IKAROS IN CALM/AF10 RELATED LEUKEMIAS
Impaired differentiation is a hallmark in cancer. Hematopoietic tumors often involve deregulation of lineage specific transcription factors such as Ikaros in the lymphoid or CEBPA in the myeloid compartment[1,2]. Mutations in these key regulators of differentiation are frequently found in leukemias which morphologically resemble immature cells of the affected lineage. For example, genomic alterations of Ikaros are found in acute lymphoblastic leukemia (ALL) and CEBPA mutations are associated with acute myeloid leukemia (AML)[3,4]. Unsurprisingly, an impaired differentiation machinery may cause accumulation of non-functional early cells. However, disturbed Ikaros function may not only lead to blocked lymphoid differentiation, but also to myeloid transdifferentiation as a result of the blocked lymphoid differentiation pathway.
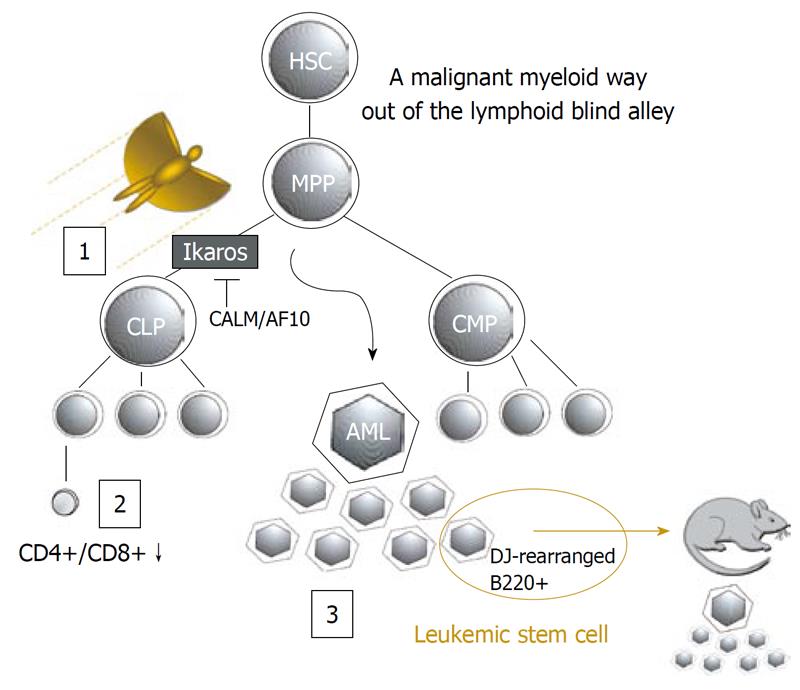
In particular, this mechanism could be relevant in CALM/AF10 positive AML, since expression of the CALM/AF10 fusion protein alters the subcellular localization of Ikaros[5]. The fusion of the CALM and AF10 genes results from the t(10;11)(p13;q14) translocation and can be found as the sole cytogenetic abnormality in ALL, AML and in malignant lymphomas and correlates with poor prognosis[6,7]. In a murine bone marrow transplantation model, CALM/AF10 expression results in the development of an aggressive bi-phenotypic leukemia. While the leukemic bulk is myeloid, the leukemia propagating cells show lymphoid traits including B220 surface markers and immunoglobulin heavy chain rearrangements[8] (Figure 1). This bi-phenotypic leukemia might be explained in part by the interaction between CALM/AF10 and Ikaros that results in increased cytoplasmic localization of Ikaros[5]. While the putative transcription factor AF10 shows a nuclear localization[9], CALM (Clathrin Assembly Lymphoid Myeloid Leukemia Gene) plays a role in endocytosis and localizes mainly to the cytoplasm[10]. Similar to CALM, the CALM/AF10 fusion protein shows a predominately cytoplasmic localization, however, both CALM and CALM/AF10 were shown to shuttle between the nucleus and the cytoplasm[11]. Thus, CALM/AF10 is able to interact with nuclear proteins such as Ikaros. The interaction of CALM/AF10 and Ikaros may tether Ikaros to the cytoplasm and thereby disturb Ikaros function as a transcription factor, and further, as a tumor suppressor. Similar to dominant negative Ikaros isoforms, CALM/AF10 may interfere with the formation of Ikaros homodimers that are essential for the recruitment of Ikaros target genes to pericentromeric heterochromatin[12,13]. If this occurs in early progenitor cells, lymphoid differentiation could be partially or completely blocked. Depending on the extent of the block, progenitors may either seek a detour towards the myeloid route or escape into the T-cell compartment. Interestingly, in Ikaros knock-out mice, B lymphocytes are absent, whereas T-lymphocytes are present, but severely defective[12,14]. In a similar manner, clinically healthy transgenic CALM/AF10 mice show impaired thymocyte differentiation[15] (Figure 1). CALM/AF10 is a common fusion transcript in T-ALL with a particularly high frequency in the TCRγδ lineage[16]. Recently, aberrant cytoplasmic Ikaros localization was reported to be common in T-ALL, while genomic deletions of Ikaros are rare in T-ALL[17]. It is tempting to speculate that CALM/AF10 may modulate lineage fate through interaction with Ikaros in a dose-dependent manner, depending on the expression levels of the two proteins, the exact developmental stage of the target cell and possible additional genetic alterations. A complete block of lymphoid differentiation would then result in AML, and a partial block would facilitate the onset of T-ALL. Considering the numerous known Ikaros isoforms including the myeloid specific isoform Ikaros X[18], it will be challenging to elucidate the role of the interaction between CALM/AF10 and Ikaros in malignancy.
Figure 1 Model of the CALM/AF10 Ikaros interaction during leukemogenesis (adapted from Greif et al[5], 2008).
Hematopoietic stem cells (HSC) give rise to multipotent progenitors (MPP) that divide into common lymphoid (CLP) and myeloid progenitors (CMP). Ikaros is required for the maturation of lymphoid progenitors. We propose that CALM/AF10 alters its subcellular localization[5] and thereby disturbs Ikaros function at this stage leading to impaired thymocyte differentiation[15] and phenotypically acute myeloid leukemia (AML) with lymphoid characteristics[8]
Altered Ikaros function is likely necessary but not sufficient to cause CALM/AF10 related leukemia. Leukemic fusion proteins are successful in causing leukemia, because they often affect several different regulatory circuits in their target cells. For example, CALM/AF10 was recently shown to cause chromosomal instability through its interaction with the histone methyltransferase DOT1L[19].
LINEAGE PROMISCUITY AND ABERRANT SIGNALING IN HEMATOPOIETIC MALIGNANCY
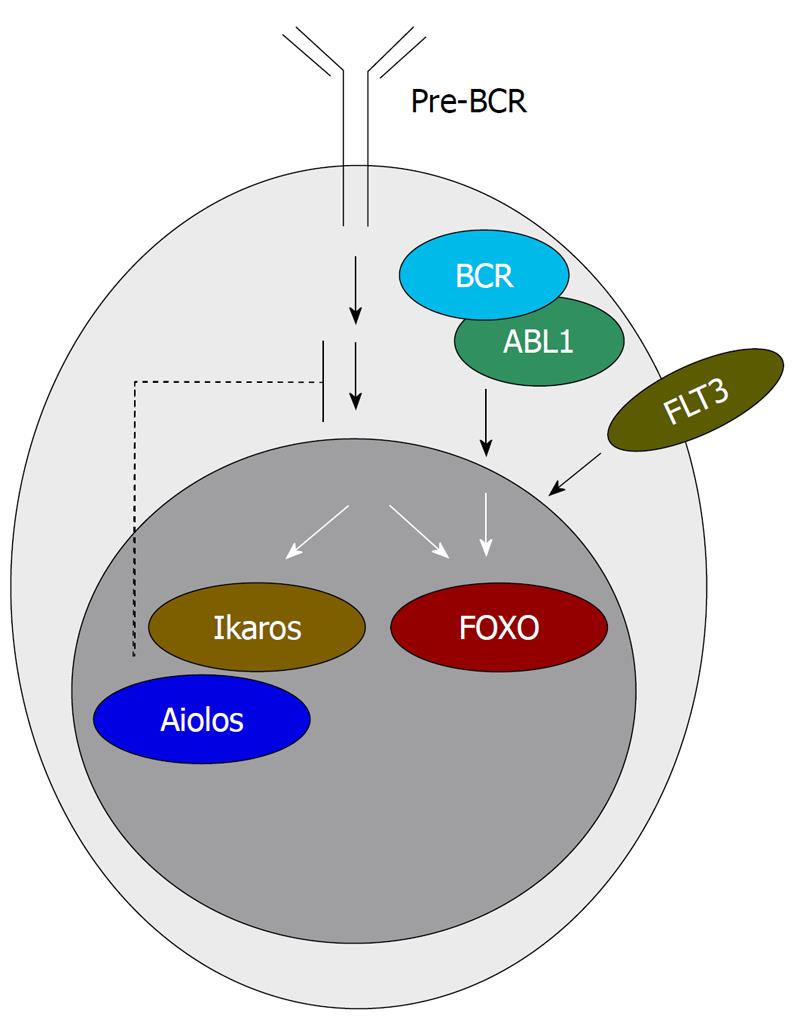
Another Ikaros-mediated lineage switch can be observed in chronic myeloid leukemia (CML). Lymphoid blast crisis during the progression of CML is often associated with acquisition of Ikaros deletions[1,20]. Apparently, in CML Ikaros mutations facilitate the development of a lymphoid blast crisis. This is in contrast to the myeloid phenotype observed in CALM/AF10-driven AML which might be a consequence of altered Ikaros function. How can these multiple consequences of disturbed Ikaros function be explained? We propose that impaired function of Ikaros might have two main consequences: (1) mutations or altered protein interactions of Ikaros prevent the cell from entering the lymphoid compartment; and (2) impaired lymphoid differentiation interrupts feed-back loops involving upstream regulators of Ikaros such as the pre-B-cell-receptor (pre-BCR). Loss of the physiological inhibition of pre-B-BCR-signaling by Ikaros and its protein family member Aiolos[21,22] (Figure 2) may result in continued or even increased signaling. Continuous pre-BCR-signaling may not only persistently push the cells towards lymphoid development, but might also cause the cell to differentiate along alternative pathways (e.g. the myeloid lineage). The pre-BCR-signaling cascade includes promiscuous players like the forkhead box O (FOXO) transcription factors[23,24] (Figure 2). In AML for example, the frequently mutated FLT3 receptor tyrosine kinase was reported to promote proliferation by signaling through FOXO proteins[25]. Increased phosphorylation of FOXO3A correlates with adverse prognosis in AML[26]. Hence, FOXO proteins might act at the interface between early lymphoid and malignant myeloid cell fate. In CML, persistent BCR/ABL1-mediated signaling towards lymphoid development might eventually break through the differentiation block after Ikaros becomes mutated resulting in lymphoid blast crisis. In particular, phosphorylation and thereby inactivation of FOXO3A through the PI3-K/Akt pathway is a potential link between BCR-ABL1 signaling and pre-BCR-signaling[27]. Gilliland and co-workers[28] postulated that at least two classes of mutations are required for leukemia development: class I mutations which increase proliferation (e.g. activating mutations in tyrosine kinases) and class II mutations which lead to a differentiation block (e.g. fusion genes like AML1/ETO)[28]. We suggest that the distinction between these two classes of mutations is often not as clear and that a single mutation might have effects on both proliferation and differentiation. One example might be mutations or functional alterations of the Ikaros protein. Ikaros mutations might not only result in a block in differentiation but might, at the same time, redirect up-stream signaling to drive the cell to differentiate along alternative pathways and thereby contribute to increased proliferation.
Figure 2 Pre-B-cell-receptor signaling is regulated by a feed-back loop via Ikaros and Aiolos.
The pre-B-cell-receptor (pre-BCR)-signaling cascade also involves FOXO transcription factors which may act as an interface between early B-cell development and FLT3-signaling that is frequently altered in acute myeloid leukemia (AML)[25]. FOXO-proteins are also regulated by downstream signaling of BCR-ABL1[27]. Disruption of Ikaros function by either deletions or protein interactions may interrupt the inhibitory feed-back loop to the pre-BCR pathway and thereby enhance pre-BCR-signaling.
In summary, the above discussed examples illustrate that the hematopoietic hierarchy is highly dynamic and that its plasticity does not only allow the diversity of specialized blood cells, but also lineage promiscuity in leukemia. The example of Ikaros demonstrates how a regulator of healthy differentiation can be converted into a driver of malignancy. There is a growing list of key hematopoietic regulators which are altered in leukemia including PAX5, GATA-1, C/EBPa and PU.1[29,30].
Ikaros is a lineage switch controlling differentiation towards the lymphoid or myeloid lineage. However, lineage fate is not always binary, rather, an intermediate position of the switch may cause the cell to be confused about its fate and become malignant. For example, the complete absence of the ets transcription factor PU.1 does not lead to the development of leukemia in knock-out mice, while lowering the levels of PU.1 to just 20% of normal levels results in leukemia[31].
If an early hematopoietic cell gets trapped in the lymphoid blind alley, it may escape through the myeloid back door and emerge as a leukemia cell.
Peer reviewer: Sherine Elsawa, PhD, Instructor in Oncology, Division of Oncology Research, Mayo Clinic, Gonda 19-300Res, 200 First St SW, Rochester, MN 55905, United States; Yu Jiang, PhD, Associate Professor, Department of Pharmacology and Chemical Biology, University of Pittsburgh, 200 Lothrop St., Pittsburgh, PA 15261, United States; Jianhui Guo, Dr., Stanley S. Scott Cancer Center, Louisiana State University Health Sciences Center, 3535 Apollo Drive, Apartment K224, Metairie, LA 70003, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM