Published online May 26, 2012. doi: 10.4331/wjbc.v3.i5.93
Revised: May 10, 2012
Accepted: May 17, 2012
Published online: May 26, 2012
Peroxisomes are intracellular organelles mediating a wide variety of biosynthetic and biodegradative reactions. Included among these are the metabolism of hydrogen peroxide and other reactive species, molecules whose levels help define the oxidative state of cells. Loss of oxidative equilibrium in cells of tissues and organs potentiates inflammatory responses which can ultimately trigger human disease. The goal of this article is to review evidence for connections between peroxisome function, oxidative stress, and inflammation in the context of human health and degenerative disease. Dysregulated points in this nexus are identified and potential remedial approaches are presented.
- Citation: Terlecky SR, Terlecky LJ, Giordano CR. Peroxisomes, oxidative stress, and inflammation. World J Biol Chem 2012; 3(5): 93-97
- URL: https://www.wjgnet.com/1949-8454/full/v3/i5/93.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i5.93
Peroxisomes are essential organelles of human cells. In this article, we review peroxisome biology; summarizing how the organelle is formed, how it functions, and what happens when these processes are compromised. In addition, we describe an emergent link between the organelle and cellular aging pathways. In the latter analysis, we connect peroxisome function with the generation and destruction of specific inflammatory mediators and speculate on the organelle’s involvement in initiating and progressing human disease.
Peroxisomes synthesize and degrade a wide variety of cellular compounds[1]. Through α- and β-oxidations, specific long-chain, very-long-chain, and 3-methyl-branched-chain fatty acids are degraded. These processes may occur entirely within the organelle or may involve participation of other organelles - e.g., mitochondria. The notion that peroxisomes shuttle metabolites for continued processing and/or anaplerotic metabolism is part of an emergent theme for the organelle; specifically, that it is integrated into a variously interacting endomembrane system responsible for a number of critical cellular processes[2].
The peroxisome’s handling of hydrogen peroxide, a reactive oxygen species produced by oxidative reactions occurring within the organelle, also bears on this point. Under most conditions, hydrogen peroxide is produced and immediately processed by the organelle’s resident marker enzyme, catalase. However, conditions exist in which the balance of hydrogen peroxide production is upset, and the potentially toxic metabolite accumulates[3-7]. As discussed further below, such phenomena set cells on a pro-aging program with potentially important health ramifications[2].
Other catabolic functions carried out by peroxisomes include degradation of polyamines, glyoxylate, certain amino acids, and several xenobiotics[1]. In addition, the organelle breaks down the arachidonic acid derivatives known as eicosanoids. Eicosanoids are critically important signaling molecules which exert tremendous control over inflammatory reactions[8]. Among the arachidonic acid derivatives under consideration here are prostaglandins, thromboxanes, leukotrienes, and prostacyclins. These compounds elicit broad ranging inflammatory reactions depending on concentration and location.
Included among the activities engendered by these molecules are modulating vasoconstriction/vasodilation and smooth muscle contraction/relaxation, controlling platelet aggregation, regulating hormone release/metabolism, and initiating pyrogenic (i.e., febrile) responses[8]. And these examples only partially represent the broad physiological effects elicited by eicosanoids. The important point is that through their ability to be metabolized by peroxisomes - organelle function is linked to the inflammatory response.
Inflammation could not be a more popular topic in current medical research. There is a sense in the clinical community that inflammation plays a major role in aging and in chronic diseases. Indeed, the term “inflammaging” has been coined and is in use. Targeting inflammation is popularly seen as a major strategy to combat these processes. Specific pathways involving the presence of reactive oxygen species and chronic activation of inflammatory pathways are examined further below.
Peroxisomes contain enzymes which contribute to the synthesis of critical cellular constituents including bile acids, ether phospholipids, and docosahexaenoic acids, among others[1]. Bile acids, derived from cholesterol, are important molecules involved in digestion through their ability to emulsify fats. Ether phospholipids, including plasmalogens, represent a vital class of membrane protective molecules, found throughout cells of the body. Myelin, the insulating layer of nerve sheaths, contains plasmalogens; absence of the ether phospholipid is associated with progressive neurological impairment[9]. Docosahexaenoic acids, peroxisomally produced omega-3 fatty acids, are the pivotal precursors of resolvins (“resolution-phase interaction products”), maresins (“macrophage mediator in resolving inflammation”), and protectins (formerly called “neuroprotectins”)[10-12]. These molecules possess potent anti-inflammatory, inflammatory resolving, and immunoregulatory activities. Importantly, conversion of docosahexaenoic acids to these biologically active mediators is accelerated by non-steroidal anti-inflammatory drugs, including aspirin, which inhibits the cyclooxygenase-2 enzyme[8].
From a functional perspective, the peroxisome is clearly a major player in cellular metabolism and a key component of organismal physiology. As to how it acquires these capacities, the answer lies in a magnificently choreographed series of biochemical processes which bring about its biogenesis[13].
Defining the origins of the peroxisome membrane has taken some time - many years in fact. Growth and division of existing organelles gained considerable early support until evidence was obtained that the endoplasmic reticulum was also providing membrane[14]. The current consensus is that both processes contribute to peroxisome membrane growth and proliferation[13]. Once assembled, the peroxisome membrane acquires additional membrane proteins including those constituting the import machinery. Although still not described in complete detail, this apparatus is known to consist of the following components: soluble receptors which recognize peroxisomal targeting signals on nascent proteins/enzymes; docking proteins which serve to concentrate and direct the receptor-ligand complex at the organelle membrane; and several molecules involved in facilitating the translocation process and recycling essential components for additional rounds of import[13]. Mechanisms are in place to recycle unneeded, damaged, or aged import factors, as well as to degrade the entire organelle when appropriate.
Many of the enzymes imported by peroxisomes oxidize substrates and produce hydrogen peroxide as a metabolic by-product. This hydrogen peroxide is normally processed to water and oxygen by catalase or other organellar peroxidases, thus maintaining oxidative balance. In recent years, several circumstances have been described in which the balance is upset and peroxisomes begin to produce excess hydrogen peroxide and related downstream reactive oxygen species. These include certain disease states in which catalase is either not produced or is unstable[15-17], as well as situations in which the enzyme is inactivated[7] or mislocalized[3,18]. Certain xenobiotics/environmental toxins appear to be able to inhibit activity of the enzyme, and aging cells are progressively less able to correctly compartmentalize the critical antioxidant enzyme.
Under conditions in which peroxisomal reactive oxygen species amass, dramatic effects on cells are seen. For example, cellular proteins, lipids, and DNA are oxidatively damaged, organelle function is compromised and metabolism is slowed[3,4,7,18].
Peroxisomes fail to form or are deficient in one or more of their constituent enzymes in a series of devastating genetic diseases described in ever greater detail over the past 30 years or so[19,20]. The severity of the clinical manifestations reflects the extent of the organelle’s impaired function. Many affected children die within the first decade of life with deficits manifest in nearly all organ systems. To date, treatment approaches have largely been limited to palliative care. Advances in gene and/or protein therapies promise to improve clinical outcomes.
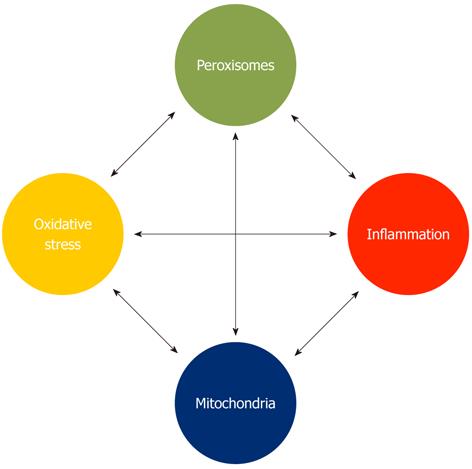
Oxidative stress and inflammation are inextricably tied processes. Chronic inflammation is associated with elevated reactive oxygen species levels; anti-inflammatory cascades are linked to diminished reactive oxygen species concentrations. And the converse is true - elevated oxidative stress triggers inflammation, whereas redox balance inhibits the cellular response. Thus, oxidative stress and inflammation may be seen as both causes and consequences of cellular pathology. We suggest here that through the peroxisome’s role in cellular redox balance, as well as its ability to synthesize various anti-inflammatory molecules and degrade pro-inflammatory mediators, the organelle is part of a critical network controlling cell function and organismal well-being (Figure 1). What is surprising is that these vital roles for the organelle have been unappreciated for so long.
We have previously argued that peroxisomes function as important communication centers - integrating signals from various sources to alter their own metabolism as well as that of other organelles, and to initiate or inhibit cellular aging programs[2]. A major redox-based interplay exists between peroxisomes and mitochondria, a relationship that warrants additional analysis. Several reports indicate that altering peroxisomal redox balance triggers oxidative stress in mitochondria - resulting in reactive oxygen species production, diminished membrane potential, and compromised organelle function[7,21]. Obviously, the cellular consequences of diminished mitochondrial function are profound. However, restoring peroxisomal redox balance - for example by supplementing (peroxisomal) catalase, renews mitochondria[18]. Mitochondria repolarize and aging cells delay appearance of senescence markers. Increasing oxidative stress in peroxisomes is “progeric” on cells; eliminating the stress revives them.
This approach of targeted antioxidant prophylaxis has also been successful in disease models. For example, in a human cell model for psoriasis, catalase supplementation reduces expression of the inflammatory cytokine, tumor necrosis factor α, that is thought to be a major initiator of the chronic inflammation seen in psoriatic tissue[22]. Similarly, in in vitro[23] and in vivo (Terlecky SR - unpublished) models of ischemia-reperfusion (heart attack), damage to cardiomyocytes and cardiac tissue is dramatically inhibited. In a rat cell model of Alzheimer’s disease, β-amyloid peptide-induced neuronal toxicity is significantly reduced (Terlecky SR - unpublished). Enhancing peroxisomal catalase also reduces inflammatory cytokine production in appropriately challenged human fibroblasts (Terlecky SR - unpublished). The evidence is mounting - peroxisome redox balance is a major determinant of cell stress and the presence or absence of cell pathology. Perhaps it is not surprising then that epidemiological studies suggest a very strong link exists between diminishing cellular catalase levels and the onset of degenerative disease[24].
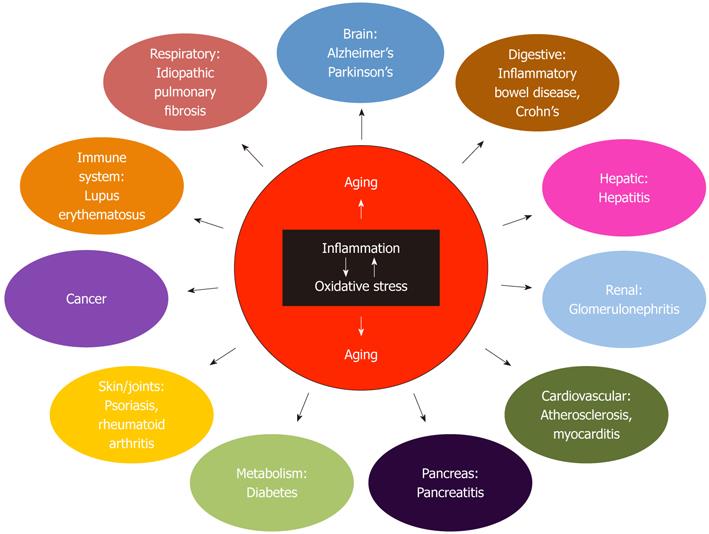
The treatment of human degenerative disease requires, in our view, a distance from the reductionist and extremely focused approaches long applied by the scientific and medical communities. Rather, we suggest a broader attack, targeting oxidative stress, chronic inflammation, and the resultant pro-aging programs initiated in cells and tissues (Figure 2). There are many ways to approach this - the direction discussed in this article focuses on the peroxisome. The organelle plays a key role in controlling inflammation and maintaining oxidative balance in cells. By targeting peroxisomes - perhaps a number of devastating diseases could be more effectively treated or prevented. We suggest enhancing peroxisome function and maintaining the organelle’s redox balance by all means possible. Mitochondrial integrity/activity will be maintained or enhanced, oxidative stress will be reduced, inflammation will be held in check, and cellular pathology will be all but eliminated. Optimistic dreams? Perhaps. But as is often said in science, “That’s why you do the experiment”.
We thank Kendra Krentz for careful reading of the manuscript.
Peer reviewers: Antonio Brunetti, MD, PhD, Professor, Cattedra di Endocrinologia, Università di Catanzaro “Magna Græcia”, 88100 Catanzaro, Italy; Manuel Vazquez-Carrera, Dr., University of Barcelona, Unitat de Farmacologia. Facultat de Farmacia. Diagoanl 643, E-08028 Barcelona, Spain
S- Editor Cheng JX L- Editor A E- Editor Zheng XM
| 1. | Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 726] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 2. | Titorenko VI, Terlecky SR. Peroxisome metabolism and cellular aging. Traffic. 2011;12:252-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Legakis JE, Koepke JI, Jedeszko C, Barlaskar F, Terlecky LJ, Edwards HJ, Walton PA, Terlecky SR. Peroxisome senescence in human fibroblasts. Mol Biol Cell. 2002;13:4243-4255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Wood CS, Koepke JI, Teng H, Boucher KK, Katz S, Chang P, Terlecky LJ, Papanayotou I, Walton PA, Terlecky SR. Hypocatalasemic fibroblasts accumulate hydrogen peroxide and display age-associated pathologies. Traffic. 2006;7:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Kawada Y, Khan M, Sharma AK, Ratnayake DB, Dobashi K, Asayama K, Moser HW, Contreras MA, Singh I. Inhibition of peroxisomal functions due to oxidative imbalance induced by mistargeting of catalase to cytoplasm is restored by vitamin E treatment in skin fibroblasts from Zellweger syndrome-like patients. Mol Genet Metab. 2004;83:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sheikh FG, Pahan K, Khan M, Barbosa E, Singh I. Abnormality in catalase import into peroxisomes leads to severe neurological disorder. Proc Natl Acad Sci USA. 1998;95:2961-2966. [PubMed] |
| 7. | Koepke JI, Wood CS, Terlecky LJ, Walton PA, Terlecky SR. Progeric effects of catalase inactivation in human cells. Toxicol Appl Pharmacol. 2008;232:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Smyth EM, FitzGerald GA. The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds. 10th ed. Basic and Clinical Pharmacology. New York: McGraw-Hill Companies 2007; 293-308. |
| 9. | Kassmann CM, Lappe-Siefke C, Baes M, Brügger B, Mildner A, Werner HB, Natt O, Michaelis T, Prinz M, Frahm J. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet. 2007;39:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 603] [Cited by in RCA: 680] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 12. | Das UN. Lipoxins, resolvins, protectins, maresins, and nitrolipids: connecting lipids, inflammation, and cardiovascular disease risk. Curr Cardio Risk Rep. 2010;4:24-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Ma C, Agrawal G, Subramani S. Peroxisome assembly: matrix and membrane protein biogenesis. J Cell Biol. 2011;193:7-16. [PubMed] [DOI] [Full Text] |
| 14. | Geuze HJ, Murk JL, Stroobants AK, Griffith JM, Kleijmeer MJ, Koster AJ, Verkleij AJ, Distel B, Tabak HF. Involvement of the endoplasmic reticulum in peroxisome formation. Mol Biol Cell. 2003;14:2900-2907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Eaton JW, Ma M. Acatalasemia. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill 1995; 2371-2383. |
| 16. | Wen JK, Osumi T, Hashimoto T, Ogata M. Diminished synthesis of catalase due to the decrease in catalase mRNA in Japanese-type acatalasemia. Physiol Chem Phys Med NMR. 1988;20:171-176. [PubMed] |
| 17. | Crawford DR, Mirault ME, Moret R, Zbinden I, Cerutti PA. Molecular defect in human acatalasia fibroblasts. Biochem Biophys Res Commun. 1988;153:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Koepke JI, Nakrieko KA, Wood CS, Boucher KK, Terlecky LJ, Walton PA, Terlecky SR. Restoration of peroxisomal catalase import in a model of human cellular aging. Traffic. 2007;8:1590-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW. Peroxisome biogenesis disorders. Biochim Biophys Acta. 2006;1763:1733-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 20. | Wanders RJ, Waterham HR. Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim Biophys Acta. 2006;1763:1707-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Ivashchenko O, Van Veldhoven PP, Brees C, Ho YS, Terlecky SR, Fransen M. Intraperoxisomal redox balance in mammalian cells: oxidative stress and interorganellar cross-talk. Mol Biol Cell. 2011;22:1440-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Undyala V, Terlecky SR, Vander Heide RS. Targeted intracellular catalase delivery protects neonatal rat myocytes from hypoxia-reoxygenation and ischemia-reperfusion injury. Cardiovasc Pathol. 2011;20:272-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Góth L, Eaton JW. Hereditary catalase deficiencies and increased risk of diabetes. Lancet. 2000;356:1820-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |