Published online Jan 7, 2019. doi: 10.4331/wjbc.v10.i1.7
Peer-review started: August 28, 2018
First decision: October 16, 2018
Revised: October 26, 2018
Accepted: November 26, 2018
Article in press: November 26, 2018
Published online: January 7, 2019
Processing time: 121 Days and 21 Hours
Patients with autism spectrum disorders (ASD) present deficits in social interactions and communication, they also show limited and stereotypical patterns of behaviors and interests. The pathophysiological bases of ASD have not been defined yet. Many factors seem to be involved in the onset of this disorder. These include genetic and environmental factors, but autism is not linked to a single origin, only. Autism onset can be connected with various factors such as metabolic disorders: including carnitine deficiency. Carnitine is a derivative of two amino acid lysine and methionine. Carnitine is a cofactor for a large family of enzymes: the carnitine acyltransferases. Through their action these enzymes (and L-carnitine) are involved in energy production and metabolic homeostasis. Some people with autism (less than 20%) seem to have L-carnitine metabolism disorders and for these patients, a dietary supplementation with L-carnitine is beneficial. This review summarizes the available information on this topic.
Core tip: Autism spectrum disorder is characterized by impaired communication, altered social skills, stereotypical behaviors and limited interests. The pathophysiological bases of autism have not been defined yet. Several publications have pointed a possible connection between autism and carnitine deficiency. Carnitine is a cofactor for a large family of enzymes: the carnitine acyl transferases. Through their action these enzymes are involved in energy production and metabolic homeostasis. Low plasma carnitine were reported in autism patients and for some of them, defects in L-carnitine metabolism have been reported. This review summarizes the available information on the possible link between autism and carnitine.
- Citation: Demarquoy C, Demarquoy J. Autism and carnitine: A possible link. World J Biol Chem 2019; 10(1): 7-16
- URL: https://www.wjgnet.com/1949-8454/full/v10/i1/7.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v10.i1.7
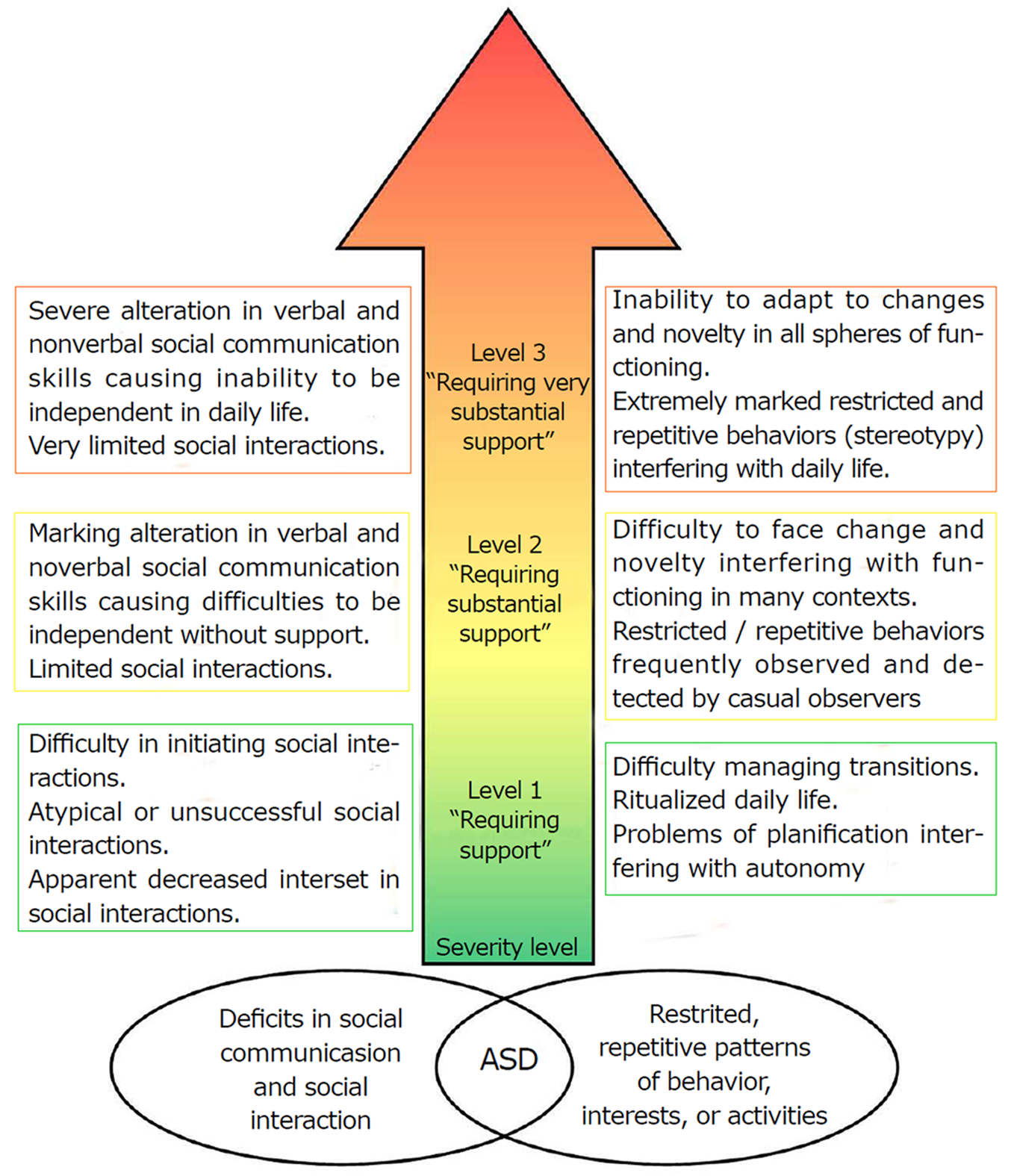
Autism is a heterogeneous neurodevelopmental disorder actually known as Autism spectrum disorders (ASD). It is characterized by a dyad of symptoms: impaired communication and altered social skills[1]. ASD symptoms include (1) persistent deficits in initiating and sustaining social interaction and social communication; and (2) limited, repetitive and stereotypical patterns of behaviors and interests.
The impact of ASD on people can be very dissimilar: some people with ASD can live independently, others require life-long care and support. The onset of this disease occurs, typically, in early childhood usually before the age of three. For some patients, the symptoms will become apparent later when daily autonomy requirements will not be handled by the patient. According to the Diagnostic and Statistical Manual of mental disorders (DSM-5®), the symptoms must appear during the childhood to be considered as autism[1].
Most people affected by autism are healthy otherwise but for some of them, autism is associated with other health problems. Among those pathologies associated with autism, one can find metabolic disorders such as phenylketonuria, chromosomal abnormalities, infectious diseases such as rubella or neurocutaneous disorders.
In most cases, deficits are severe enough to affect the personal life: the different aspects of social, educational, occupational life are generally affected in people affected by ASD. The spectrum is large and among patients, a full range of mental abilities and communication skills can be observed[2].
In the literature, some theories explain autism by cognitive deficit. Baron-Cohen studied the hypothesis of a deficit of theory of mind and showed that patients with ASD have difficulties in imputing mental states (beliefs, desires, intentions, emotions, etc.) to others[3]. It has been proposed that people with ASD have a deficit of executive functions. Evaluations of cognitive functions objectivize deficits in inhibition, cognitive flexibility and working memory. Happé and Frith[4] developed another theory indicating that a person with ASD has a weak central coherence; this theory suggested that a person with autism tends to be more focused on details: the perception of a person with ASD can be defined as fragmented. There are other theories that have been developed to explain what happens in ASD patients: like the perception and sensory theory[5].
When ASD is diagnosed in childhood, the symptoms tend to persist during adolescence and adulthood.
With appropriate interventions, the autistic spectrum can be improved: behavioral treatment can improve communication and social behavior, usually associated with a positive impact on wellbeing for people with ASD and their family.
In the 60th, the prevalence of ASD was estimated to be around 4-5 in 10000, today this number is around 100 (or even more) in 10000 people[6,7] and some authors, even reported a prevalence of 3.6%[7]. This increase may not find its origin in genetic and thus, environmental factors may play a role in the onset of ASD[8]. This rise may also be due to a more efficient diagnosis and a better detection of the disease. For ASD, a male-to female ratio of 3.75:1 has been found[9].
ASD can be diagnosed as early as 2 years old, but most children are not diagnosed with ASD until the age of 4. Usually, the age of diagnosis depends of the severity of ASD. The DSM-5 defined 3 severity levels which depend on the requiring support. A schematic representation of the major features found in ASD patients is summarized in Figure 1.
Parents who have a child with ASD have a 2%-18% risk of having a second child who is also affected[10]. This data can be linked to the genetic aspects of ASD.
ASD is found in every country and in every ethnic group and in both sexes. Reports indicate that prevalence might be different according to the ethnical origin[11] even if contradictory data suggest that more standardized protocol for diagnosing are required. The differences among these numbers in various countries may also be due to the lack of homogeneity that exists all around the world for diagnosing autism. Professionals agreed to homogenize diagnostic criteria. The main diagnostic guides i.e., the DSM-5 edited by the American Psychological Association (APA) and the International Classification of Diseases 11th Revision (ICD-11) published by the World Health Organization (WHO) are considering this problem.
For several years, ASD is recognized as a public health problem, some people consider ASD as becoming epidemic and as a target for health policy. The support of people with ASD is costly; in fact, people with ASD need more medical examinations and drug prescriptions than most healthy people. An important proportion of children with ASD requires special educational services and some stay in health institution at adulthood. In 2014, in the United States, the total cost for children with ASD was estimated between 11.5 billion to 60.9 billion United States dollars per year[12].
The pathophysiological origin of ASD has not been defined yet. Many factors have been suggested including genetic and environmental factors. These aspects are extensively detailed in a recent review[13]. What seems clear today is the fact that autism is not linked to a single origin. Autism can be associated with many factors, and among those, metabolic disorders can possibly increase the risk of the development of autism.
Some people with ASD also have other health problems, including anxiety and depression, epilepsy, attention deficit hyperactivity disorder (ADHD). In people with ASD, the intellectual level is extremely variable, ranging from profound impairment to higher levels.
In most cases, the etiology of ASD is not known, but a genetic factor, involving possibly 15 or more loci, is widely has been proposed for contributing to the development of ASD[14]. ASD traits are also found in patients affected by several genetic diseases such as Rett syndrome or Angelman syndrome[15].
The strong heterogeneity among individuals with ASD has limited the pure genetic implication[16].
Among potential environmental factors the role of perinatal factors was studied by Gardener et al[17], they performed a meta-analysis of the association between perinatal and neonatal factors and the risk of autism. In this study they described associations between more than 60 potential perinatal risk factors and ASD[17].
Durkin et al[18] showed that the age of the parents is associated with the risk of ASD for the children. They noticed that the risk is even more pronounced for the elder children. Premature birth has been identified as a risk factor[19]. Prematurity (infants born at < 37 wk of gestation) and low birth weight (< 2500 g) have also been examined as a risk factor for the development of ASD, among several other potential risk factors[20]. Recently a few studies have associated ASD and pesticides: pesticide exposures during pregnancy is a risk factor for ASD[21].
Some people with ASD have metabolic disorders and/or health problems such as mitochondrial dysfunction and gastrointestinal abnormalities. More than thirty years ago, Coleman and Blass[22] suggested an abnormality in carbohydrate metabolism in individuals with ASD, several years later, it was proposed that ASD may be a disorder associated with an impairment in mitochondrial function[23]. Recently a meta-analysis examined the possible link between mitochondria and autism, the conclusions of this study are that it is not clear if mitochondrial dysfunction contributes to the development or pathogenesis of ASD or if mitochondrial dysfunction is just an epiphenomenon of ASD[14].
Carnitine occurs in two racemic forms: L- and D-carnitine. In the human body, only the L-isomer is present. L-carnitine is an amino acid derivative found in almost any cell in the body. When discovered, a century ago, L-carnitine was considered as a vitamin as it was shown that the development of a worm (Tenebrio Molitor) was dependent on L-carnitine. Several decades later, it was shown that mammals are able to synthesize L-carnitine and subsequently, L-carnitine was not considered as a vitamin anymore.
L-carnitine is mainly found in muscles where it plays a major role in the use of fatty acid for energy production and carnitine found in the human body can either come from an endogenous synthesis or from the foodstuffs.
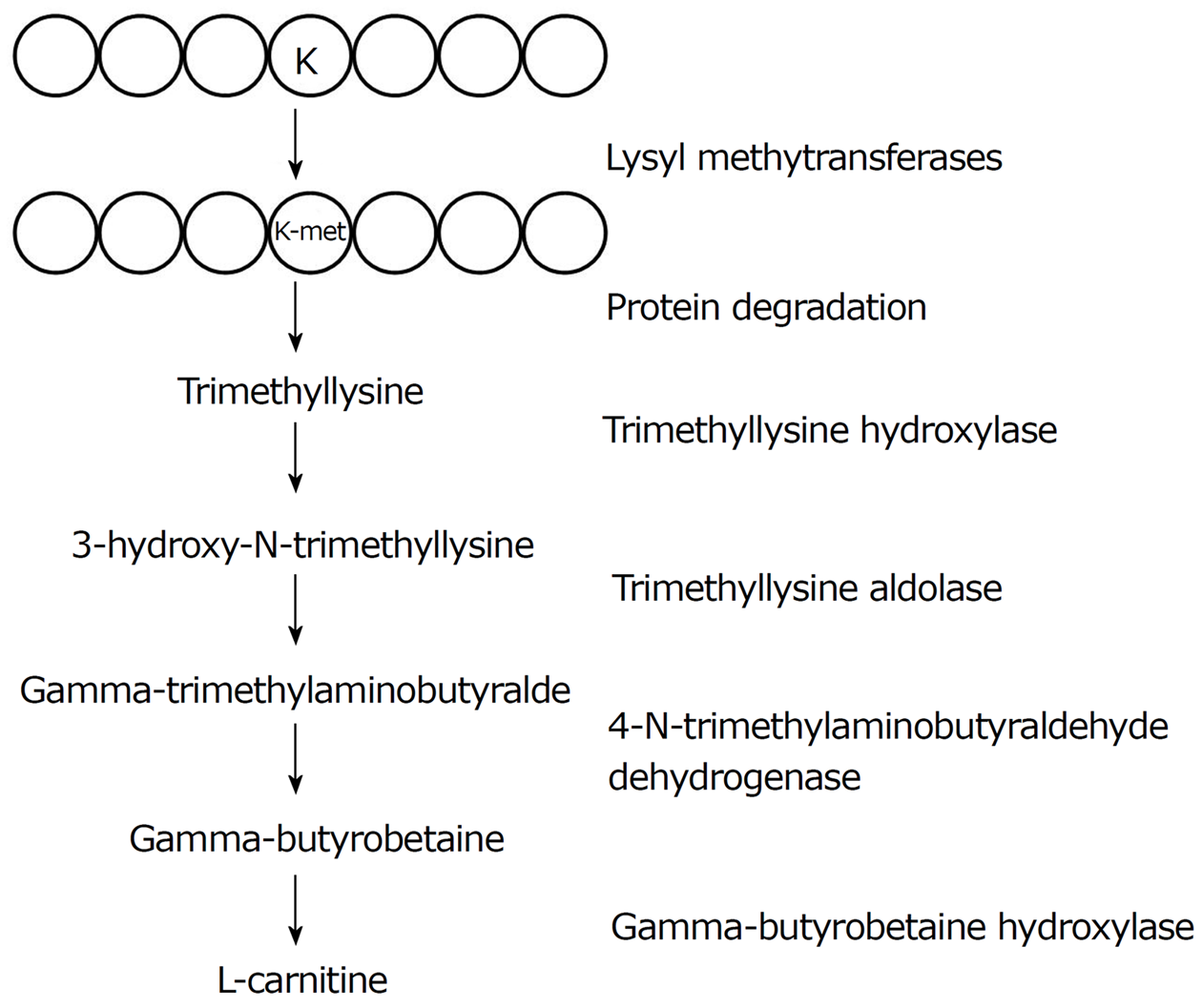
L-carnitine biosynthesis is performed with 2 ultimate precursors: lysine and methionine and the enzymatic reactions involved in this synthesis requires several cofactors: vitamin C, iron, vitamin B6 and niacin (Figure 2).
The first step corresponds to the methylation of lysyl residues included in various proteins such as histones, cytochrome c or calmodulin. This reaction is catalyzed by enzymes known as protein lysyl methyltransferases. The product of this reaction is trimethyllysyl residues which are released from proteins by protein hydrolysis as free trimethyllysine (TML).
Subsequently, TML enters the mitochondria and interacts with the trimethyllysine hydroxylase (TMLD, encoded by the trimethyllysine hydroxylase epsilon gene: TMLHE) which converts TML into 3-hydroxy-N-trimethyllysine[24]. 3-hydroxy-N-trimethyllysine is then cleaved into gamma-trimethylaminobutyraldehyde, a reaction catalyzed by hydroxyl N-trimethyllysine aldolase (HTMLA)[25].
Gamma-trimethylaminobutyraldehyde is then dehydrogenated and forms gamma-butyrobetaine a reaction catalyzed by the 4-trimethylammoniobutyraldehyde dehydrogenase. Finally, L-carnitine is formed by the hydroxylation of gamma-butyrobetaine a reaction catalyzed by the gamma-butyrobetaine hydroxylase (BBOX1).
L-carnitine biosynthesis involves different organelles (the nucleus, the mitochondria, the peroxisome and the cytosol) in various tissues and organs: kidney, liver, brain, etc.[26]. Between 1 and 2 μmol of carnitine are synthesized/kg b.w. per day in a human body.
L-carnitine is mainly present in meat and meat products, dairy and fishes provide also a significant amount of carnitine. Most fruits and vegetables are not riche in L-carnitine. An omnivorous diet brings about 50 to 100 mg of carnitine per day, 80% coming from meat while a vegetarian diet brings around 10 mg of carnitine/day.
For regular foods, L-carnitine bioavailability varies from 54% to 87%[27] and for dietary supplements, the bioavailability is only around 15%.
In Human, L-carnitine concentration in muscles is around 3 and 6 µmol of per gram making muscle the major reservoir for L-carnitine in the body, however, it has been shown that L-carnitine present in the muscle does exchange easily with the plasma and muscle is unable to synthesize carnitine and relies on L-carnitine synthesized elsewhere in the body or from the dietary carnitine. In contrast, L-carnitine level in the liver is much less than in the muscle (around 0.5 to 1 µmol of L-carnitine per g of tissue) but the hepatic carnitine can be quickly released in the plasma. High levels of L-carnitine are also found in the testes and the brain.
There is no evidence for a catabolism of L-carnitine in mammalian cells and L-carnitine is eliminated as it from the body in the urine.
L-carnitine can be synthesized in several organs (liver, kidney, testis and brain), and can be provided by the foodstuffs. In the human body, carnitine is mainly found in muscles and as muscles are unable to synthesize carnitine they rely on an active transport across the sarcolemma to provide L-carnitine to muscle cells. L-carnitine transport across membrane is done by transporters. The major transporters for L-carnitine belongs to the organic cation transporter (OCTN) family. L-carnitine transport is done through the activity of three transporters: OCTN1 (SLC22A4) and OCTN2 (SLC22A5) in humans and animals and Octn3 (Slc22a21) in mice. A defect in OCTN2 is known to induce primary systemic carnitine deficiency (SCD) a defect that leads to alteration in beta-oxidation of long-chain fatty acids, causing various symptoms, such as myopathy, cardiomyopathy, fatty liver and male infertility[28].
Secondary carnitine deficiency is due to defects in other metabolic pathways or to drugs that impair intestinal or renal absorption of L-carnitine. The consequences of this deficiency are basically the same than those observed in primary deficiency.
The major actions of carnitine are due to the fact that carnitine acts as a cofactor for a large family of enzymes: the carnitine acyltransferases. These enzymes are responsible for the esterification of carnitine with acyl groups, allowing the formation of acyl-carnitines. The carnitine acyltransferases are widely distributed in the cell. Carnitine acetyltransferase, is an enzyme that catalyzes the esterification of carnitine into short chain acyl-coenzyme A (acyl-CoA); this activity was described in different organelles: the cytosol, the mitochondrion, the peroxisome and the endoplasmic reticulum in various tissues: the heart, the brain, the kidney, the sperm cells and the liver[29]. Carnitine octanoyltransferase (CrOT) is an enzyme required for the peroxisomal metablism of very long-chain fatty acids and branched-chain fatty acids[30]. Carnitine palmitoyltransferase 1 (CPT 1) is an enzyme located on the outer mitochondrial membrane: it catalyzes the esterification of long chain acyl-coenzyme A to acyl-carnitine. Carnitine palmitoyltransferase 2 (CPT 2) is present in the inner mitochondrial membrane; it catalyzes the conversion of acyl-carnitine back to acyl-coenzyme A and together with CPT 1 and a transporter the carnitine acyl-carnitine translocase allows for the transport of acyl-Co across mitochondrial membranes. Once in the mitochondrial matrix, acyl-CoA can be used in several metabolic pathways: primarily the beta-oxidation.
Mitochondrial metabolism of long chain fatty acids: The mitochondrial metabolism of long chain fatty acids requires several steps: one of them is the entry of fatty acids inside the mitochondria. This transport is done through a system known as the carnitine system. This 3-protein complex is composed of 2 enzymes: the carnitine palmitoyl transferases 1 and 2 and a transporter: the carnitine acyl-carnitine translocase (CACT). Together these 3 proteins allow the transfer of activated fatty acids from the cytosol to the mitochondrial membrane. In this process, L-carnitine is a key compound that is involved in all the steps of this pathway. Once in the mitochondria matrix, activated fatty acids can enter the beta oxidation pathway and generate energy[31].
Mitochondrial acyl-CoA/free CoA ratio control: Coenzyme A is one of the key compounds in cell physiology and many pathways use this cofactor. Coenzyme A may be present in the cell either as a free compound or bound to various molecules (e.g. acyls). Inside the cell, a stable equilibrium between free and acylated CoA exists but this equilibrium may be destabilized. To restore this equilibrium, several process can be activated, one involves L-carnitine. This process involves (1) an increase in acyl-carnitine synthesis which leads to (2) an increase in the mitochondrial beta oxidation. Together these two events induce an increase in the level of free coenzyme and restore the free/acylated coenzyme A ratio[32].
Peroxisomal beta oxidation: In the peroxisome; the β-oxidation of very long chain fatty acids leads to the formation of medium chain-acyl CoAs and acetyl CoA, and is sometimes considered as incomplete contrarily to the mitochondrial beta oxidation which leads to the formation of acetyl-coA only[31]. In the peroxisome, two enzymes dependent on L-carnitine are involve in the beta oxidation of fatty acids. These enzymes are the carnitine acetyltransferase and the carnitine octanoyltransferase. These enzymes seem to be necessary for the exit of medium-chain and short-chain acyl-CoAs from the peroxisomal matrix to the cytosol[30] but the precise role of these enzymes is not clearly defined yet.
Acetylation of histones: Acetylation and deacetylation of histones are crucial mechanisms for the regulation of the transcription. Acetylation requires available acetyl-CoA and it has been suggested that acetyl-carnitine formed in mitochondria[32] can enter the nucleus and be converted into acetyl-CoA and then be used for histone acetylation[33].
Free radical production: Free radicals may interact with various molecular species: lipids, nucleic acids or proteins leading to altered cell function. Several studies have proposed that a dietary supplementation in L-carnitine may exert a protective effect against the deleterious effects of free radicals[34], however, the mechanisms involved in this potential protective effect remain unclear[35].
Although the spectrum of autism is thought to be highly heritable, and may result from multigene susceptibility interactions, no single gene has been identified that can adequately explain the disorder’s complex heterogeneity and alarmingly increasing prevalence.
Researches carried out on this topic have pinpointed many genetic variations, including genes involved in carnitine biosynthesis and glutamatergic transmission, which may augment susceptibility to neurodevelopmental disorders like ASD.
The links between L-carnitine and autism rely on 3 major observations (1) the alteration of mitochondrial function occurring in patients with ASD. This aspect has been several times reviewed in the literature and will not be detailed in the present paper (for more information: see ref. [36]); (2) the relationships between L-carnitine levels and the severity of autism and (3) the genetic aspects of autism associated with L-carnitine metabolism. These two aspects are detailed in the present review.
A few publications have looked at carnitine levels in patients with ASD and one single publication has studied the effect of a L-carnitine supplementation on ASD patients.
The study of Filipek et al[37] measured the level of total and free carnitine in control and ASD patients. They observed that total and free carnitine values were significantly reduced in the autistic individuals with a P < 0.001 and that more than 80% of ASD patients have total and free carnitine levels below the reference value. This information was also reported by another group in 2005[38]. Mostafa et al[38] also reported a decrease in L-carnitine levels in ASD patients. They described a decrease of almost 50% in L-carnitine levels in ASD individuals. They also reported decreases in lactate levels and poly unsaturated fatty acid levels. This study was done on a significant number of individuals (30 control vs 30 ASD).
Besides this purely quantitative aspect, Frye et al[39] analyzed the acyl-carnitine profile of ASD patients, in a large cohort (n = 213), these authors reported that 17% of children with ASD exhibited an elevation in short-chain and long-chain, but not medium-chain, acyl-carnitines. These authors indicated that this pattern of acyl-carnitine abnormalities is similar to what is observed in the brain of propionic acid rodent, model of ASD. This defect in L-carnitine levels is associated with mitochondrial dysfunction.
The acyl-carnitine composition was studied in a population of Chinese children with ASD. The goal of this publication was to identify a possible relation between the acyl-carnitine profile and the intelligence level. This study was carried out on 90 children: 60 with ASD and 30 control. The intelligence level was assessed using the Chinese Wechsler Young Children Scale of Intelligence (C-WYCSI). Blood analysis for L-carnitine derivatives showed that glutaryl carnitine and carnosyl carnitine were significantly decreased in ASD group and the authors of this publication suggested that those 2 compounds may be potential biomarkers for diagnosis of ASD. For these authors, these alterations indicate a potential mitochondrial dysfunction leading to an abnormal fatty acid metabolism in children with ASD[40].
L-carnitine was used as a potential treatment for patients diagnosed with autism. In the study published by Fahmy, 30 children diagnosed with autism (median age 69 mo, ranging from 29 mo to 103 mo) were randomly allotted into either the placebo (n = 14) group or the group receiving 100 mg/kg bodyweight per day of liquid L-carnitine (n = 16) for 6 mo. Several parameters were analyzed in these children: parameters associated with autism such as the childhood autism rating scale (CARS) form and parameters related to carnitine such as free and total carnitine levels. The results presented by the authors revealed a significant improvement in CARS scores in patients receiving L-carnitine, this improvement was associated with an increase in total and free carnitine levels. The authors concluded about (1) the good tolerance of the treatment; and (2) the improvement of autism severity in patient treated for 6 mo with L-carnitine, they also concluded that subsequent studies will be welcome[41].
In conclusion, the analysis of studies carried out on patients with autism for parameters related to L-carnitine suggest that L-carnitine may be lowered in patients with ASD. Furthermore, a L-carnitine supplementation seems to improve the symptoms of autism in patients. However, the relatively low number of publications describing these parameters implies some moderation.
With the overall goal to identify exonic copy number variants in the genome of ASD patients, Celestino-Soper and coauthors[42] analyzed 3743 samples for detecting disease-causing copy number variants (CNVs) that are not detected by most techniques used in conventional research and clinical diagnosis laboratories. This was done on 297 samples from 99 trios (probands with ASD, mother, father). Fifty-five potentially pathogenic CNVs were identified and validated and in a male proband, an exonic deletion of the TMLHE.
Using genome-wide chromosomal analysis methods, Celestino-Soper and coauthors[43] identified 55 potentially pathogenic copy number variants, among those and in the male samples, a deletion of exon 2 of the TMLHE gene (trimethyllysinehydroxylase epsilon) was found, this gene encodes the first enzyme in the biosynthesis of carnitine which is located in mitochondria. The lack of this enzyme leads to a decrease in plasmatic levels of 3-hydroxy-6-N-trimethyllysine andγ-butyrobetaine and in an increase in 6-N-trimethyllysine concentration in the plasma.
In the same time, the entire chromosome X exome was analyzed by next-generation sequencing in 12 unrelated families with ASD affected males. Thirty-six possibly deleterious variants were found located in 33 candidate genes. Among those a mutation in TMLHE, was identified in two brothers with autism. The screening of the TMLHE coding sequence in 501 male patients with ASD allowed the identification of 2 additional missense substitutions. These mutations were shown to induce a loss-of-function and led to an increase in trimethyllysine in the plasma of patients[44].
Based on these observations, a 4-year-old male with a mutation in the TMLHE gene and developing an autism spectrum disorder was supplemented with L-carnitine (200 mg/kg per day). In this young patient, the levels of carnitine were very low. The authors reported that two weeks after the initiation of the treatment, his family reported “noticeable increases in language, non-verbal expression, and engagement with others”[45]. The evolution was positive as the patient's regression ended and even started progressing. In the same time, the levels of carnitine in the plasma increased. Under such circumstances (with a TMLHE deficit) a supplementation in L-carnitine seemed efficient.
More recently, another potential mechanism involving the role of carnitine in the onset of autism has been propose, it implies a defect in the transport of L-carnitine into the cells. The amino acid transporter SLC7A5, also able to transport carnitine, has been recently shown to be associated with the onset of autism[46]. The same transporter seems also to be implicated in the metabolism of drugs such as Risperidone given for limiting the symptoms in ASD patients. Depending on the polymorphism of the gene, the drug can be differently catabolized[47]. One might notice that the transporter SLC7A5 is not purely a transporter of carnitine but a protein able to transport various amino acids.
A few aspects in the relation between carnitine and autism should be highlighted: (1) Low plasma carnitine is reported in autism, but not systematically. Furthermore, it seems that if some affected infants have low plasma carnitine during the early childhood, their plasma carnitine return to normal when measured a few years later[48]; (2) Typically, L-carnitine levels are measured in the plasma, but it is very likely that the important levels of L-carnitine for brain development should be measured in the brain. And little to no information are available on these values; and (3) In any cases, not all patients with ASD have altered levels of carnitine.
In conclusion, some patients with ASD might have L-carnitine synthesis disorders (around 10%-20%) and for these patients, a supplementation with L-carnitine is beneficial. It is still remaining 80%-90% of the patients who have no L-carnitine synthesis or transport defects and for whom the origin of the disease should be find elsewhere.
Manuscript source: Invited manuscript
Specialty type: Biochemistry and molecular biology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Haggar M, Cheng TH S- Editor: Ma YJ L- Editor: A E- Editor: Wu YXJ
| 1. | American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013. |
| 2. | Lincoln AJ, Courchesne E, Kilman BA, Elmasian R, Allen M. A study of intellectual abilities in high-functioning people with autism. J Autism Dev Disord. 1988;18:505-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 104] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a "theory of mind"? Cognition. 1985;21:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4327] [Cited by in RCA: 3306] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 4. | Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1974] [Cited by in RCA: 1480] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 5. | Bogdashina O. Sensory perceptual issues in autism and asperger syndrome: different sensory experiences-different perceptual worlds. Jessica Kingsley Publishers. Jessica Kingsley Publishers, 2016. |
| 6. | Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 982] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 7. | Fombonne E. Editorial: The rising prevalence of autism. J Child Psychol Psychiatry. 2018;59:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Grabrucker AM. Environmental factors in autism. Front Psychiatry. 2013;3:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1-20. [PubMed] |
| 10. | Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488-e495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1031] [Cited by in RCA: 895] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 11. | Becerra TA, von Ehrenstein OS, Heck JE, Olsen J, Arah OA, Jeste SS, Rodriguez M, Ritz B. Autism spectrum disorders and race, ethnicity, and nativity: a population-based study. Pediatrics. 2014;134:e63-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Lavelle TA, Weinstein MC, Newhouse JP, Munir K, Kuhlthau KA, Prosser LA. Economic burden of childhood autism spectrum disorders. Pediatrics. 2014;133:e520-e529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 13. | Fakhoury M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int J Dev Neurosci. 2015;43:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17:290-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 675] [Cited by in RCA: 604] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 15. | Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | Rossignol DA, Bradstreet JJ. Evidence of mitochondrial dysfunction in autism and implications for treatment. Am J Biochem Biotech. 2008;4:208–217. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 417] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 18. | Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, Kirby RS, Leavitt L, Miller L, Zahorodny W. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168:1268-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Ouss-Ryngaert L, Alvarez L, Boissel A. [Autism and prematurity: state of the art]. Arch Pediatr. 2012;19:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Darcy-Mahoney A, Minter B, Higgins M, Guo Y, Williams B, Head Zauche LM, Birth K. Probability of an Autism Diagnosis by Gestational Age. Newborn Infant Nurs Rev. 2016;16:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect. 2014;122:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 22. | Coleman M, Blass JP. Autism and lactic acidosis. J Autism Dev Disord. 1985;15:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Lombard J. Autism: a mitochondrial disorder? Med Hypotheses. 1998;50:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 412] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 25. | Strijbis K, Vaz FM, Distel B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life. 2010;62:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Rigault C, Le Borgne F, Demarquoy J. Genomic structure, alternative maturation and tissue expression of the human BBOX1 gene. Biochim Biophys Acta. 2006;1761:1469-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Evans AM, Fornasini G. Pharmacokinetics of L-carnitine. Clin Pharmacokinet. 2003;42:941-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Demarquoy J. L‐carnitine: Structure and Function. In: eLS John Wiley Sons Ltd, Chichester 2011; . [DOI] [Full Text] |
| 29. | Bloisi W, Colombo I, Garavaglia B, Giardini R, Finocchiaro G, Didonato S. Purification and properties of carnitine acetyltransferase from human liver. Eur J Biochem. 1990;189:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Le Borgne F, Ben Mohamed A, Logerot M, Garnier E, Demarquoy J. Changes in carnitine octanoyltransferase activity induce alteration in fatty acid metabolism. Biochem Biophys Res Commun. 2011;409:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Demarquoy J, Le Borgne F. Crosstalk between mitochondria and peroxisomes. World J Biol Chem. 2015;6:301-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 32. | Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, Kroemer G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 2015;21:805-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 977] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 33. | Madiraju P, Pande SV, Prentki M, Madiraju SR. Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics. 2009;4:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Le Borgne F, Ravaut G, Bernard A, Demarquoy J. L-carnitine protects C2C12 cells against mitochondrial superoxide overproduction and cell death. World J Biol Chem. 2017;8:86-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Surai , P.F . Antioxidant action of carnitine: molecular mechanisms and practical applications. EC Veterinary Science. 2015;vol. 2, no 1, p. 66-84. |
| 36. | Rose S, Niyazov DM, Rossignol DA, Goldenthal M, Kahler SG, Frye RE. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol Diagn Ther. 2018;22:571-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 37. | Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Mostafa GA, El-Gamal HA, El-Wakkad AS, El-Shorbagy OE, Hamza MM. Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatry. 2005;2:179–188. |
| 39. | Frye RE, Melnyk S, Macfabe DF. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry. 2013;3:e220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 40. | Lv QQ, You C, Zou XB, Deng HZ. Acyl-carnitine, C5DC, and C26 as potential biomarkers for diagnosis of autism spectrum disorder in children. Psychiatry Res. 2018;267:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Fahmy SF, El-hamamsy MH, Zaki OK, Badary OA. l-Carnitine supplementation improves the behavioral symptoms in autistic children. Res Autism Spectr Disord. 2013;7:159–66. [DOI] [Full Text] |
| 42. | Celestino-Soper PB, Shaw CA, Sanders SJ, Li J, Murtha MT, Ercan-Sencicek AG, Davis L, Thomson S, Gambin T, Chinault AC. Use of array CGH to detect exonic copy number variants throughout the genome in autism families detects a novel deletion in TMLHE. Hum Mol Genet. 2011;20:4360-4370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Celestino-Soper PB, Violante S, Crawford EL, Luo R, Lionel AC, Delaby E, Cai G, Sadikovic B, Lee K, Lo C. A common X-linked inborn error of carnitine biosynthesis may be a risk factor for nondysmorphic autism. Proc Natl Acad Sci USA. 2012;109:7974-7981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Nava C, Lamari F, Héron D, Mignot C, Rastetter A, Keren B, Cohen D, Faudet A, Bouteiller D, Gilleron M. Analysis of the chromosome X exome in patients with autism spectrum disorders identified novel candidate genes, including TMLHE. Transl Psychiatry. 2012;2:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Ziats MN, Comeaux MS, Yang Y, Scaglia F, Elsea SH, Sun Q, Beaudet AL, Schaaf CP. Corrigendum to "Improvement of regressive autism symptoms in a child with TMLHE deficiency following carnitine supplementation". Am J Med Genet A. 2015;167A:2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Tărlungeanu DC, Deliu E, Dotter CP, Kara M, Janiesch PC, Scalise M, Galluccio M, Tesulov M, Morelli E, Sonmez FM. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell. 2016;167:1481-1494.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 47. | Medhasi S, Pinthong D, Pasomsub E, Vanwong N, Ngamsamut N, Puangpetch A, Chamnanphon M, Hongkaew Y, Pratoomwun J, Limsila P. Pharmacogenomic Study Reveals New Variants of Drug Metabolizing Enzyme and Transporter Genes Associated with Steady-State Plasma Concentrations of Risperidone and 9-Hydroxyrisperidone in Thai Autism Spectrum Disorder Patients. Front Pharmacol. 2016;7:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Beaudet AL. Brain carnitine deficiency causes nonsyndromic autism with an extreme male bias: A hypothesis. Bioessays. 2017;39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |