Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.188
Revised: May 15, 2010
Accepted: May 22, 2010
Published online: May 26, 2010
AIM: To evaluate the ability of anti-ricin A-chain antibodies, delivered intracellularly, to protect against ricin-induced cytotoxicity in RAW264.7 cells.
METHODS: Anti-deglycosylated ricin A-chain antibody and RAC18 anti-ricin A-chain monoclonal antibody were delivered intracellularly by encapsulating in liposomes or via conjugation with the cell-penetrating MTS-transport peptide. RAW264.7 cells were incubated with these antibodies either before or after ricin exposure. The changes in cytotoxicity were estimated by MTT assay. Co-localization of internalized antibody and ricin was evaluated by fluorescence microscopy.
RESULTS: Internalized antibodies significantly increased cell viability either before or after ricin exposure compared to the unconjugated antibodies. Fluorescence microscopy confirmed the co-localization of internalized antibodies and ricin inside the cells.
CONCLUSION: Intracellular delivery of antibodies to neutralize the ricin toxin after cellular uptake supports the potential use of cell-permeable antibodies for post-exposure treatment of ricin intoxication.
- Citation: Wu F, Fan S, Martiniuk F, Pincus S, Müller S, Kohler H, Tchou-Wong KM. Protective effects of anti-ricin A-chain antibodies delivered intracellularly against ricin-induced cytotoxicity. World J Biol Chem 2010; 1(5): 188-195
- URL: https://www.wjgnet.com/1949-8454/full/v1/i5/188.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i5.188
Ricin, a natural product of the castor bean (Ricinus communis), is the most toxic substance in the plant kingdom[1]. It has the potential to be used as a biological weapon because of its stability and ease of production worldwide[2]. It can be injected into a target, used to contaminate food and water, or can be dispersed as an aerosol. Ricin poisoning can cause severe tissue damage and inflammatory reaction and can result in death. Rauber et al[3] documented more than 750 cases of accidental or deliberate ricin intoxication following castor bean ingestion, and 14 of them resulted in a fatality. A fatal dose through ingestion is considered to be 5-6 castor beans for a child and 20 beans for an adult[2]. The ingested toxin causes initial symptoms including nausea, vomiting, diarrhea, and abdominal pain. In severe poisoning, the symptoms progress to gastrointestinal bleeding with necrosis of the liver, spleen and kidneys, and even cardiovascular collapse[4]. Ricin administered intragastrically to mice induced villus atrophy and epithelial damage in the proximal small intestine, coincident with an inflammatory response of the intestinal mucosa[5].
Ricin is a heterodimeric protein consisting of two polypeptide chains, the A-chain (RTA) and the B-chain (RTB), linked by a disulfide bond. RTB binds to galactose residues on cell surface receptors, facilitating cell entry and endocytosis of the toxin. Upon entering the endosome, the toxin-receptor complex can either be delivered toward lysosomes, where it would be destroyed, or it can be recycled back to the cell surface. As a result, only a small fraction of ricin (about 5%) will finally enter the cytosol[6]. It is not clear yet exactly when the disulfide bond is dissociated to separate the A-chain and B-chain yet, after it occurs, the A-chain can be taken up by the Golgi apparatus and transported retrogradely to the rough endoplasmic reticulum, where it exerts its toxic effect[7]. The A-chain enzymatically cleaves an adenine residue near the 3’ end of the 28S ribosomal RNA[8]. This deletion causes elongation factor-2 to fail to bind, thereby blocking protein synthesis[9]. One molecule of RTA is sufficient to block protein synthesis in the cell, resulting in the inactivation of about 2000 ribosomes per minute[10]. The irreversible poisoning of the ribosome and inhibition of protein synthesis may lead to eventual cell death.
Since there are currently no antidotes to ricin for humans, the discovery of antitoxins is a high priority. Ricin ribotoxicity can be counteracted by several different types of antitoxins including neutralizing anti-ricin antibodies, small molecule RTA inhibitors, polynucleotide active site inhibitors and polynucleotide substrate analogues[11,12]. We have previously shown that a 31-nucleotide RNA aptamer that contained all sequences and structures necessary for forming high affinity complexes with RTA and blocking enzymatic activity of RTA in vitro neutralized the inhibitory effects of ricin on translation inhibition in cell-free and cell-based luciferase assays and a ricin-induced cytotoxicity assay[13].
In animal models, passive antibody administration (producing immediate immunity) had been reported to be effective in protecting against ricin-induced lethality[14-16]. In our previous study[17], we developed a lung aspiration mouse model for evaluating the therapeutic index of antibodies against the RTA for post-exposure treatment against ricin-induced lung toxicity. Briefly, to examine the effects of post-exposure antibody treatment, polyclonal antibody specific for deglycosylated RTA [deglycosylated ricin A-chain antibody (dgA Ab)] or RAC18 anti-ricin A-chain monoclonal antibody (RAC18 mAb)[18] was administered between 0-24 h after a ricin (16 μg/kg) lethal challenge via lung aspiration. Aspiration of polyclonal dgA Ab (50 μg) up to 18 h after a ricin challenge fully protected against ricin lethality (100% survival). In contrast, only 30% protection was observed when dgA Ab treatment was delayed to 24 h after ricin challenge. Similarly, RAC18 mAb offered complete protection (100%) when administered at early time points. The survival rate decreased to 60% and 50% when RAC18 mAb treatment was delayed to 18 and 24 h after ricin exposure, respectively[17]. The failure to rescue ricin-challenged mice at later time points may be due to the fact the toxin is internalized and that these antibodies are not capable of entering into the cells to neutralize the intracellular ricin toxin.
MTS is a hydrophobic peptide derived from the Kaposi fibroblast growth factor signal peptide[19]. The MTS peptide conjugated to antibodies has been shown to facilitate entry of antibodies into living cells without causing toxicity[20]. MTS-conjugated anti-caspase 3 antibody had been shown to inhibit actinomycin-induced apoptosis in human T lymphoma cells[21]. Many other cationic and hydrophobic cell penetrating peptides (CPPs) have been reported to perform intracellular delivery of proteins into cultured cells[22], as well as in vivo delivery of enzymes such as galactosidase and Cre recombinase to tissues[23,24]. Also CPPs significantly enhanced retention of the antibody by tumors[25]. More importantly, most CPPs are nontoxic when used at low doses[26].
Therefore, we hypothesize that cytosolic delivery of neutralizing antibodies that inhibit intracellular ricin activity will prolong the therapeutic window and/or increase the therapeutic index at later time points after ricin exposure. The purpose of this study is to evaluate the protective effects of neutralizing anti-ricin A-chain antibodies with membrane-penetrating properties; i.e. cell-permeable antibodies, against ricin toxicity before further validation in animal studies.
Murine macrophage RAW264.7 cells were obtained from ATCC (Manassas, USA) and cultured in RPMI 1640 plus 10% fetal bovine serum, penicillin and streptomycin. Cells were cultured in 5% CO2 humidified atmosphere at 37°C. Ricin (Ricinus Communis Agglutinin II or RCA60) was obtained from Vector Laboratories Inc. (Burlingame, USA). Anti-dgA Ab was IgG purified by protein-A sepharose from pooled polyclonal antisera obtained from mice hyperimmunized with dgA (obtained from Dr. Martha Hale, USAMRIID, Fort Detrick, MD, USA). The RAC18 monoclonal antibody (RAC18 mAb) against ricin A-chain was purified using protein G-agarose column (Gibco-BRL) as described previously[18]. Control mouse IgG was obtained from Sigma-Aldrich (St. Louis, USA).
Fluorescent labeling of ricin and RAC18 monoclonal antibody were performed as follows. One milligram of ricin or purified RAC18 mAb were mixed with 20 × molar excesses of the carboxylic acid, succinimidyl esters of Alexa Fluor 488 or 594 (Invitrogen-Molecular Probes, Eugene, USA), respectively, in a volume of 0.5 mL of phosphate buffered saline. The mixture was slowly stirred for 1 h. Dye-conjugated protein was separated from unconjugated dye on 2 mL Zeba desalting spin columns (Pierce, Rockford, USA).
Liposome encapsulation of antibodies was performed using Ab-DeliverIN™ (Boca Scientific, Boca Raton, USA) in accordance with the protocol provided by the manufacturer. Briefly, 0.4 μL of Ab-DeliverIN™ and 4 μL of antibody (100 μg/mL, diluted with PBS) were mixed and incubated for 10-15 min at room temperature. Then 20 μL of serum-free medium was added to the antibody/Ab-DeliverIN™ mixture and added immediately to cells cultured in 96-well plate in 100 μL culture medium. The MTS peptide (KGEGAAVLLPVLLAAPG) was synthesized by Sigma-Genosys (The Woodlands, USA). The MTS peptide-antibody conjugate was generated in accordance to Zhao et al[21]. Briefly, antibodies were dialyzed against PBS (pH 6.0), oxidized by adding 1/10 volume of 200 mmol/L NaIO4 and incubated at 4°C for 30 min in the dark. Glycerol was added to a final concentration of 30 mmol/L to terminate the oxidation step. Samples were subsequently dialyzed at 4°C for 1 h against PBS (pH 6.0). The MTS peptide was added to the antibodies and samples were incubated at 37°C for 1 h. The resulting antibody-peptide conjugate was dialyzed against PBS (pH 7.4).
Cytotoxicity of RAW264.7 cells induced by ricin was quantified using the CellTiter 96® Aqueous Non-Radioactive Cell Proliferation (MTT) Assay from Promega (Madison, USA) in accordance with the manufacturer’s instructions. The plate was read using a 492 nm absorbance filter in a Perkin Elmer HTS700 BioAssay plate reader (Waltham, USA). The optical density of untreated controls was set at 100%. The number of viable cells after a ricin challenge was determined by measuring optical density and expressing viability as percent of untreated controls. For cell viability assays involving pretreatment with antibodies, RAW264.7 cells (104 cells/well) were seeded in a 96-well plate overnight and then incubated with liposome-encapsulated antibodies or control IgG for 8 h to allow time for antibody internalization. After 3 washes with PBS to remove extracellular antibodies that have not been internalized, ricin (100 ng/mL) was added and incubated for another 24 h before cell viability quantification. For cell viability assays involving post-exposure treatment with MTS-conjugated dgA Ab, RAW264.7 cells grown in 96-well plates (104/well) were first exposed to ricin (100 ng/mL) for 1 h, 2 h or 4 h. After 3 washes with PBS to remove extracellular ricin that had not been internalized into the cells, MTS-conjugated dgA Ab (1 μg/well) was added and incubated for another 48 h before quantitation of cell viability using the MTT assay. As controls, unconjugated dgA Ab was added to cells at 1 h, 2 h or 4 h after ricin treatment.
To detect the intracellular localization of deliverIN-encapsulated RAC18 mAb and co-localization with ricin in cells pre-treated with deliverIN-encapsulated RAC18 mAb, RAW264.7 cells cultured in chamber slides were first incubated with naked or deliverIN-encapsulated Alexa 594-labeled RAC18 mAb for 8 h. Cells were washed 3 times to remove extracellular antibodies and Alexa 488-labeled ricin was added for an additional 12 h before fixation and visualization under the fluorescent microscope. As a control, cells were pretreated with Alexa-594-labeled RAC18 mAb without liposome encapsulation before the addition of Alexa 488-labeled ricin. To detect the intracellular localization of MTS-conjugated dgA Ab and co-localization with ricin in cells after post-treatment with MTS-conjugated dgA antibody, RAW264.7 cells grown in chamber slides were first exposed to Alexa 488-labeled ricin for 12 h, washed 3 times with PBS to remove extracellular ricin that had not been internalized before the addition of MTS-conjugated dgA Ab or unconjugated (or naked) dgA Ab for an additional 24 h. To detect MTS-conjugated dgA antibody, fixed cells were stained with TRITC-labeled goat anti-mouse IgG (Sigma-Aldrich, St. Louis, USA). Fluorescent microscopy was performed with a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan) with an Optronics Magnafire digital camera (Optronics, Goleta, USA). Digital image analysis was performed with ImagePro Plus (Media Cybernetics, Silver Spring, USA).
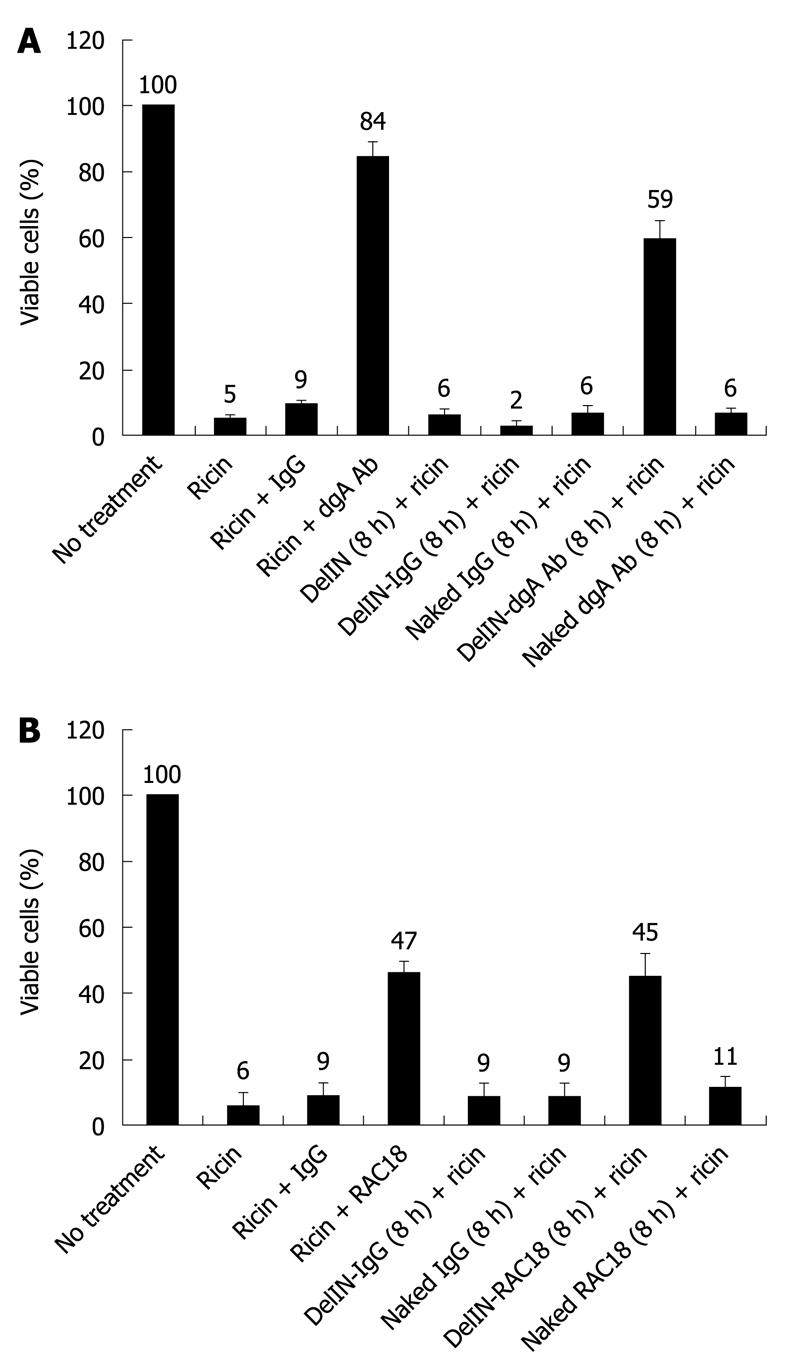
To examine the protective effects of anti-ricin A-chain antibodies against ricin-induced cytotoxicity, RAW264.7 cells were treated with ricin alone or ricin co-incubated with polyclonal dgA Ab (Figure 1A) or monoclonal RAC18 mAb (Figure 1B). 84% and 47% of ricin-treated cells were viable when ricin was co-incubated dgA Ab and RAC18 mAb, respectively, compared to ricin alone (5%-6%) or ricin plus control IgG (9%). These results demonstrated that dgA Ab and RAC18 mAb can neutralize ricin toxicity, presumably before internalization of the toxin.
To ascertain the protective effects of intracellular anti-ricin antibodies against ricin cytotoxicity, dgA Ab and RAC18 mAb were first delivered inside RAW264.7 cells via encapsulation with the liposome Ab-DeliverIN™ (abbreviated as DelIN), a lipid-based formulation that forms non-covalent complexes with antibodies. These liposome-antibody complexes are internalized by cells and antibodies are released into the cytoplasm. To ensure sufficient time for antibody uptake by cells, RAW264.7 cells were pre-incubated for 8 h with dgA Ab, RAC18 mAb or control IgG encapsulated by DelIN liposome, denoted as DelIN-dgA Ab, DelIN-RAC18 or DelIN-IgG, respectively, or naked antibodies (without DelIN formulation). After 8 h, antibodies were removed and cells were washed multiple times to remove residual extracellular antibodies before ricin exposure. Only cells pretreated with DelIN-dgA Ab and DelIN-RAC18 mAb were protected against ricin toxicity, 59% (Figure 1A) and 45% viable cells (Figure 1B), respectively. In contrast, no protection against ricin cytotoxicity was observed when cells were pretreated with dgA Ab without DelIN (naked dgA Ab + ricin, 6% viable cells) or RAC18 mAb without DelIN (naked RAC18 + ricin, 11% viable cells). Pretreatment with naked IgG, DelIN alone or DelIN-IgG complex also offered no protection against ricin toxicity. Since ricin was added after the removal of extracellular or naked antibodies, only dgA Ab and RAC18 mAb, which had been imported intracellularly by liposomes, can encounter and neutralize intracellular ricin A-chain activity. On the other hand, both naked dgA Ab and RAC18 mAb offered protection when co-incubated with ricin, presumably because interactions of ricin and antibodies could occur extracellularly before the toxin was internalized. Once the toxin was internalized, only intracellular antibodies could offer protection.
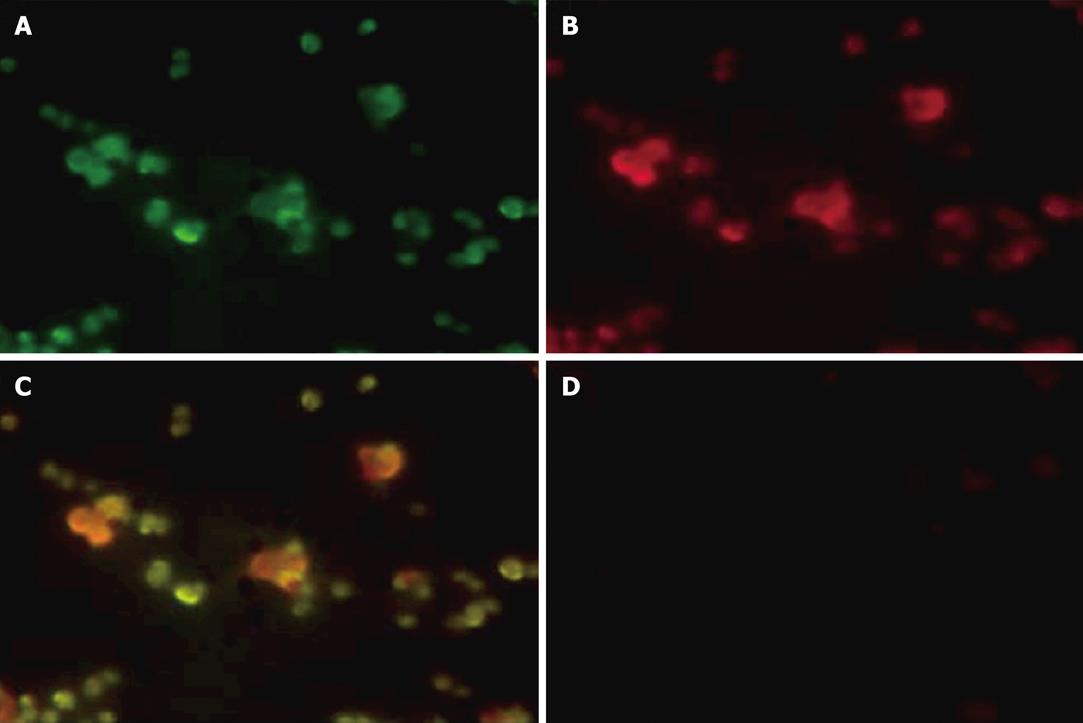
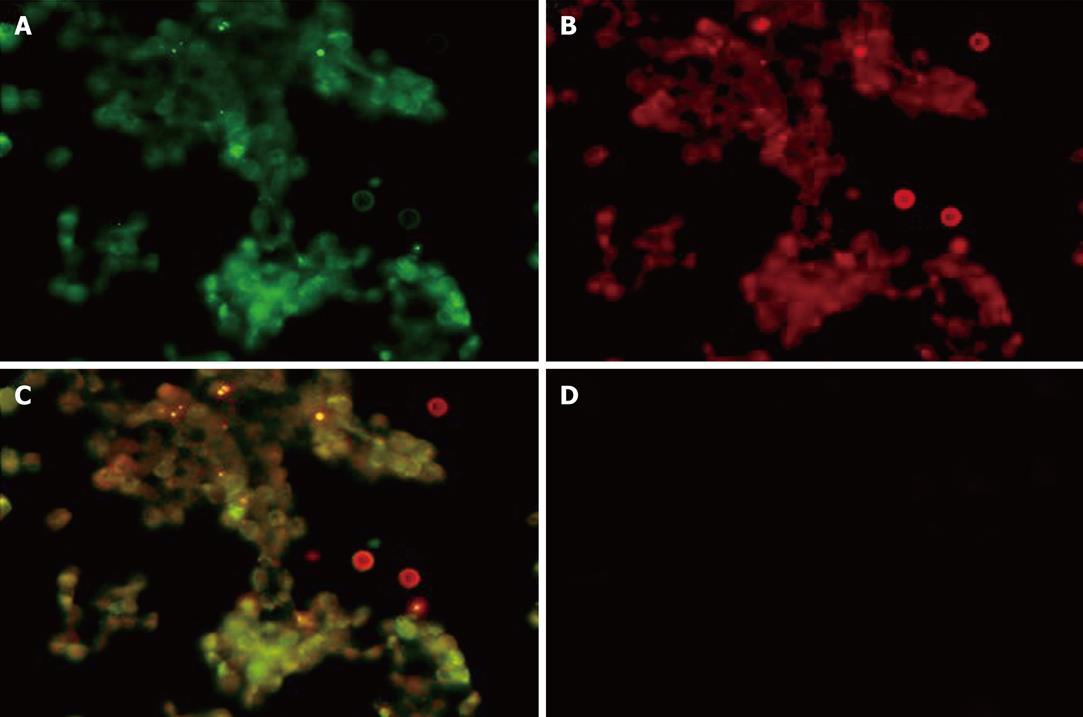
Next, we examined the intracellular localization of fluorescent-labeled ricin and fluorescent-labeled DelIN-encapsulated RAC18 mAb by immunofluorescent staining (Figure 2, Panels A-C). As shown in Figure 2, green and red fluorescent staining in the cytosol was observed in cells treated with Alexa 488-labeled ricin (Panel A) and DelIN-encapsulated Alexa 594-labeled RAC18 mAb (Panel B), respectively, and co-localization was observed as yellow staining (Panel C) after superposition of A and B. In contrast, no fluorescent staining was observed when cells were treated with naked Alexa 594-labeled RAC18 mAb (Panel D). These results confirm that naked RAC 18 mAb cannot enter into cells without encapsulation by liposomes, and that internalized DelIN-encapsulated RAC18 mAb co-localize with ricin inside the cells.
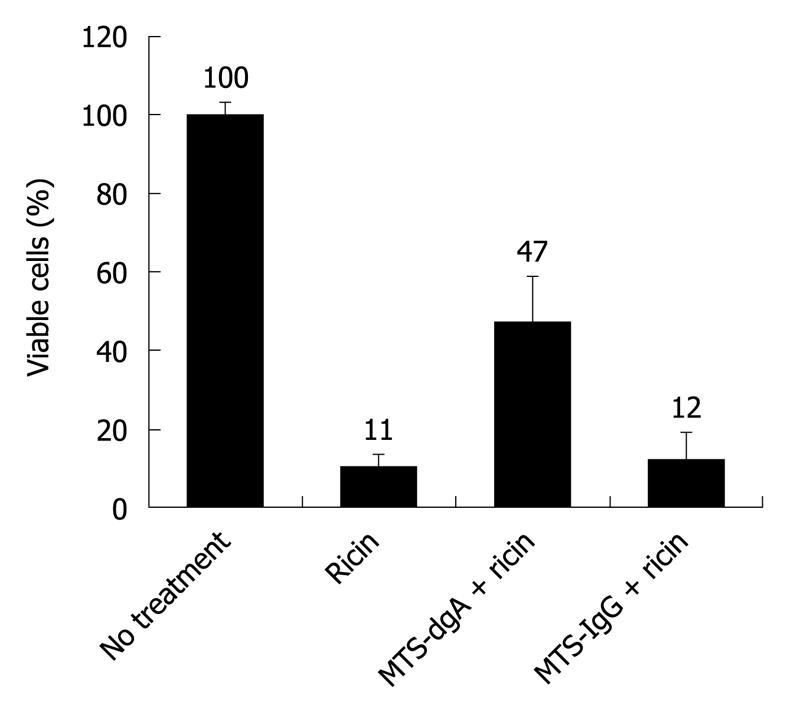
To determine if cell-permeable antibody imported by CPP maintains a neutralizing function, polyclonal dgA Ab or control IgG was covalently attached to the CPP MTS peptide by chemical conjugation (as described in Materials and Methods) and evaluated for a neutralizing function. Cell penetrating peptides are short peptides that facilitate cellular uptake of various molecular cargoes including antibodies. As shown in Figure 3, when RAW264.7 cells were co-incubated with ricin and MTS-conjugated control IgG, only 12% of the cells were viable. However, when cells were treated with ricin and MTS-conjugated dgA Ab, 48% cell survival was observed. Hence, MTS-conjugated dgA Ab maintained neutralizing activity against ricin toxicity.
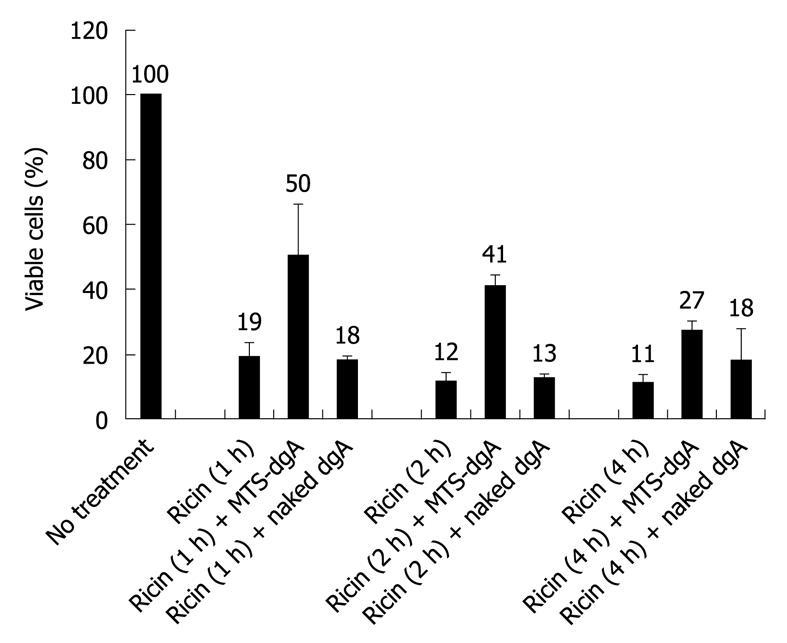
To mimic human exposure whereby passive antibody treatment will occur after ricin exposure, we investigated the protective effects of post-exposure treatment with MTS-conjugated dgA Ab at early time points after ricin exposure. RAW264.7 cells were first exposed to ricin for 1 h, 2 h or 4 h, followed by removal of extracellular ricin and several washings to remove residual extracellular ricin. MTS-conjugated dgA Ab or naked (unconjugated) dgA Ab was then added and cell viability was measured. As shown in Figure 4, only 19% of cells survived after a brief treatment with ricin for 1 h and subsequent treatment with MTS-conjugated dgA Ab increased cell survival to 50% while naked dgA Ab did not offer any protection (18% viable cells). When ricin exposure was extended to 2 h, increased cell death was observed leading to only 12% viable cells. Similarly, subsequent treatment with MTS-dgA Ab but not naked dgA Ab offered protection against ricin cytotoxicity (41% vs 13% viable cells, respectively). However, the protective effect of MTS-dgA Ab was reduced to 27% viable cells when antibody treatment was delayed to 4 h after ricin exposure. These results suggested that only MTS-conjugated dgA Ab but not naked dgA Ab could neutralize the toxicity of ricin when administered after ricin exposure after extracellular ricin had been removed. In addition, the protective effects of MTS-dgA Ab decreased at later time points after ricin exposure.
To confirm intracellular uptake of MTS-dgA Ab, the co-localization of Alexa 488-labeled ricin and MTS-dgA Ab was verified by immunofluorescence microscopy. As shown in Figure 5, intracellular staining of Alexa 488-labeled ricin (Panel A, green staining) and MTS-dgA Ab detected with TRITC-labeled anti-mouse IgG (Panel B, red staining) was observed. As anticipated, no intracellular staining was observed with naked anti-dgA Ab (Panel D). Superposition of panels A and B demonstrated co-localization of ricin with MTS-dgA Ab within the cells (Panel C, yellow staining).
We have provided evidence for proof-of-concept for our hypothesis that intracellular delivery of neutralizing antibodies into the cytosol can protect against ricin-induced cytotoxicity. We used two different methods for intracellular delivery of antibodies to RAW246.7 cells. The first method was mediated via encapsulation of antibodies within the liposomes (Ab-DeliverIN™), which represents one of the oldest nanotechnologies for drug delivery[27]. The second method was through conjugation of the antibody with membrane transporting peptides, also called cell-penetrating peptides (CPPs), which represents a novel strategy to deliver biological active molecules into living cells[28]. RAW264.7 cells was used because macrophages are highly sensitive to ricin intoxication due to abundant mannose receptors present on the cell surface that bind ricin, and macrophage apoptosis had been shown to be one of the early events that occurred in the lungs after ricin inhalation[29]. Two neutralizing anti-ricin antibodies were used in this study, namely, the polyclonal anti-dgA Ab and monoclonal antibody RAC18 mAb, which had been proven to offer protection against lethal challenge by ricin in our previous study both in vitro in RAW264.7 cells and in vivo in a lung aspiration animal model.
Immunofluorescent and co-localization studies demonstrated that Ab-DeliverIN™ and MTS conjugation indeed transported the antibodies into the cells, where internalized antibodies interacted with intracellular ricin (Figures 2 and 5). When pretreated with antibodies before ricin exposure, these internalized antibodies protected cells from ricin toxicity compared to the naked antibodies (Figures 1 and 3). DelIn-encapsulated dgA Ab (59%) was more protective than DelIn-encapsulated RCA18 mAb (47%), which is consistent with what we observed in vivo. When antibodies were given at 18 h after a ricin lethal lung challenge, dgA Ab treatment resulted in 100% mice survival, while administration of RAC18 mAb only offered 50%-60% survival. The difference in therapeutic efficacy may be due to the fact that monoclonal antibodies only have a single epitope while polyclonal antibodies offered multiple epitopes for neutralization. However, through various methods, like sub-clone selection or combinatorial library engineering, the most efficient monoclonal antibody can be obtained. Compared to polyclonal antibodies, monoclonal antibodies offered the advantage of defined specificity and activity and ease in the production of large quantities for human therapeutics.
Although CPPs as a potent delivering tool is very promising, precise understanding of their internalization mechanism is needed. Cellular internalization mechanisms determine the cell-compartment destination. Clathrin-mediated endocytosis implies that the degradative route is early endosomes to late endosomes and ultimately to lysosomes. On the other hand, if the caveolin-mediated route is taken, then the vesicles are targeted either to the Golgi apparatus or to the endoplasmic reticulum (ER)[25,30]. Better understanding of these mechanisms will help us choose the right CPP for transporting the cargo to the appropriate cell targets.
In summary, results from this initial study support our novel hypothesis that intracellular delivery of neutralizing antibodies can inhibit the activity of RTA after the ricin toxin has been internalized into the cells. More importantly, these results support further development of cell-permeable antibodies as novel antidotes for the post-exposure treatment of ricin intoxication. Further study will be needed for evaluation of therapeutic efficacy and safety of using cell-permeable anti-ricin A-chain monoclonal antibodies transported by cell-penetrating peptides in animal models.
Ricin is a plant toxin from castor beans that inhibits protein synthesis and induces cell cytotoxicity. Accidental ingestion of toxin or deliberate use of aerosolized ricin as a bioweapon can cause sever tissue injury and result in death. Currently, there are no antidotes for the treatment of ricin intoxication in humans. In animal models, passive antibody administration (producing immediate immunity) had been reported to be effective in reducing ricin-induced lethality. However, the effective therapeutic window is around 24 h post-exposure, probably due to the fact the toxin is internalized and that these antibodies are not capable of entering into the cells to neutralize the intracellular ricin toxin. To improve and extend the post-exposure therapeutic window, the protective effects of anti-ricin A-chain antibodies delivered intracellularly to neutralize the ricin toxin after cellular uptake were evaluated.
Cell penetrating peptides (CPPs) have been used widely for the import of molecules into mammalian cells in basic and applied biomedical research. CPPs are short peptides that enable proteins to translocate across the cell membrane and internalized within the cytosol through atypical secretory and internalization pathways. Hence, CPPs have been used to overcome the lipophilic barrier of the cell membranes and to deliver a large variety of cargoes inside the cell including proteins, DNA, antibodies, contrast agents and drugs. The delivery of therapeutic anti-ricin antibodies to neutralize ricin after cellular uptake offers proof-of-concept for further development of cell-penetrating or cell-permeable antibodies as a novel strategy for post-exposure treatment of ricin intoxication.
The therapeutic window for post-exposure treatment of ricin intoxication by passive antibody treatment is limited by the short duration before cellular uptake of the toxin. Once the toxin is internalized, antibodies that function only extracellularly can no longer be effective. Two methods were utilized to shuttle therapeutic antibodies to neutralize the ricin toxin after cellular uptake, namely via liposome encapsulation and conjugation with the cell-penetrating MTS-transport peptide. Hence, the development of effective methods to shuttle therapeutic antibodies to neutralize intracellular ricin is a major breakthrough for the development of ricin antidotes for the post-exposure treatment of ricin intoxication.
The development of cell-permeable antibodies for neutralizing intracellular targets, such as the ricin toxin, is an innovative approach for post-exposure treatment of ricin and other intracellular toxins. Cell-permeable anti-ricin antibodies have the potential to extend the post-exposure therapeutic window and improve therapeutic index at delayed time points after ricin intoxication.
A liposome is a small vesicle that can be used to encapsulate therapeutic agents for the intracellular delivery of drugs and antibodies against intracellular targets. CPPs are short peptides that have been used to overcome the lipophilic barrier of the cell membranes for the delivery of a variety of cargoes into mammalian cells. Conjugation or cross-linking of CPPs to therapeutic antibodies enables the antibodies to be shuttled into living cells without harming them. These cell-penetrating or cell-permeable antibodies open new therapeutic windows.
The paper is interesting and well written.
Peer reviewer: Bruno Stieger, Professor, Department of Medicine, Division of Clinical Pharmacology and Toxicology, University Hospital, Zurich 8091, Switzerland
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
| 1. | Ellenhorn MJ. Ellenhorn’s Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore: Williams & Wilkins 1997; 1847-1849. |
| 2. | Maman M, Yehezkelli Y. Ricin: A possible, Noninfectious Biological Weapon. Bioterrorism and Infectious Agents: A New Dilemma for the 21st Century. New York: Springer Science+Business Media Inc 2005; 205-215. |
| 3. | Rauber A, Heard J. Castor bean toxicity re-examined: a new perspective. Vet Hum Toxicol. 1985;27:498-502. |
| 4. | Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294:2342-2351. |
| 5. | Yoder JM, Aslam RU, Mantis NJ. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect Immun. 2007;75:1745-1750. |
| 7. | Lord JM, Deeks E, Marsden CJ, Moore K, Pateman C, Smith DC, Spooner RA, Watson P, Roberts LM. Retrograde transport of toxins across the endoplasmic reticulum membrane. Biochem Soc Trans. 2003;31:1260-1262. |
| 8. | Endo Y, Tsurugi K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J Biol Chem. 1988;263:8735-8739. |
| 9. | Wool IG, Glück A, Endo Y. Ribotoxin recognition of ribosomal RNA and a proposal for the mechanism of translocation. Trends Biochem Sci. 1992;17:266-269. |
| 10. | Sandvig K, van Deurs B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943-5950. |
| 11. | Rainey GJ, Young JA. Antitoxins: novel strategies to target agents of bioterrorism. Nat Rev Microbiol. 2004;2:721-726. |
| 12. | Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005;57:1424-1439. |
| 13. | Fan S, Wu F, Martiniuk F, Hale ML, Ellington AD, Tchou-Wong KM. Protective effects of anti-ricin A-chain RNA aptamer against ricin toxicity. World J Gastroenterol. 2008;14:6360-6365. |
| 14. | Colombatti M, Johnson VG, Skopicki HA, Fendley B, Lewis MS, Youle RJ. Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. J Immunol. 1987;138:3339-3344. |
| 15. | Poli MA, Rivera VR, Pitt ML, Vogel P. Aerosolized specific antibody protects mice from lung injury associated with aerosolized ricin exposure. Toxicon. 1996;34:1037-1044. |
| 16. | Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13:417-421. |
| 17. | Pratt TS, Pincus SH, Hale ML, Moreira AL, Roy CJ, Tchou-Wong KM. Oropharyngeal aspiration of ricin as a lung challenge model for evaluation of the therapeutic index of antibodies against ricin A-chain for post-exposure treatment. Exp Lung Res. 2007;33:459-481. |
| 18. | Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol. 2004;172:6221-6228. |
| 19. | Hawiger J. Noninvasive intracellular delivery of functional peptides and proteins. Curr Opin Chem Biol. 1999;3:89-94. |
| 20. | Zhao Y, Lou D, Burkett J, Kohler H. Chemical engineering of cell penetrating antibodies. J Immunol Methods. 2001;254:137-145. |
| 21. | Zhao Y, Brown TL, Kohler H, Müller S. MTS-conjugated-antiactive caspase 3 antibodies inhibit actinomycin D-induced apoptosis. Apoptosis. 2003;8:631-637. |
| 22. | Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449-1452. |
| 23. | Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569-1572. |
| 24. | Jo D, Nashabi A, Doxsee C, Lin Q, Unutmaz D, Chen J, Ruley HE. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nat Biotechnol. 2001;19:929-933. |
| 25. | Jain M, Chauhan SC, Singh AP, Venkatraman G, Colcher D, Batra SK. Penetratin improves tumor retention of single-chain antibodies: a novel step toward optimization of radioimmunotherapy of solid tumors. Cancer Res. 2005;65:7840-7846. |
| 26. | Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57:637-651. |
| 27. | Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532-1555. |
| 28. | Juliano RL, Alam R, Dixit V, Kang HM. Cell-targeting and cell-penetrating peptides for delivery of therapeutic and imaging agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:324-335. |
| 29. | Cook DL, David J, Griffiths GD. Retrospective identification of ricin in animal tissues following administration by pulmonary and oral routes. Toxicology. 2006;223:61-70. |
| 30. | Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116:1059-1071. |