INTRODUCTION
Neuronal stimulation induces transient changes in the levels of free intracellular calcium [Ca2+]i which in turn plays an important regulatory role in crucial nerve cell functions such as the release of neurotransmitters, signal transduction, induction of gene expression, synaptic plasticity, and learning and memory formation[1]. Following the transduction of the Ca2+ signal, neurons instantaneously return the [Ca2+]i to baseline levels in order to allow cells to respond to a new stimulus and to prevent the cytotoxicity associated with prolonged exposure to elevated Ca2+ levels[2]. The various mechanisms for restoring baseline resting [Ca2+]i following neuronal excitation include sequestration into the endoplasmic reticulum and mitochondria, buffering by Ca2+ binding proteins, and extrusion across the plasma membrane by the Na+/Ca2+ exchangers (NCXs) and the plasma membrane Ca2+-ATPases (PMCAs)[3,4]. The PMCA pumps are regulated by the Ca2+ sensor protein calmodulin (CaM), which binds to the C terminus, resulting in conformational changes that displace the autoinhibitory domain away from the catalytic site, thus relieving autoinhibition and causing several-fold stimulation[5,6]. The PMCAs represent the major transport system at the plasma membrane responsible for the long term regulation of resting free [Ca2+]i and counteracting transient increases that occur during Ca2+ signaling[7]. The PMCAs are encoded by four different genes that give rise to four distinct isoforms PMCA 1-4; further specialization is achieved by alternative splicing of the primary mRNA transcripts. Neurons are unique in that they express an unusually wide array of PMCA subtypes, including all four isoforms and multiple splice variants, attesting to the complexity of Ca2+ handling in these cells.
DISRUPTION OF CALCIUM HOMEOSTASIS IN THE AGING BRAIN: CONTRIBUTION OF THE PMCAs
The hypothesis that brain aging is linked with alterations in neuronal Ca2+ homeostasis was first proposed by Khachaturian[8-11]. Since then, a wide constellation of experimental evidence has emerged supporting the assertion that a disruption in the precise regulation of [Ca2+]i may be a final common pathway leading to altered neuronal function and cell death[12-16]. In the last decade, we have witnessed an extensive growth in the literature providing compelling evidence indicating that Ca2+ regulating systems in brain neurons are altered with increasing age[17-21]. Brain aging is associated with increased activation of the voltage-gated Ca2+ channels, altered Ca2+ transport across the mitochondria, and decreased activity of the NCXs and the sarco-endoplasmic reticulum Ca2+-ATPases[22-25]. It is not clear whether any single event by itself has a greater impact, or the multiplicity of defects in Ca2+ regulating systems is required before the overall disruption of Ca2+ homeostasis is observed in aged neurons.
Age-related alterations in the PMCAs were first assessed by Michaelis and coworkers[26-30]. These authors demonstrated for the first time a significant age-associated decline in the PMCA enzyme activity and ATP-dependent Ca2+ transport activity in synaptic plasma membranes (SPMs) isolated from Fisher 344 rats. We further confirmed the age-dependent decrease in PMCA activity in the more hardy Fisher 344/Brown Norway rats[31], a hybrid strain with a longer average life span and remarkable resistance to a variety of age-related pathologies[32]. The decline in PMCA activity monitored in SPMs isolated from rats at five different ages (5, 14, 22, 30 and 34 mo) representing young adults, middle-aged and aged animals is progressive with increasing biological age and does not appear to be the result of the end-stage of the brain aging process. Decrease in PMCA catalytic activity is associated with a statistically significant decrease in maximum velocity (Vmax) with no appreciable change in the affinity of the enzyme for Ca2+ (Kact)[31]. Efforts to investigate the mechanism underlying lowered PMCA activity showed that the decrease is due in part to the reduction in PMCA protein levels present in the SPMs of aged rats. An approximately 20% reduction in PMCA protein is observed at 34 mo, the highest age we tested, compared to the 5 mo young adults[31]. Age-related reduction in the SPM PMCA may be attributed to a variety of reasons such as decreased synthesis, altered stability, abnormal targeting to synaptic membranes, structural alterations leading to enhanced removal from the synaptic terminals, or modifications leading to reduced immunoreactivity, although none of these possibilities have been experimentally validated. Given that the pan PMCA antibodies we used in these studies recognized all four isoforms, it is unclear if specific isoforms/splice variants of the PMCAs are selectively influenced by the aging process or the decrease is evenly represented across all four isoforms. Lowered PMCA activity and protein levels in SPMs as observed with increasing age is likely to contribute to the disruption of Ca2+ homeostasis, a hallmark of aged neurons.
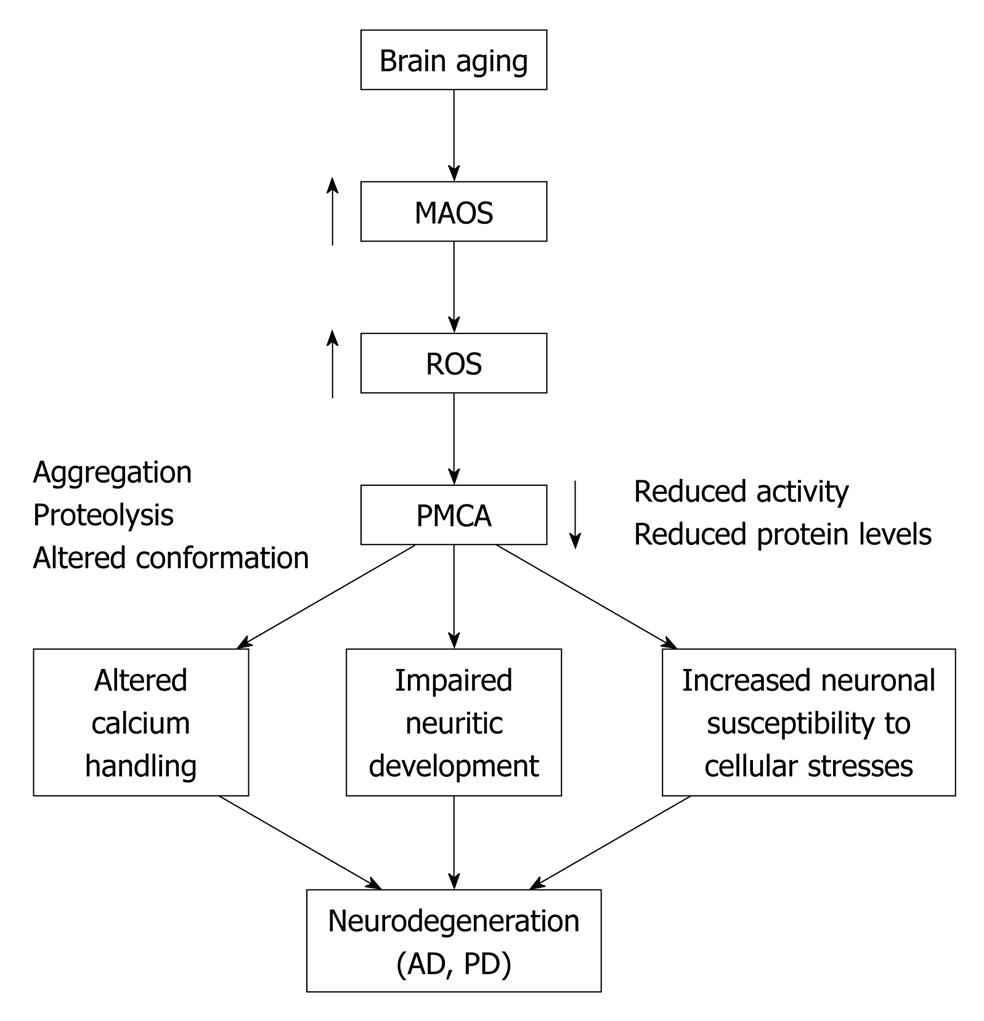
A substantial body of evidence implicates a progressive reduction in antioxidant capacity as an important contributor to the subtle alterations in cellular function associated with aging[33]. Such an imbalance in the levels of cellular antioxidants, in conjunction with an accelerated rate of generation of free radicals, can lead to lipid peroxidation and/or post-translational modification of proteins resulting in their conformational instability, structural modification and often times the accumulation of inactive or less active forms of enzyme molecules[34,35]. The approximately 40% decrease in PMCA enzyme activity observed in aged SPMs may reflect both the presence of inactive PMCA molecules in the membrane as well as enhanced clearance of the damaged protein, whereas the approximately 20% lower immunoreactivity may reflect only the enhanced removal of the PMCAs in aged brain[31]. One potential source of modification in the activity of integral membrane proteins such as the PMCAs is the possibility of alterations in the membrane lipid environment in which the protein is embedded and/or the accumulation of structural changes to the protein itself, particularly in view of its unusually long half life[36]. The high abundance of oxidation-sensitive polyunsaturated fatty acyl chains in synaptosomal membranes makes it likely that the age-related loss in PMCA catalytic turnover may be due to lipid peroxidation and consequent changes in the physicochemical properties of the membrane bilayer. Our studies, utilizing HPLC analysis of the 2, 4-dinitrophenylhydrazone derivatives present in synaptic membranes isolated from young and aged rat brain, reveal no significant change in the content of reactive aldehydes (malondialdehyde, formaldehyde, acetaldehyde or acetone), which would comprise potential breakdown products of lipid peroxidation[37]. Electron paramagnetic resonance measurements employing 5- and 12-stearic acid spin labels with the nitroxide reporter groups at two depths in the bilayer also show no significant changes in fatty acid chain dynamics or membrane fluidity in the synaptic membranes that could correlate with the observed age-associated inhibition of PMCA activity. The absence of any detectable end-products of lipid peroxidation or alterations in fatty acyl chain dynamics strongly indicate that the age-dependent changes in PMCA are likely the result of direct alteration of the protein and that the PMCAs may be targets of reactive oxygen and/or nitrogen species (Figure 1).
Figure 1 A schematic depiction of the cellular events that link brain aging and neurodegenerative disorders.
MAOS: Membrane-associated oxidative stress; ROS: Reactive oxygen species; PMCA: Plasma membrane Ca2+-ATPase; AD: Alzheimer’s disease; PD: Parkinson’s disease.
THE PMCAs: TARGETS OF OXIDATIVE STRESS
Biochemical studies
Free radical-induced oxidative damage to proteins is an important factor in the pathophysiology of several human diseases including Alzheimer’s disease, Parkinson’s disease, ischemia-reperfusion injury and also in normal biological aging. Oxidative modification of proteins may involve altered conformation, misfolding, enhanced propensity for aggregation and oxidation of amino acid residues[32,33]. To determine the susceptibility of the PMCAs to oxidative stress and to identify the molecular signatures of oxidative modification that may appear on the protein upon exposure to oxidants, we carried out a series of studies employing several experimental paradigms[38-40]. Exposure of SPMs to oxidants such as H2O2, peroxynitrite and the azo-initiators 2,2’-Azobis 2-aminopropane dihydrochloride and 4,4’-Azobis 14-cyanovaleric acid (peroxyl radical generating agents) for a short period of 10 min result in a rapid loss of Ca2+-activated ATPase activity of the PMCAs[39]. As observed in aging brain SPMs, lowered PMCA activity is attributed to a diminished Vmax with no significant change in Kact. Although all three oxidants led to significant loss of PMCA activity, the effect of peroxynitrite was the most potent, followed by peroxyl radicals and H2O2. The major structural change observed in the PMCA is the appearance of high molecular weight aggregates observed in immunoblots run under non-reducing conditions of electrophoresis. The PMCA adducts can be reversed by the addition of reducing agents and chaotropic agents and therefore appear to be generated by the oxidation of cysteine residues to form intermolecular disulfide bonds and increased hydrophobic interactions between PMCA molecules, respectively[39,41].
Given that the studies described above were conducted in SPMs, it is not clear whether oxidant-mediated changes in PMCA are due to direct effects on the protein and/or due to secondary effects resulting from peroxidation of the polyunsaturated fatty acids abundant in SPMs. Thus, in the next series of studies, the erythrocyte PMCA was purified from its native membrane environment and reconstituted into mixed micelles made of phosphatidylcholine and functional and structural alterations in the isolated protein investigated following a brief exposure to H2O2[38]. The purified PMCA preparations allowed us to perform structural characterization of the protein (PMCA 4 being the predominant isoform present in erythrocyte membranes) and shed light on the molecular mechanisms involved in H2O2-mediated PMCA inactivation. Exposure of the protein to H2O2 (25-100 μmol/L, 10 min, 37°C) inhibits both basal and CaM-stimulated PMCA activity at nearly identical rates. Neither the concentration-dependent stimulation of PMCA activity by CaM nor the binding of CaM to the H2O2-exposed PMCA is disrupted suggesting that the inhibitory effect of the oxidant is apparently not mediated through a direct effect on the CaM binding domain. H2O2 quenches PMCA tryptophan fluorescence, an indicator of global conformational changes in the protein, with a rate similar to that of PMCA inactivation. A novel finding in these studies was the protection offered by CaM against the deleterious effects of H2O2. Pretreatment of PMCA with CaM prior to the addition of H2O2 completely protects the enzyme against oxidant-mediated inactivation suggesting the existence of a CaM-induced conformational state resistant to oxidation. As observed in SPMs, exposure of the purified PMCA to H2O2 also generates high molecular weight aggregates. Although the PMCA adducts are significantly reversed by dithiothreitol, there is no recovery in PMCA activity suggesting that the oxidation-induced conformational changes are not fully reversed even when the disulfide bonds are reduced. Quantification of cysteine residues in the PMCA using the fluorescent maleimide probe ThioGlo-1[42] shows labeling of only 7 of the 21 known cysteines present in PMCA 4, presumably due to the existence of intramolecular disulfide bonds and/or mixed protein-glutathione disulfide bonds. As expected, exposure to H2O2 significantly reduces cysteine labeling (40% reduction). Amino acid analysis reveals no chemical change in any other amino acid except for methionine residues, a fraction of which are oxidized to methionine sulfoxide (0.06 mol methionine sulfoxide/mol PMCA or 0.002 methionine sulfoxide/mol methionine), as there are 28 methionine residues in PMCA 4[38].
Single molecule spectroscopy approaches
Given that one of the structural alterations observed in the PMCA following exposure to H2O2 is altered conformation, we used single molecule spectroscopy to interrogate conformational changes in oxidant-modified PMCA molecules to better understand the mechanism underlying its inactivation[43]. Single molecule spectroscopic strategies have been developed and successfully used by Johnson and coworkers to investigate conformational changes in the interaction between the PMCA and CaM at the molecular level, a feat that cannot be achieved by conventional ensemble kinetic and biochemical methods given the vast heterogeneity in PMCA conformational states[43-48]. Previous work by Yao et al[49] demonstrated that fluorescently labeled CaM bound to the autoinhibitory domain of PMCA exhibits a rotational correlation time much shorter than expected for the whole PMCA enzyme, consistent with segmental motion of the CaM binding domain. Subsequent polarization modulation studies on single molecule complexes of PMCA bound to tetramethylrhodamine-labeled CaM show the existence of two major conformational distributions, a high orientational mobility population present at 25 μmol/L Ca2+, a concentration sufficient for full activation by CaM (attributed to PMCA with a fully dissociated CaM binding domain) and a second state with low orientational mobility which appears at a low Ca2+ concentration (0.15 μmol/L) (corresponding to autoinhibited PMCA-CaM complexes with a non-dissociated autoinhibitory domain)[45]. The existence of a PMCA-CaM state with the autoinhibitory domain not fully dissociated is indicative of a far greater complexity in PMCA-CaM interactions than previously realized. Binding and hydrolysis of the substrate ATP at the nucleotide-binding site of PMCA drive structural motions of the enzyme that result in the transfer of the terminal phosphate of ATP to a highly conserved aspartate residue in the phosphorylation domain. Consistent with this, addition of ATP (but not its non-hydrolysable analogs) to the PMCA-CaM complexes eliminates the low orientational mobility conformational state and abolishes the Ca2+ dependence in modulation depth. The ATP-dependence of the autoinhibitory domain mobility, a reflection of the structural interaction between the PMCA catalytic core and CaM binding domain, offers new insights into the functional relationships of the various PMCA-CaM ligands on the pump activity[43].
To investigate the conformational changes in PMCA-CaM complexes following oxidative modification by H2O2, we performed single molecule polarization modulation spectroscopy on PMCA exposed to 100 μmol/L H2O2 as described[38,43]. In the absence of ATP, the orientational mobility populations for native and oxidant-treated PMCA-CaM complexes are nearly the same, indicating that CaM binding and coupling of the autoinhibitory domain with the enzymatic core is unaltered by H2O2, observations that are consistent with our biochemical studies[38]. However, in the presence of 1 mmol/L ATP, marked differences are evident in the oxidant-treated PMCA molecules. The most interesting observation is the appearance of a population of PMCA-CaM complexes with low orientational mobility distribution present even at high Ca2+ (25 μmol/L), suggestive of PMCA molecules with autoinhibitory domain associated with the catalytic site[45]. Thus, H2O2 appears to disrupt the structural coupling between ATP binding and hydrolysis and the autoinhibitory domain, suggesting that the loss of PMCA enzyme activity resulting from oxidative damage is correlated with a reduced dissociation of the autoinhibitory domain from the nucleotide binding site. This interpretation is further substantiated by proteolysis studies showing reduced accessibility of chymotrypsin to the CaM binding domain of H2O2-treated PMCA[43]. Oxidative modification at or near the nucleotide binding site of the PMCA such as the formation of disulfide bonds may alter the structural geometry of ATP binding and impair protein conformational changes associated with the productive utilization of ATP[43]. Interestingly, the only chemical change observed in H2O2-treated PMCA is cysteine oxidation[38]. Of the twenty-one known cysteine residues present in PMCA 4, ten are located in the cytoplasmic loop between transmembrane four and five, a region that contains the active site of the enzyme. Of these, cysteine 537 is located on the stretch of residues comprising the receptor region that interacts with the CaM-binding domain[50,51], while cysteine 601 is a few residues away from the ATP binding site at lysine 591. Oxidative modification of one or more of these crucial cysteines is likely to alter conformational interactions between the catalytic site and the CaM binding domain, resulting in nonproductive binding of ATP and subsequent enzyme inactivation[43].
Effects of oxidative stress on the PMCAs in neurons
While in vitro studies on the PMCAs present in SPMs and purified protein preparations provide valuable information on oxidation-induced alterations in PMCA activity, structure and conformation, they do not address the sensitivity of the PMCA pumps to oxidative stress as it would occur in intact cells with its array of antioxidant enzymes that can counteract the deleterious effects of oxidants. A number of studies have indeed addressed this issue and investigated the effects of neurotoxins, excitotoxic insults, and reactive oxygen species (ROS) on the PMCAs present in neurons[40,52-56], and in non-neuronal cells[57-59]. Acute exposure of cerebrocortical neurons to N-methyl D-aspartate (NMDA), kainate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, okadaic acid or maitotoxin results in accelerated cell death, which correlates with the activation of the Ca2+-dependent protease calpain and subsequent PMCA proteolysis[54]. PMCA degradation in cells is consistent with earlier observations showing its susceptibility to in vitro proteolysis by exogenous calpain, which cleaves the protein at the autoinhibitory CaM binding domain resulting in irreversible activation[53,60,61]. Although the studies by Hajimohammadreza et al[54] did not assess the consequence of calpain-mediated cleavage on PMCA activity, they were the first to report PMCA degradation in degenerative neuronal cultures.
The response of the PMCAs (PMCA 2 and PMCA 4, in particular) to excitotoxic agents was further highlighted in hippocampal neurons exposed to glutamate[55]. This manipulation reduces PMCA activity as evidenced by a significantly lower rate of PMCA-mediated Ca2+ efflux[55]. Interestingly, lowered PMCA function correlates with a loss of the protein from the plasma membrane. Calpain inhibitors abrogate these effects signifying the role of this Ca2+-activated protease in glutamate-mediated PMCA inactivation and clearance from the plasma membrane. In contrast to in vitro studies showing irreversible activation of the purified PMCA upon exposure to exogenous calpain, in situ activation of the protease in cells reduces both PMCA function and protein levels. Calpain-triggered down-regulation of PMCA may be due to degradation, endocytosis or possible internalization of the protein en route to degradation in lysosomes[55]. Although not experimentally proven, the possibility of PMCA internalization/recycling being part of a physiological Ca2+ signaling cascade was suggested by the relatively small degree of PMCA internalization observed in healthy cells in response to a non-excitotoxic concentration of glutamate. It is not clear if the effects of calpain are due to direct proteolysis of the PMCA protein or mediated by some other cytoskeletal component(s) involved in its retrieval from the membrane. PMCA down-regulation and internalization are also seen in the cell body and neurites of hippocampal neurons treated with H2O2[56] suggesting that this may be part of a concerted response mounted by cells in response to conditions of excitotoxicity and oxidative stress.
The above mentioned series of studies demonstrate the sensitivity of the PMCAs to exogenously added excitotoxic agents and oxidants. In a recent study, we assessed the effects of ROS generated within neurons using the cells own machinery as would likely occur in vivo[40]. Primary cortical neurons were treated with paraquat, a redox cycling agent that utilizes the cellular microsomal cytochrome P450 enzyme system to generate intracellular superoxide free radicals[62]. Exposure of neurons to paraquat results in an almost instantaneous generation of superoxide free radicals as monitored by the inactivation of aconitase, an enzyme with iron-sulfur centers believed to be one of the most sensitive targets of ROS[63]. A 24 h exposure of primary cortical neurons to paraquat (5-100 μmol/L) results in marked alterations in PMCA activity which exhibits a biphasic response[40]. While low concentrations of paraquat (5-25 μmol/L) stimulate CaM-independent PMCA activity by approximately two-fold and abolish its sensitivity to CaM, higher concentrations (50-100 μmol/L) inhibit both basal and CaM-stimulated PMCA activity. As observed before with exogenously added toxic agents[54-56], paraquat treatment also leads to calpain-mediated PMCA proteolysis[40]. It is notable that PMCA stimulation and loss of sensitivity to CaM occur under conditions that precede its proteolysis leading us to speculate that low concentrations of paraquat may cause structural changes in the PMCA that promote the dissociation of the autoinhibitory CaM binding domain, resulting in PMCA stimulation. Paraquat-induced conformational changes may expose the calpain cleavage site on the PMCA thus making it more accessible to proteolytic degradation. Paraquat-treated cells also exhibit PMCA aggregation, a consistent signature of PMCA oxidation observed in SPMs and the purified protein[38,39].
Increase in oxidative stress and elevations in [Ca2+]i have also been linked to the activation of caspases which mediate programmed cell death or apoptosis[64]. The link between the PMCAs and apoptosis was discovered when the PMCA 4b subtype was shown to be a substrate of caspase 3 in the early stages of apoptosis[65-67]. In contrast to calpain, which can form several products of different sizes depending on the site of cleavage[53,60,68], cleavage by caspase occurs precisely at an aspartate (consensus site 1077DEID1080), a few residues upstream of the CaM binding domain. The single 120 kDa proteolytic fragment is fully active even in the absence of CaM[65,66], a condition that would help the cell to respond more efficiently to an increased Ca2+ load[67]. The effects of caspase-mediated PMCA cleavage on its activity and function appear to yield mixed results. In a study by Schwab et al[67], 2002, PMCA proteolysis by caspases inactivated PMCA 2 and 4 in both neurons and non-neuronal cells undergoing apoptosis, resulting in impaired [Ca2+]i handling and consequent Ca2+ overload. Expression of non-cleavable PMCA mutants prevents the disruption in Ca2+ handling, slows down the kinetics of apoptotic cell death, and significantly delays necrosis. A number of cellular conditions, such as exposure to excitotoxins, oxidative stress and ischemic injury, may activate both calpains and caspases. The predominance of one proteolytic pathway vs the other and ensuing pattern of PMCA proteolysis and effects on pump activity appear to depend largely on the type of insult and its intensity and duration. If the stress is mild, increase in PMCA activity may counteract the increased Ca2+ load and protect cells against death. However, under severe and more chronic conditions, the PMCAs may be down-regulated further impairing Ca2+ homeostasis and promoting cell death (Figure 1).
FUNCTIONAL EFFECTS OF ALTERED PMCA EXPRESSION
Given the technical challenges associated with culturing adult and aged neurons and the dearth of an appropriate technology that allows the assessment of PMCA function in live animals, there is limited information on the functional consequences of altered PMCA levels such as those that occur in the synaptic terminals in the aging brain[31]. A number of approaches have been made by various laboratories (including ours) to experimentally manipulate PMCA expression and determine the downstream consequences on various aspects of cell function. This is especially pertinent given the critical role of the PMCAs in maintaining neuronal [Ca2+]i and also its newly assigned role in modulating cellular signaling pathways[69]. The PMCAs have been shown to interact with a number of signaling proteins such as nNOS, calcineurin, and various members of the membrane microdomain organizing proteins of the membrane-associated guanylate kinase family[70-77]. More recently, the PMCAs have been shown to be localized in neuronal lipid rafts, cholesterol-enriched microdomains in the plasma membrane[78,79], believed to be local centers for cell signaling events. PMCA activity is significantly reduced in response to cellular cholesterol depletion suggesting the possibility of local regulation of the pump activity in lipid rafts[78].
Antisense-(AS) plasmid-mediated reduction of specific isoforms has yielded valuable information on the role of individual PMCA subtypes in regulating the dynamics of cellular Ca2+ handling and also their contribution to a diverse array of neuronal functions[80-84]. For example, blockade of PMCA 1 causes no change in the levels of resting free [Ca2+]i or its release from intracellular stores but results in a significantly slower rate of Ca2+ clearance following release from intracellular stores[80]. However, the most striking effect of lowered PMCA 1 in PC6 cells is the impairment in neurite extension elicited by nerve growth factor (NGF)[80]. Cells with reduced PMCA 1 levels have fewer and shorter neurites and a conspicuous absence of defined growth cones in response to NGF. Interestingly, these effects are not mediated by a loss of NGF signaling but are rather attributed to downstream consequences of altered cellular Ca2+ transients. Further clues regarding the role of PMCA 1 in neuritic development were unraveled in a subsequent study showing a significant down-regulation in the expression of the integrin receptor alpha subunit in cells with blocked PMCA 1[81]. These studies indicate a close relationship between PMCA 1 and the regulation of cell-extracellular matrix attachment and contact-dependent growth.
Transient reduction of PMCA 2 using AS techniques prolongs the effects of NMDA and increases the sensitivity to inhalational anesthetics[83]. We utilized an RNA interference strategy to experimentally lower PMCA 2 expression[82]. Short interference RNA treatment led to 80% reduction of PMCA 2 expression, which remained suppressed throughout a 6 d period. siRNA-treated cells exhibit marked changes in total PMCA activity, with a shift from Michaelis-Menten kinetic properties, a three-fold increase in Kact for Ca2+ and 22% suppression of Vmax[82]. Ca2+ imaging studies using Fura 2 show that neurons with lowered PMCA 2 have (1) elevated resting [Ca2+]i; (2) a significantly slower recovery following depolarization; and (3) inability to return to their own resting levels present prior to depolarization, signifying the importance of PMCA 2 in maintaining resting [Ca2+]i. Cells with elevated Ca2+ levels would likely exhibit an increased vulnerability to various metabolic and oxidative insults. Consistent with this idea, PMCA 2 deficient cells are more susceptible to cellular stresses particularly those involving Ca2+ overload[82].
Although experimental lowering of the PMCAs in cultured cells cannot be extrapolated to the in vivo situation that exists in brain, they greatly emphasize the importance of maintaining normal levels of PMCA protein in neuronal survival and growth. Given that lowered PMCA expression increases the vulnerability of cells to various insults, the reverse condition, i.e. increased PMCA levels, would likely offer neuroprotection. Consistent with this notion, cells over-expressing PMCA 2 and 4 protect cells against Ca2+-mediated cytotoxicity[85,86]. Coincidently, PMCA levels are up-regulated during the maturation of hippocampal neurons[87] and in neuroblastoma cells undergoing differentiation[88]. Analysis of PMCA expression profile in the developing brain shows that distinct PMCA subtypes are expressed at different levels in various brain regions, with a noticeably higher expression in cellular compartments characterized by a greater number of synapses, suggesting a key role of the PMCAs in synaptogenesis and in the maturation of neuronal electrophysiological properties[89-91].
THE PMCAs AND NEURODEGENERATION
A role for neuronal Ca2+ dysregulation in age-related neurodegenerative disorders was suggested almost three decades ago[8,92] and has been experimentally validated since then by a large body of literature[20,93-95]. However, the contribution of the PMCAs in neurodegeneration is just beginning to be elucidated. There are two principal means by which the PMCAs may either impact or be impacted by neurodegenerative diseases. Altered PMCA function may disrupt neuronal Ca2+ homeostasis and increase cellular Ca2+ load which may in turn influence the metabolism and production of pathological peptides/proteins such as the amyloid beta peptide in Alzheimer’s disease and alpha synuclein in Parkinson’s disease. Conversely, events downstream from the accumulation of the pathological forms of these peptides/proteins may disrupt energy homeostasis, increase membrane excitability, elevate membrane-associated oxidative stress, and activate proteolytic enzymes which may consequently have an inhibitory effect on PMCA function (Figure 1). Convincing experimental support for either one or both of these possibilities is still lacking.
The PMCAs have been found to be significantly down-regulated in models of global ischemia-reperfusion injury and seizures[65,73-77]. Evidence suggests that the suppression of PMCA activity is not simply due to the disruption of ion gradients and lowered ATP levels associated with these conditions, but rather due to oxidative modification and degradation of the PMCA protein[96]. The relationship between the PMCAs and Alzheimer’s disease, the most common age-dependent neurodegenerative disorder, was first suggested by a reduction in PMCA activity in neurons exposed to the amyloid beta peptide[97-99]. Recent studies have further established the involvement of the PMCA in Alzheimer’s disease[100]. PMCA activity in human brain tissue from Alzheimer’s disease patients shows an altered dependence on Ca2+ compared to control brain with stimulation of activity at lower concentrations and less inhibition at high Ca2+ concentrations[100] suggesting that the affinity of the stimulatory site for Ca2+ increases and that for the inhibitory site decreases in Alzheimer’s brain. More interestingly, addition of amyloid beta peptide to control brain altered its Ca2+ dependency to resemble that of Alzheimer’s disease brain[100]. In preliminary studies on human brain tissue from Parkinson’s disease, we have observed a significant reduction in PMCA activity compared to age-matched controls[101]. PMCA enzyme and protein levels are also lowered in SH-SY5Y neuroblastoma cells exposed to the Parkinsonian mimetics methyl phenyl pyridinium[101] and 6-hydroxydopamine (our unpublished observations). Overall, the findings presented here suggest that the PMCAs are altered in a number of neurodegenerative conditions. Further studies are needed to elucidate the underlying mechanisms and determine whether the observed changes in the PMCA pumps are a cause or consequence of disease progression. Therapeutic approaches that can protect the PMCAs and stabilize [Ca2+]i homeostasis may be capable of slowing or even preventing neuronal degeneration. The PMCAs are therefore emerging as a new class of drug targets for therapeutic interventions in various chronic degenerative disorders.
CONCLUSION
The PMCA pumps are critical to the maintenance of precise levels of intracellular Ca2+, quintessential to the functioning of nerve cells. The PMCAs in the SPMs diminish progressively with increasing age. This may be due to elevated levels of oxidative stress present in aged neurons. Lowered PMCA expression disrupts neuronal Ca2+ handling and increases the vulnerability of nerve cells to stresses involving Ca2+ overload. Age- and oxidation-related downregulation of the PMCAs may play an important role in compromised neuronal function in the aging brain and its several-fold increased susceptibility to age-associated neurodegenerative disorders.
Peer reviewers: Luca Munaron, PhD, Associate Professor, Department of Animal and Human Biology, University of Torino, Via Accademia Albertina 13, 10123 Torino, Italy; Dong Min Shin, DDS, PhD, Professor, Division of Physiology, Yonsei University College of Dentistry, 134 Sinchon-dong, Seodaemoon-ku, Seoul 120-752, South Korea
S- Editor Cheng JX L- Editor Negro F E- Editor Zheng XM