INTRODUCTION
Sphingosine-1-phosphate (S1P) is a bioactive lipid that has been implicated in regulating a variety of cell biological responses including cell proliferation, survival, differentiation and migration. S1P signals through a family of five G-protein-coupled receptors originally called EDG receptors but now renamed S1P1-5, that couple to a variety of G proteins and downstream pathways[1]. In addition, S1P has less-well-defined, receptor-independent intracellular functions including release of Ca2+ from intracellular stores[2] and regulation of histone acetylation[3].
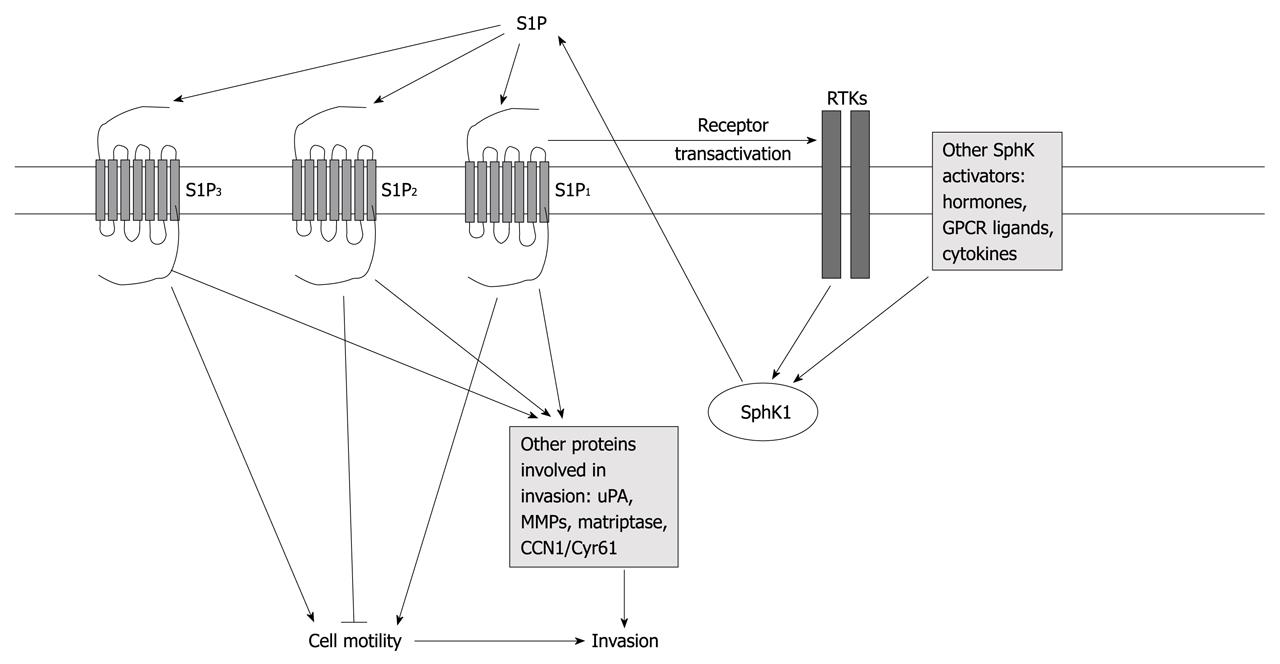
Numerous studies have linked S1P and sphingosine kinase (SphK), the enzyme that forms S1P, to cancer[4]. Many of these studies have implicated SphK/S1P signaling in enhancing cancer cell proliferation, preventing apoptosis, increasing drug resistance and stimulating tumor angiogenesis. Although a number of studies have also described the regulation of cancer cell migration, invasion and metastasis by S1P, the effects of S1P on these behaviors vary between different cancers and even among different models of the same cancer type. Thus, in some cases, S1P enhances migration and invasion, while in others, it is inhibitory. This review focuses on the effects of S1P on cancer cell migration, invasion and metastasis, and the mechanisms behind these pleiotropic responses. Figure 1 shows a simplified diagram of the interactions discussed below.
Figure 1 Diagram depicting mechanisms of regulation of cell motility and invasion by sphingosine-1-phosphate.
Signaling pathways are simplified for clarity. See text for more details and references. RTKs: Receptor tyrosine kinases; S1P: Sphingosine-1-phosphate; uPA: Urokinase plasminogen activator; MMPs: Matrix metalloproteinases.
DIFFERENTIAL REGULATION OF CELL MIGRATION BY S1P RECEPTORS
Initial studies have shown that S1P is primarily inhibitory to cancer cell migration. Thus, S1P blocks motility of melanoma and osteosarcoma[5] as well as breast cancer cell lines[6]. These responses are primarily thought to be mediated by receptor-independent effects of S1P, as in some studies they were not mimicked by dihydro-S1P, which also acts as an agonist for S1P receptors, and a photolyzable caged S1P which increases intracellular S1P was effective[7]. Nevertheless, another study has shown that S1P immobilized on glass beads can inhibit F10 melanoma cell motility[8], which suggests that cell surface receptors are involved at least in some cell types. Most of these early studies used high concentrations of S1P, in the micromolar range. The discovery of S1P receptors has revealed that the affinity of these receptors for S1P is in the nanomolar range[9]. As with a number of agents that can induce cell motility, S1P commonly stimulates cell migration in a bell-shaped dose-response curve with less effect or even inhibition at high concentrations[10,11]. Whether the decreased effects of S1P at high concentrations are due to overstimulation of receptors or to intracellular effects of S1P is not entirely clear.
Nevertheless, subsequent studies clearly have indicated that different S1P receptors have different effects on cell motility. Thus, S1P signaling decreases cell migration through Rho activation and induction of stress fibers in B16/F10 melanoma cells, which express predominantly the S1P2 receptor[12]. Furthermore, in CHO cells that overexpress individual S1P receptors and in B16 melanoma cells, using an S1P2 antagonist, S1P2 signaling has been shown to block cell migration through activation of Rho, which leads to inhibition of Rac[13,14]. These effects are functional in vivo as well, because S1P2 overexpression in melanoma cells decreases metastasis in a tail vein injection model, while S1P1 overexpression enhances metastasis[15].
In contrast to the anti-migratory effect of S1P2, S1P1 and S1P3 stimulate cell migration[14,16,17]. S1P receptor expression profile correlates with the effect of S1P on migration in gastric cancer cells, with S1P2 dominating in cells in which S1P blocks migration, and S1P1 and S1P3 dominating in cells in which S1P stimulates migration[18]. A similar correlation has been seen in glioblastoma cell lines[10,19]. Furthermore, S1P has been found to inhibit migration of glioma cells that predominantly express S1P2, but to stimulate migration after S1P2 is knocked down by siRNA, thus allowing the pro-migratory effects of S1P1 and S1P3[20]. The pro-migratory effects of S1P1 and S1P3 are thought to be due to the coupling of these receptors to Gi proteins[21,22]. Although S1P2 can couple to Gi, it favors coupling to G12/13, which leads to strong Rho activation[23].
There is some uncertainty about the mechanism by which S1P2 blocks cell migration. Lepley et al[19] have found that S1P2 signaling does not inhibit Rac in glioma cells, as has previously been shown in melanoma cells, but instead a Rho kinase mediated signal is necessary to block cell migration. Furthermore, Rho-dependent regulation of the lipid phosphatase PTEN has been shown to be necessary for S1P2-mediated block of migration of fibroblasts and endothelial cells[24]. Nevertheless, S1P2 is able to block migration of PTEN-negative glioma cells[25], which suggests that multiple pathways downstream of Rho can mediate the anti-migratory effect of S1P2 depending upon cellular context.
SphK SIGNALING AND RECEPTOR CROSSTALK REGULATING CANCER CELL MIGRATION
Several factors that lead to cancer cell migration have been shown to function at least in part through activation of SphK and subsequent production of S1P. SphK1 has been known for some time to be activated by platelet-derived growth factor (PDGF) signaling[26]. In a seminal paper, Spiegel’s group has shown that activation of SphK1 and subsequent stimulation of the S1P1 receptor by secreted S1P is necessary for PDGF-induced Rac activation and cell migration[27]. Subsequently a number of other studies have demonstrated the involvement of SphK and S1P signaling in signaling pathway crosstalk and receptor transactivation to regulate cell migration in a variety of cell types, as described below[28-35].
A number of studies have examined the interaction of SphK with epidermal growth factor receptor (EGFR) signaling in cell migratory responses. Activation and translocation of SphK1 to the plasma membrane in response to EGFR activation has been shown to be necessary for EGF-induced migration in MCF-7 breast cancer cells[28,29]. However, the signaling crosstalk could also proceed in the opposite direction, that is, SphK activation and subsequent S1P receptor activation that leads to transactivation of EGFR. Thus, also in MCF-7 cells, estrogen has been shown to activate SphK1 that leads to production and release of S1P. S1P then activates the S1P3 receptor, which in turn transactivates EGFR[30]. S1P also transactivates EGFR in another breast cancer cell line, MDA-MB-231, and stimulates cell migration through activation of both EGFR-dependent and independent signaling pathways[31]. S1P can also transactivate EGFR, c-met and HER2 in gastric cancer cells[32,33] and PDGF receptor in ovarian cancer cells through S1P3 signaling[34]. Furthermore, in follicular thyroid carcinoma cells, S1P transactivates vascular endothelial growth factor receptor (VEGFR)-2, and S1P receptors, and VEGFR-2 then cooperates to activate Akt, which leads to cell migration[35].
A recent study has indicated that expression level of SphK1 can have unexpected effects on migratory responses. Thus, in MCF-7 cells, S1P-induced activation of S1P3 stimulates migration through translocation of extracellular signal-regulated kinase (ERK) to membrane ruffles and the nucleus. However, in MCF-7 cells that overexpress the EGFR family member HER2, SphK1 expression is increased, and high levels of SphK1 expression lead to decreased expression of p21-activated protein kinase-1 (PAK1) and thus desensitize MCF-7 breast cancer cells to S1P-mediated stimulation of migration[36]. Knockdown of SphK1 with siRNA or overexpression of PAK1 reverses the effect and allows S1P to stimulate migration[36]. This unusual signaling pathway crosstalk appears to have important effects on tumor behavior because the same study has shown that, although high expression of SphK1 is correlated with reduced survival time in a large cohort of estrogen-receptor-positive breast cancer patients, within patients who are positive for HER1, HER2 or HER3, a high HER1-3/SphK1 ratio correlates with poorer prognosis than does a low HER1-3/SphK1 ratio[36]. Thus, the interplay of SphK and S1P with growth factor receptor signaling can be complex, and the details must be carefully examined in each cell type to understand the contributions of these pathways to cancer, and which pathways might be useful for therapeutic targeting.
Although most work on the involvement of SphK in cell migration has focused on SphK1, SphK2 may also be involved in some cases. EGF activates SphK2 via ERK-mediated phosphorylation[37]. Although SphK1 is necessary for EGF-induced migration of HEK293 cells, and SphK2 is not involved, EGF-induced migration of MDA-MB-453 breast cancer cells requires activation of both SphK1 and SphK2, because siRNA for either kinase prevents migration[38].
Although most of the work discussed above has focused on interactions of SphK/S1P signaling with growth factor receptor tyrosine kinase signaling, other types of receptors also interact with S1P to regulate cell migration. Other hormones and cytokines besides estrogen can activate SphK1[39-41]. For example, SphK1 is also necessary for migration of MCF-7 cells in response to prolactin or 17-β-estradiol[39]. Furthermore, G-protein-coupled receptor agonists are also known to activate SphK1, and in gastric cancer cells, lysophosphatidic acid, which signals through G-protein-coupled receptors that are closely related to S1P receptors, transactivates EGFR, which in turn, upregulates SphK1, which leads to S1P3-dependent migration and invasion[40]. In esophageal cancer cells, transforming growth factor (TGF)-β activates SphK1 to cause ERK activation that leads to enhanced migration[41]. This response requires S1P2, because siRNA to S1P2 blocks TGF-β-induced migration and invasion. The authors have suggested that this apparent contradictory pro-migratory effect of S1P2 in this case was due to its coupling primarily to Gi rather than G12/13 in these cells[41].
EFFECTS OF S1P ON INVASION AND METASTASIS
SphK and S1P have been shown to enhance invasiveness of a number of different cancer cells in in vitro models including, T lymphoma cells[42], Wilms tumor cells through S1P1 signaling[43], thyroid cancer cells by autocrine signaling in cells that overexpress SphK1[44], and in glioma cells[10]. S1P has also been found to regulate invasiveness of ovarian cancer cells in complex ways. S1P stimulates migration and invasiveness of ovarian cancer cells[45], but has no effect on migration of normal ovarian epithelial cells[46]. Furthermore, although low concentrations of S1P stimulate invasion of Dov13 ovarian carcinoma cells, high concentrations of S1P block migration via excess stress fiber formation and N-cadherin expression[47,48].
Although cell motility responses as discussed above are an important aspect of cancer invasion and metastasis, they are only a part of the entire process. Another important part of invasion by cancer cells is degradation of extracellular matrix materials through protease secretion. The enhancement of invasion of ovarian carcinoma cells mentioned above by low S1P concentrations appears to be mediated through Rac, which leads to regulation of the urokinase plasminogen activator (uPA) and matrix metalloproteinase-2[49]. Similarly, in glioblastoma cells, we and others have shown that S1P regulates a number of proteases that may be important for invasion and metastasis. Thus, S1P induces expression of uPA and its receptor, as well as plasminogen activator inhibitor, which leads to increased uPA activity[50,51]. Furthermore, neutralizing antibodies to uPA blocked S1P-receptor-induced invasion of glioblastoma cells in an in vitro spheroid invasion assay[51]. Upregulation of the uPA system appears to be mediated by S1P1 and S1P2 receptors[50,51]. Thus, although S1P2 is generally anti-migratory, it may contribute to invasion of glioma cells. In agreement, although S1P2 overexpression decreases motility of U-118 MG glioma cells, it enhances invasion of these cells through Matrigel[52]. This correlates with enhanced adhesion of S1P2-overexpressing U-118 MG cells to Matrigel, as well as induction of the matricellular protein CCN1/Cyr61[52], which is known to increase cell motility, invasiveness, and adhesion, as well as angiogenesis[53]. Neutralizing antibodies to CCN1 decrease S1P2-stimulated invasion of glioblastoma cells[51,52]. Another protease that is activated by S1P is matriptase[54]. Matriptase activates uPA and may thereby contribute to invasion[55]. Thus, S1P may contribute to invasion by multiple mechanisms in addition to regulation of cell motility, including regulation of proteases as well as cell adhesion.
LINKS OF MIGRATION/INVASION TO S1P-INDUCED ANGIOGENESIS
Another mechanism by which S1P signaling could affect metastasis is through induction of angiogenesis. S1P is well known to be a pro-angiogenic signal (see reviews by Takuwa and Agraves in this volume for more details). Angiogenesis provides tumors with needed nutrients and oxygen, however, it can also provide a means of access for cancer cells to the bloodstream for the purpose of metastasis. Angiogenic signaling is induced in tumors by hypoxia, which upregulates hypoxia-inducible factor (HIF)-1 and HIF-2. Hypoxia can also induce enhanced invasiveness of tumor cells as a means of escaping the stressful low oxygen environment by leading to induction of pro-metastatic gene expression[56]. Hypoxia has also been shown to induce expression of SphK1 and SphK2 in smooth muscle cells[57]. Hypoxia in U-87 glioma cells causes upregulation of SphK1 expression via HIF-2, which results in release of S1P[58]. Furthermore, conditioned media from hypoxic glioma cells causes an angiogenic response in human umbilical vein endothelial cells, which is mediated by S1P receptors[58].
Hypoxia has also been found to lead to short-term SphK1 activation that is mediated by reactive oxygen species and precedes HIF induction in a number of different cancer cells[59]. Furthermore, this SphK1 activation is necessary for HIF-1α upregulation[59]. Thus, SphK1 appears to be involved upstream of HIFs as a regulator of HIF-1α induction and downstream as a target for HIF-2α-mediated transcription[58,59]. Ultimately, upregulation of SphK1 under hypoxic conditions could lead to induction of angiogenesis and enhanced survival of tumor cells under stressful conditions. In agreement with this idea, hypoxia induces SphK2 expression in A549 lung cancer cells, which leads to S1P release and resistance to chemotherapeutics[60]. Given the above evidence that S1P release from cancer cells can modulate tumor cell migration and invasiveness, it is tempting to speculate that SphK1 activation/induction and S1P release in response to hypoxia can also promote tumor cell invasion.
Endothelial cell SphK1 may also be involved in tumor angiogenesis. Hypoxia in endothelial cells upregulates SphK1 expression via HIF1-α and HIF2-α, which results in enhanced endothelial cell migration[61]. Thus, SphK1 may be a key regulator of the angiogenic process at several levels, and therefore have important effects on tumor survival, growth and invasion.
THERAPEUTIC TARGETING
A number of groups have been examining mechanisms to target SphK/S1P signaling for cancer therapy[62-67]. Approaches include inhibition of SphK with pharmacological agents, blocking S1P with an anti-S1P antibody, and targeting S1P receptors using the sphingosine analog FTY720. These studies have shown promising efficacy in animal models; often leading to potent inhibition of tumor growth, induction of tumor cell apoptosis, and in some cases, inhibition of metastasis. A recent detailed and excellent review has discussed these therapies at length[4], and thus they are not considered further here.
CONCLUSION
In addition to its effects on cell proliferation, cell survival and chemotherapeutic resistance and tumor angiogenesis, SphK/S1P signaling clearly plays several roles in the regulation of cancer cell motility and invasiveness. The mechanisms behind these effects are complex. They involve reciprocal interactions with several other signaling pathways that may often be tumor and cell type specific, and include effects on cell migration, protease secretion and activation and cell adhesion. Although much of the work on S1P regulation of cancer cell invasion has been done in vitro, some work in animal models suggests that these effects are important in vivo as well. However, future work will need to explore these responses further in vivo, because in vitro models of invasion, although significantly easier to work with and quantitate, often do not effectively mimic the in vivo situation.
Lastly, the ability of S1P signaling to regulate invasiveness positively and negatively may have important implications for the eventual targeting of this pathway in cancer therapy. Thus, a full understanding of the effects of S1P on a particular cancer type and the mechanisms and receptors involved are necessary to design future therapeutics that have the desired effect.
Peer reviewers: Christine Blattner, PhD, Principal Investigator, Karlsruher Institut für Technologie, ITG-Institute of Toxicology and Genetics, PO Box 3640, 76021 Karlsruhe, Germany; Takuji Tanaka, MD, PhD, Professor, FIAC, Director of The Tohkai Cytopathology Institute: Cancer Research and Prevention,5-1-2 Minami-Uzura, Gifu 500-8285, Japan; Evangelia Papadimitriou, PhD, Associate Professor in Molecular Pharmacology, Laboratory of Molecular Pharmacology, Department of Pharmacy, University of Patras, GR 26504, Greece
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM