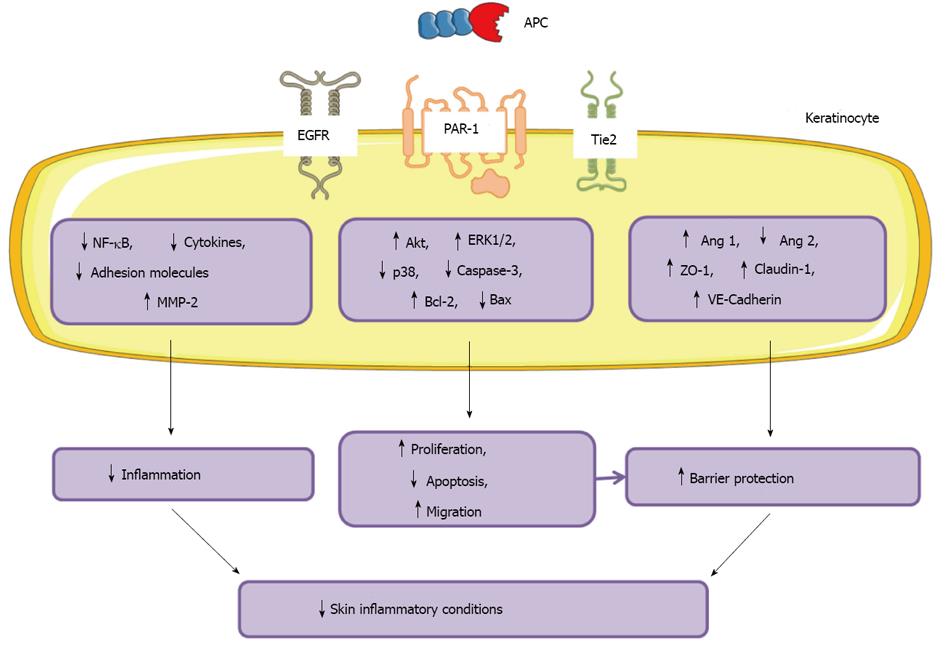
The anti-inflammatory, barrier stabilisation and anti-apoptotic properties of APC make it an appealing treatment for skin diseases associated with inflammation, barrier disruption and keratinocyte dysfunction. A summary of the actions of APC on keratinocytes and skin inflammatory disorders is shown in Figure 5.
In late 2011, rhAPC (Xigris; drotrecogin alfa [activated]; Eli Lily) was withdrawn from the market after failure to significantly improve patient outcome in a clinical trial of septic shock[101], in an attempt to replicate earlier favourable results[102]. One concern was the observation of serious bleeding in patients, although there was no significant difference between patients treated with rhAPC and placebo[101,102]. Most in vivo studies, including our own, show that systemic rhAPC does not induce any bleeding side-effects[71,82,100,103-105]. Bleeding has occurred in a subset of near-death sepsis patients with recent surgery and although APC efficacy and safety is controversial in treatment of sepsis patients, it is beneficial and safe in clinical trials for chronic wound healing[82,100], acute lung injury[106,107], and solid organ transplantation[108]. Recently APC mutants (3K3A-APC and APC-2Cys) with minimal anticoagulant activity, but normal cytoprotective activity have been generated[109,110] and shown pre-clinically to be safe[12,111-116]. Although both variants are yet to be assessed in the field of skin inflammatory diseases. The notion that rhAPC may increase bleeding during wound healing could be circumvented by use of APC variants lacking anticoagulant activity.
Nevertheless, the future for utilising exogenous APC as a topical treatment for skin inflammatory conditions remains a novel and exciting avenue of investigation.
P- Reviewers: Huang Y, Vijayan KV, Wang Z S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ
| 1. | Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486-26491. [PubMed] |
| 2. | Okajima K, Koga S, Kaji M, Inoue M, Nakagaki T, Funatsu A, Okabe H, Takatsuki K, Aoki N. Effect of protein C and activated protein C on coagulation and fibrinolysis in normal human subjects. Thromb Haemost. 1990;63:48-53. [PubMed] |
| 3. | Gruber A, Griffin JH. Direct detection of activated protein C in blood from human subjects. Blood. 1992;79:2340-2348. [PubMed] |
| 4. | Baker WF, Bick RL. Treatment of hereditary and acquired thrombophilic disorders. Semin Thromb Hemost. 1999;25:387-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Yuksel M, Okajima K, Uchiba M, Horiuchi S, Okabe H. Activated protein C inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappa B and activator protein-1 in human monocytes. Thromb Haemost. 2002;88:267-273. [PubMed] |
| 7. | Lay AJ, Donahue D, Tsai MJ, Castellino FJ. Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood. 2007;109:1984-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Xue M, March L, Sambrook PN, Jackson CJ. Differential regulation of matrix metalloproteinase 2 and matrix metalloproteinase 9 by activated protein C: relevance to inflammation in rheumatoid arthritis. Arthritis Rheum. 2007;56:2864-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 900] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 10. | McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 575] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002;169:2643-2647. [PubMed] |
| 12. | Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, Isobe H, Suda T. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Bretschneider E, Uzonyi B, Weber AA, Fischer JW, Pape R, Lötzer K, Schrör K. Human vascular smooth muscle cells express functionally active endothelial cell protein C receptor. Circ Res. 2007;100:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Xue M, Thompson P, Kelso I, Jackson C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp Cell Res. 2004;299:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Guo H, Zhao Z, Yang Q, Wang M, Bell RD, Wang S, Chow N, Davis TP, Griffin JH, Goldman SA. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-Akt pathway. J Neurosci. 2013;33:6181-6190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Thiyagarajan M, Fernández JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788-12797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Cerbák R, Stĕtka F, Filkuka J, Utrata P, Rubácek M, Dominik J, Nicovský J, Bednarík M. [Type II atrial septal defects in adulthood]. Vnitr Lek. 1989;35:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kurata T, Hayashi T, Yoshikawa T, Okamoto T, Yoshida K, Iino T, Uchida A, Suzuki K. Activated protein C stimulates osteoblast proliferation via endothelial protein C receptor. Thromb Res. 2010;125:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Xue M, Smith MM, Little CB, Sambrook P, March L, Jackson CJ. Activated protein C mediates a healing phenotype in cultured tenocytes. J Cell Mol Med. 2009;13:749-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Xue M, Minhas N, Chow SO, Dervish S, Sambrook PN, March L, Jackson CJ. Endogenous protein C is essential for the functional integrity of human endothelial cells. Cell Mol Life Sci. 2010;67:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Hemmer CJ, Löbermann M, Unverricht M, Vogt A, Krause R, Reisinger EC. Activated protein C protects vascular endothelial cells from apoptosis in malaria and in sepsis. Trop Med Int Health. 2011;16:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 410] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 24. | Yen YT, Yang HR, Lo HC, Hsieh YC, Tsai SC, Hong CW, Hsieh CH. Enhancing autophagy with activated protein C and rapamycin protects against sepsis-induced acute lung injury. Surgery. 2013;153:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Cheng T, Liu D, Griffin JH, Fernández JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 434] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 26. | Liu D, Cheng T, Guo H, Fernández JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379-1383. [PubMed] [DOI] [Full Text] |
| 27. | Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J. 2003;373:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199-11203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 484] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 29. | Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002;30:S288-S293. [PubMed] |
| 30. | Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernández JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 32. | Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286-17293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 33. | Minhas N, Xue M, Fukudome K, Jackson CJ. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2010;24:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Xue M, Chow SO, Dervish S, Chan YK, Julovi SM, Jackson CJ. Activated protein C enhances human keratinocyte barrier integrity via sequential activation of epidermal growth factor receptor and Tie2. J Biol Chem. 2011;286:6742-6750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Vetrano S, Ploplis VA, Sala E, Sandoval-Cooper M, Donahue DL, Correale C, Arena V, Spinelli A, Repici A, Malesci A. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc Natl Acad Sci USA. 2011;108:19830-19835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Esmon CT. Structure and functions of the endothelial cell protein C receptor. Crit Care Med. 2004;32:S298-S301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 38. | Madhusudhan T, Wang H, Straub BK, Gröne E, Zhou Q, Shahzad K, Müller-Krebs S, Schwenger V, Gerlitz B, Grinnell BW. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood. 2012;119:874-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Feistritzer C, Mosheimer BA, Sturn DH, Riewald M, Patsch JR, Wiedermann CJ. Endothelial protein C receptor-dependent inhibition of migration of human lymphocytes by protein C involves epidermal growth factor receptor. J Immunol. 2006;176:1019-1025. [PubMed] |
| 40. | Gramling MW, Beaulieu LM, Church FC. Activated protein C enhances cell motility of endothelial cells and MDA-MB-231 breast cancer cells by intracellular signal transduction. Exp Cell Res. 2010;316:314-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120:1971-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | White TC, Berny MA, Tucker EI, Urbanus RT, de Groot PG, Fernández JA, Griffin JH, Gruber A, McCarty OJ. Protein C supports platelet binding and activation under flow: role of glycoprotein Ib and apolipoprotein E receptor 2. J Thromb Haemost. 2008;6:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1176] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 44. | Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001;114:3069-3070. [PubMed] |
| 45. | Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 46. | Weinstein GD, McCullough JL, Ross P. Cell proliferation in normal epidermis. J Invest Dermatol. 1984;82:623-628. [PubMed] |
| 47. | Kirschner N, Bohner C, Rachow S, Brandner JM. Tight junctions: is there a role in dermatology? Arch Dermatol Res. 2010;302:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | O’Neill CA, Garrod D. Tight junction proteins and the epidermis. Exp Dermatol. 2011;20:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Watson RE, Poddar R, Walker JM, McGuill I, Hoare LM, Griffiths CE, O’neill CA. Altered claudin expression is a feature of chronic plaque psoriasis. J Pathol. 2007;212:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Peltonen S, Riehokainen J, Pummi K, Peltonen J. Tight junction components occludin, ZO-1, and claudin-1, -4 and -5 in active and healing psoriasis. Br J Dermatol. 2007;156:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 52. | Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361-1370. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 53. | Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2038] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 54. | Luger TA, Schwarz T. Epidermal cell-derived secretory regulins. Boca Raton (FL): CRC Press 1989; 217-253. |
| 55. | Köllisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, Bauer S, Jakob T, Mempel M, Ollert M. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 56. | Watanabe H, Gaide O, Pétrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 57. | Xue M, Campbell D, Sambrook PN, Fukudome K, Jackson CJ. Endothelial protein C receptor and protease-activated receptor-1 mediate induction of a wound-healing phenotype in human keratinocytes by activated protein C. J Invest Dermatol. 2005;125:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Jackson DE, Mitchell CA, Bird P, Salem HH, Hayman JA. Immunohistochemical localization of thrombomodulin in normal human skin and skin tumours. J Pathol. 1995;175:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Krebs M, Uhrin P, Vales A, Prendes-Garcia MJ, Wojta J, Geiger M, Binder BR. Protein C inhibitor is expressed in keratinocytes of human skin. J Invest Dermatol. 1999;113:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Xue M, Campbell D, Jackson CJ. Protein C is an autocrine growth factor for human skin keratinocytes. J Biol Chem. 2007;282:13610-13616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Mammen EF, Thomas WR, Seegers WH. Activation of purified prothrombin to autoprothrombin I or autoprothrombin II (platelet cofactor II or autoprothrombin II-A). Thromb Diath Haemorrh. 1960;5:218-249. [PubMed] |
| 62. | Kisiel W, Canfield WM, Ericsson LH, Davie EW. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977;16:5824-5831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 356] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Artuc M, Hermes B, Algermissen B, Henz BM. Expression of prothrombin, thrombin and its receptors in human scars. Exp Dermatol. 2006;15:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Raife TJ, Lager DJ, Madison KC, Piette WW, Howard EJ, Sturm MT, Chen Y, Lentz SR. Thrombomodulin expression by human keratinocytes. Induction of cofactor activity during epidermal differentiation. J Clin Invest. 1994;93:1846-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Suzuki K, Deyashiki Y, Nishioka J, Toma K. Protein C inhibitor: structure and function. Thromb Haemost. 1989;61:337-342. [PubMed] |
| 66. | Li W, Adams TE, Kjellberg M, Stenflo J, Huntington JA. Structure of native protein C inhibitor provides insight into its multiple functions. J Biol Chem. 2007;282:13759-13768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Elisen MG, von dem Borne PA, Bouma BN, Meijers JC. Protein C inhibitor acts as a procoagulant by inhibiting the thrombomodulin-induced activation of protein C in human plasma. Blood. 1998;91:1542-1547. [PubMed] |
| 68. | Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA. 1996;93:10212-10216. [PubMed] |
| 69. | Taylor FB, Peer GT, Lockhart MS, Ferrell G, Esmon CT. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001;97:1685-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 70. | Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1911] [Cited by in RCA: 1876] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 71. | Julovi SM, Xue M, Dervish S, Sambrook PN, March L, Jackson CJ. Protease activated receptor-2 mediates activated protein C-induced cutaneous wound healing via inhibition of p38. Am J Pathol. 2011;179:2233-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Hudson LG, McCawley LJ. Contributions of the epidermal growth factor receptor to keratinocyte motility. Microsc Res Tech. 1998;43:444-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Makinde T, Agrawal DK. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J Cell Mol Med. 2008;12:810-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 455] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 75. | Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, Reed JC. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 1997;57:1605-1613. [PubMed] |
| 76. | Hague A, Eveson JW, MacFarlane M, Huntley S, Janghra N, Thavaraj S. Caspase-3 expression is reduced, in the absence of cleavage, in terminally differentiated normal oral epithelium but is increased in oral squamous cell carcinomas and correlates with tumour stage. J Pathol. 2004;204:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Lippens S, Kockx M, Knaapen M, Mortier L, Polakowska R, Verheyen A, Garmyn M, Zwijsen A, Formstecher P, Huylebroeck D. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218-1224. [PubMed] |
| 78. | Du ZJ, Yamamoto T, Ueda T, Suzuki M, Tano Y, Kamei M. Activated protein C rescues the retina from ischemia-induced cell death. Invest Ophthalmol Vis Sci. 2011;52:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Sakar A, Vatansever S, Sepit L, Ozbilgin K, Yorgancioglu A. Effect of recombinant human activated protein C on apoptosis-related proteins. Eur J Histochem. 2007;51:103-109. [PubMed] |
| 80. | Ando Y, Jensen PJ. Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J Invest Dermatol. 1993;100:633-639. [PubMed] |
| 81. | Jackson CJ, Xue M, Thompson P, Davey RA, Whitmont K, Smith S, Buisson-Legendre N, Sztynda T, Furphy LJ, Cooper A. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen. 2005;13:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Whitmont K, Reid I, Tritton S, March L, Xue M, Lee M, Fulcher G, Sambrook P, Slobedman E, Cooper A. Treatment of chronic leg ulcers with topical activated protein C. Arch Dermatol. 2008;144:1479-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Whitmont K, McKelvey KJ, Fulcher G, Reid I, March L, Xue M, Cooper A, Jackson CJ. Treatment of chronic diabetic lower leg ulcers with activated protein C: a randomised placebo-controlled, double-blind pilot clinical trial. Int Wound J. 2013;Jul 15; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Starodubtseva NL, Sobolev VV, Soboleva AG, Nikolaev AA, Bruskin SA. [Expression of genes for metalloproteinases (MMP-1, MMP-2, MMP-9, and MMP-12) associated with psoriasis]. Genetika. 2011;47:1254-1261. [PubMed] [DOI] [Full Text] |
| 85. | McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160-1167. [PubMed] |
| 86. | Ravanti L, Kähäri VM. Matrix metalloproteinases in wound repair (review). Int J Mol Med. 2000;6:391-407. [PubMed] |
| 87. | Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 88. | Schönbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340-3346. [PubMed] |
| 89. | Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 939] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 90. | Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernández JA. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278-1285. [PubMed] |
| 91. | Proksch E, Brasch J. Abnormal epidermal barrier in the pathogenesis of contact dermatitis. Clin Dermatol. 2012;30:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 92. | Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 93. | Koster MI. Making an epidermis. Ann N Y Acad Sci. 2009;1170:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 94. | Gutowska-Owsiak D, Schaupp AL, Salimi M, Selvakumar TA, McPherson T, Taylor S, Ogg GS. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol. 2012;21:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 95. | Bangert C, Brunner PM, Stingl G. Immune functions of the skin. Clin Dermatol. 2011;29:360-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 96. | Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7:803-813; quiz 814-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 97. | Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med. 2011;39:1521-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 98. | Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 939] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 99. | Garcia-Valladares I, Cuchacovich R, Espinoza LR. Comparative assessment of biologics in treatment of psoriasis: drug design and clinical effectiveness of ustekinumab. Drug Des Devel Ther. 2011;5:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, Jackson CJ. Combination of activated protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds. 2011;10:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 930] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 102. | Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4217] [Cited by in RCA: 3824] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 103. | Shorr AF, Janes JM, Artigas A, Tenhunen J, Wyncoll DL, Mercier E, Francois B, Vincent JL, Vangerow B, Heiselman D. Randomized trial evaluating serial protein C levels in severe sepsis patients treated with variable doses of drotrecogin alfa (activated). Crit Care. 2010;14:R229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Sadaka F, O’Brien J, Migneron M, Stortz J, Vanston A, Taylor RW. Activated protein C in septic shock: a propensity-matched analysis. Crit Care. 2011;15:R89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Xue M, Dervish S, Harrison LC, Fulcher G, Jackson CJ. Activated protein C inhibits pancreatic islet inflammation, stimulates T regulatory cells, and prevents diabetes in non-obese diabetic (NOD) mice. J Biol Chem. 2012;287:16356-16364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 106. | Tuinman PR, Dixon B, Levi M, Juffermans NP, Schultz MJ. Nebulized anticoagulants for acute lung injury - a systematic review of preclinical and clinical investigations. Crit Care. 2012;16:R70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Cornet AD, Hofstra JJ, Vlaar AP, Tuinman PR, Levi M, Girbes AR, Schultz MJ, Groeneveld AB, Beishuizen A. Activated protein C attenuates pulmonary coagulopathy in patients with acute respiratory distress syndrome. J Thromb Haemost. 2013;11:894-901. [PubMed] |
| 108. | Funk DJ, Palma Vargas J, Tuttle-Newhall J, Moretti EW. The use of recombinant human activated protein C (drotrecogin alpha) in solid organ transplant recipients: case series and review of the literature. Transpl Infect Dis. 2011;13:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 110. | Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J Biol Chem. 2007;282:9251-9259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 111. | Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 2012;18:4215-4222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439-2448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 113. | Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 114. | Guo H, Singh I, Wang Y, Deane R, Barrett T, Fernández JA, Chow N, Griffin JH, Zlokovic BV. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009;29:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 115. | Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J Thromb Haemost. 2011;9:1308-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 116. | Costa R, Morrison A, Wang J, Manithody C, Li J, Rezaie AR. Activated protein C modulates cardiac metabolism and augments autophagy in the ischemic heart. J Thromb Haemost. 2012;10:1736-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |