Published online Jun 27, 2017. doi: 10.4240/wjgs.v9.i6.153
Peer-review started: October 27, 2016
First decision: November 22, 2016
Revised: April 2, 2017
Accepted: May 18, 2017
Article in press: May 20, 2017
Published online: June 27, 2017
Processing time: 234 Days and 20.9 Hours
To assess the impact of multi-disciplinary teams (MDTs) management in optimising the outcome for rectal cancers.
We undertook a retrospective review of a prospectively maintained database of patients with rectal cancers (defined as tumours ≤ 15 cm from anal verge) discussed at our MDT between Jan 2008 and Jan 2011. The data was validated against the national database to ensure completeness of dataset. The clinical course and follow-up data was validated using the institution’s electronic patient records. The data was analysed in terms of frequencies and percentages. Significance of any differences were analysed using χ2 test. A Kaplan-Meier analysis was performed for overall survival and disease free survival.
Following appropriate staging, one hundred and thirty-three patients were suitable for potentially curative resections. Seventy two (54%) were upper rectal cancer (URC) - tumour was > 6 cm from the anal verge and 61 (46%) were lower rectal cancers (LRC) - lower extent of the tumour was palpable ≤ 6 cm. Circumferential resection margin (CRM) appeared threatened on pre-operative MRI in 19/61 (31%) patients with LRC requiring neo-adjuvant therapy (NAT). Of the 133 resections, 118 (89%) were attempted laparoscopically (5% conversion rate). CRM was positive in 9 (6.7%) patients; Median lymph node harvest was 12 (2-37). Major complications occurred in 8 (6%) patients. Median follow-up was 53 mo (0-82). The 90-d mortality was 2 (1.5%). Over the follow-up period, disease related mortality was 11 (8.2%) and overall mortality was 39 (29.3%). Four (3%) patients had local recurrence and 22 (16.5%) patients had distant metastases.
Management of rectal cancers can be optimized with multi-disciplinary input to attain acceptable long-term oncological outcomes even when incorporating a laparoscopic approach to rectal cancer resection.
Core tip: Recently, management of rectal cancer has undergone a process of standardization with introduction of total mesorectal excision and use of neo-adjuvant long course chemo-radiotherapy. In the United Kingdom, multimodal therapy is provided under the auspices of multi-disciplinary teams (MDTs). This is the first study to report on the benefits of managing patients jointly within such an MDT.
- Citation: Dhruva Rao PK, Peiris SPM, Arif SS, Davies RA, Masoud AG, Haray PN. Value of multi-disciplinary input into laparoscopic management of rectal cancer - An observational study. World J Gastrointest Surg 2017; 9(6): 153-160
- URL: https://www.wjgnet.com/1948-9366/full/v9/i6/153.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i6.153
Rectal cancer accounts for a third of patients with large bowel cancer[1,2]. Historically, management of rectal cancers has been of variable standard with significant differences in local recurrence rates[3-6]. The Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the National Institute for Health and Care Excellence (NICE) have both recommend that rectal cancer should be managed by a multi-disciplinary team (MDT)[7,8]. This has led to initiatives to standardize MDT practises across the country.
Currently, nearly 90% of patients with colorectal cancer undergo discussion and treatment planning at an MDT in the United Kingdom[2]. Total mesorectal excision (TME) has been established as the gold standard for the management of mid and lower rectal cancers over the last few years following the results of numerous trials such as the MR CR07 and Dutch TME trials[5,6,9]. The role of neo-adjuvant therapy is also well established in patients with threatened margins[7,8].
We have had an established MDT team managing colorectal cancer since 1997. Our unit has been performing laparoscopic rectal resection under the auspices of the MDT since 2000, initially in selected cases and since 2008, with increased experience, as the default approach. NICE recommends laparoscopic rectal resection by experienced surgeons[10].
We undertook this retrospective analysis of a prospectively maintained database to assess the effectiveness of our MDT rectal cancer management outcomes.
Rectal cancer = All cancers ≤ 15 cm from anal verge as measured during rigid sigmoidoscopic examination were classified as rectal cancers. These were further categorized as below: Lower rectal cancer (LRC) = All palpable tumours (≤ 6 cm from anal verge); upper rectal cancer (URC) = All other tumours (6-15 cm from anal verge); Circumferential resection margin (CRM) positivity = if CRM < 1 mm (Both on pre-op MRI and at histopathology); Local or distant metastasis was defined on the basis of radiological evidence.
Our MDT consists of 3 colorectal surgeons, 1 specialist GI clinical oncologist, 2 specialist radiologists, 1 pathologist, 1 colorectal specialist nurse, 1 enhanced recovery co-ordinator, 2 enterostomal therapists, 1 palliative care consultant/specialist nurse and 2 gastroenterologists. This team meets every week and has been active since 1997 with a track record of publications, awards and innovative solutions to enhancing quality of care and patient experiences[11-13]. Non clinical business meetings of the team are held to facilitate the formulation and agreement of local protocols for colorectal cancer diagnosis, investigations and treatment.
All patients diagnosed with rectal cancer were staged with a computerized tomography (CT) scan of thorax, abdomen and pelvis. They also underwent either a colonoscopy or a CT colonogram (done as a part of staging CT). All patients with LRC and some with URC underwent a magnetic resonance imaging (MRI) of rectum for local staging as per the T2 weighted fast spin echo protocol, in 5 mm slices in the axial, coronal and sagittal planes in addition to oblique axials targeted at right angles to the axis of the tumour, using 3 mm slices and smaller “Field of View” for maximal resolution. As per common practice in the United Kingdom, none of our patients underwent endo-rectal ultrasound scanning.
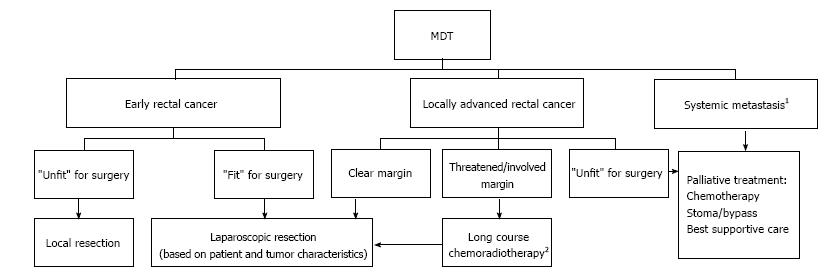
The staging investigations of all patients were reviewed by the MDT and treatment plans formulated according to the MDT protocol (Figure 1). Patients with threatened CRM were offered neo-adjuvant therapy (NAT) given as a pre-operative Long Course Chemo-Radiotherapy (LCRT), 45 Gy in 25 fractions to the pelvis over 5 wk with concurrent Capecitabine chemotherapy. In addition, short course radiotherapy 25 Gy in 5 fractions over 1 wk was considered in patients with moderate risk rectal cancers. The patients were then restaged and reviewed at MDT prior to surgery. Cases considered suitable for resection were scheduled for surgery 6-10 wk following NAT.
All patients with URC were planned for an anterior resection (AR). Planned surgical options for patients with LRC were either total mesorectal excision with de-functioning ileostomy (TME + I) or when the sphincters or levators were threatened, an abdomino-perineal excision (APER).
Post-operative histology was reviewed by the MDT and clinically fit patients with poor prognostic features on histology were offered adjuvant treatment (AT) with Oxaliplatin and 5 fluorouracil based combination chemotherapy.
The default surgical approach was laparoscopic resection except when the patient had had multiple previous surgery, anaesthetic considerations precluded a laparoscopic approach and occasionally due to technical issues such as particularly obese male patients with bulky tumours not responsive to neo-adjuvant treatment. We defined conversion as previously published[12]: (1) If the final incision made was longer than planned pre-operatively; (2) If the incision needed to be made at an earlier stage of the operation than planned pre-operatively; and (3) If the incision was made at a site other than that planned pre-operatively.
All laparoscopic procedures were performed by one of two consultant surgeons (each with experience of over 100 colorectal resections at the beginning of the study period) or by senior trainees under direct supervision (consultant scrubbed). All procedures were performed with the patient in the Lloyd Davies position with steep Trendelenburg tilt, following a step-wise approach (Table 1)[14,15]. The open procedures and the converted cases followed a similar step-wise approach through a midline laparotomy.
| 1 | Port positions: 10-12 mm - sub-umbilical, RUQ (camera), RIF and LIF; patient in Lloyd-Davies position |
| 2 | Omentum to supracolic compartment and small bowel stacking |
| 3 | Identify right ureter |
| 4 | Start medial dissection at the promontory |
| 5 | Identify left ureter, then left gonadal, pelvic nerves |
| 6 | Protect left ureter with surgicel® and Pedicle dissection |
| 7 | Identify ureter through both windows of mesentery either side of pedicle |
| 8 | Transect pedicle, confirm haemostasis |
| 9 | Left lateral dissection, identify left ureter and proceed up to peritoneal reflection; IMV high tie and splenic flexure mobilisation, if required |
| 10 | Mesorectal Dissection and preparation of rectum for division1 |
| Right mesorectal dissection up to peritoneal reflection | |
| Posterior dissection (presacral plane down to levator), keep left ureter in view | |
| Divide peritoneal reflection anteriorly and dissect till seminal vesicles/vaginal fornix | |
| Complete both lateral dissection, identify the ureters all the way | |
| Anterior dissection keeping to the plane just posterior to the vesicles/vagina | |
| Rectal Cross stapling (achieve antero-posterior staple line) or proceed to perineal dissection1 | |
| 11 | Intra-corporeal cross stapling of rectum at appropriate level protecting lateral and anterior structures and Grasp stapled end of specimen |
| 12 | Left iliac fossa port extended as a transverse incision for specimen delivery; protect wound and deliver specimen by the stapled end |
| 13 | Complete mesenteric ligation, proximal bowel division and prepare proximal bowel for anastomosis |
| 14 | Close wound, re-establish pneumoperitoneum |
| 15 | Intra-corporeal bowel anastomosis with no tension, no twist and vital structures protected |
| 16 | Close incisions |
All patients were reviewed initially at 6 wk after their surgery. The follow up protocol was a 6 monthly clinical review with haematological and biochemical tests including tumour marker CEA for 5 years, an annual CT scan of thorax, abdomen and pelvis for 3 years and a surveillance colonoscopy at 3 and 6 years. The length of follow-up was recorded in months from the date of operation.
After appropriate institutional approvals, all patients with rectal cancer discussed at our MDT meeting between Jan 2008 and Jan 2011 were identified and the patient demographics, treatment, post-operative histology and follow-up data were studied.
The primary outcome measures of the study were local recurrence rates and disease free survival. The secondary outcome measures included post-operative length of stay, major complications and overall survival.
The data was analysed in terms of frequencies and percentages. Significance of any differences were analysed using χ2 test. A Kaplan-Meier analysis was performed for overall survival and disease free survival.
During these 3 years, a total of 141 patients [median age 67 years (range 45-89); M:F = 1.7:1] were diagnosed with rectal cancer. Of these, there were 2 patients with locally advanced disease invading prostate and so were referred for exenteration elsewhere. A further 6 patients went on to have palliative treatment due to either advanced presentation or significant medical co-morbidities. The remaining 133 patients were staged as suitable for potentially curative resections. Of these, 72 (54%) were upper rectal tumours (URC) and 61 (46%) were lower rectal tumours (LRC). Three (2%) patients had resectable metastases at diagnosis and were treated with primary rectal resection, followed by chemotherapy and surgery for metastases.
The pre-operative (putative) CRM was threatened in 19 (14%) patients (4 patients due to presence of nodes close to the CRM). Of these, 14 patients had LCRT; 1 had short course radiotherapy (25 Gy in 5 fractions over 1 wk). Four patients did not receive any Neoadjuvant therapy: 1 female patient with an anterior tumour where there was lack of consensus on preoperative staging being T2 vs T4 and 3 patients where there was a small node of doubtful significance threatening the margin.
Interval between completion of NAT and surgery was a median 10 (6-24) wk. One patient had a radiological complete response to neo-adjuvant therapy and opted initially for a watch and wait policy prior to eventually opting to receive surgery.
Table 2 summarizes the operations performed. All 72 patients with URC underwent an AR. Of the 61 with LRC, 29 had TME + I, 1 patient had a TME Hartmann’s procedure and 27 had APER. Four patients had TME and anastomosis without covering ileostomy. Surgery following NAT was either APER (8/15) or TME + I (7/15).
| Operations | Laparoscopic (conversion) | Open | Total |
| Anterior resections | 66 (2) | 6 | 72 |
| TME | 4 | 4 | |
| TME + I | 25 (1) | 4 | 29 |
| TME Hartmann’s | 1 (1) | 1 | |
| APER | 26 (2) | 1 | 27 |
Laparoscopic resection was attempted in 118/133 (89%). Conversion rate was 5% (6 out of 118 patients). The reasons for conversion were uncontrollable bleeding from the IM pedicle (n = 1), low tumour in a male pelvis, requiring a suprapubic incision rather than the planned left iliac fossa incision for specimen delivery (n = 1) and dense adhesions (n = 4), requiring incisions either larger than planned or at an earlier stage of the operation). The remaining 15 patients (11%) underwent a planned open procedure due to previous extensive surgery, locally advanced tumour in an android pelvis or poor response to LCRT.
Median post-operative length of stay was 5 d (3-49). Major complications needing re-operation within 30 d occurred in 8 (6%) patients [Anastomotic leak: 2, Pelvic haemorrhage requiring packing: 2, Small Bowel Obstruction: 2 (1 - port site; 1 - pelvic), Intra-abdominal collection: 1, Wound dehiscence: 1].
Post-operative histology is shown in Table 3. One hundred and twenty four patients (93.3%) had R0 resection and 9 (6.7%) had an R1 resection (CRM positive - 6 due to tumour, 3 due to nodes). There were no R2 resections in this cohort. Median LN harvest was 12 for the laparoscopic group and 10 for the open group (P < 0.01). Of the 9 patients with positive CRM 4 were URC and 5 were LRC. The pre-operative MRI had accurately predicted this in all 5 LRC patients, 4 of whom had received NAT. None of the URCs had had pre-operative MRI as per our practice at that time and so could not be predicted and they did not receive any NAT.
| Post-op stage | n |
| R0 resection | 124 |
| R1 resection (CRM + ve) | 9 |
| R2 resection | 0 |
| T1 | 14 |
| T2 | 42 |
| T3 | 58 |
| T4 | 17 |
| N0 | 85 |
| N1 | 31 |
| N2 | 15 |
Fifty-six patients had adverse features on histology making them eligible for adjuvant therapy (AT). Of these, 13 were unfit and 3 declined the offer of further chemotherapy. The remaining 40 patients underwent AT.
Median follow up was for 53 mo (0-82). Long-term complications occurred in 9 (6.7%) patients (parastomal hernia: 6, port site hernia: 1, anastomotic stricture: 1, late onset left ureteric obstruction due to fibrosis: 1).
The 90-d mortality was 1.5% (2 patients: 1 in-hospital due to anastomotic leak; 1 patient post discharge - cause unknown). Disease related mortality over the follow-up period was 11 (8.2%); however, overall mortality for the follow-up period was 39 (29.3%).
Four patients (3%) had local recurrence. The durations to development of local recurrence were 15, 23, 33 and 39 mo. On further analysis of the sub-group with local recurrence, only 1 patient had had a histologically positive CRM. This patient had an upper rectal tumour and had not been considered for NAT. The other 3 patients having local recurrence were all T3 URC and all had had a R0 resection with CRM clearance of between 1-2 mm. In this cohort, we had no local recurrence in any patients with LRC.
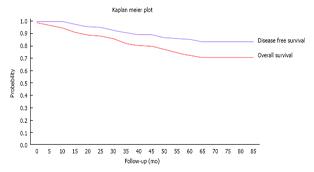
Twenty two patients (16.5%) developed distant metastases and one patient developed metachronous colonic cancer. Four of these had no poor prognostic factors on histology such as node positive disease, extra-mural lympho-vascular invasion and/or poor differentiation. Of the 18 with poor prognostic markers, 3 had declined and 5 had been deemed unfit for AT. Figure 2 shows the Kaplan- Meier curve for our cohort.
Patients in our unit have been receiving care under the MDT umbrella since 1997. Our unit has a relatively high uptake of laparoscopic rectal resections with 89% undergoing laparoscopic resection with a relatively low conversion rate using strict definitions for conversion. The median length of stay was 5 d and is comparable to most enhanced recovery programmes. Oncological results too are acceptable with a CRM positivity rate of 4% for sphincter saving resections (4 out of 106 patients) and 18% for APER (5 out of 27 patients). LNH was higher following laparoscopic resection, in keeping with other studies[16].
MDT management is a concept propagated by practice with no “research/trial” based evidence. There is no level 1 evidence that supports MDT, no grade of recommendation is provided for its use in national guidelines and yet, this concept is gaining acceptance worldwide. MDT management has been a mandatory requirement for treatment of cancers in United Kingdom since 2000. For this reason, we cannot perform a meaningful comparative analysis of patients who have not received care under the MDT umbrella. The management of the rectal cancer has also undergone a significant change over this period. This precludes use of a historical cohort for comparison as there could be other confounding factors that influence outcomes.
We believe that this the first observational study attempting to clarify the role of various MDT members who make individual specialist contributions, based on consensual decisions arrived at by a group of experts, resulting in improved clinical effectiveness.
Lap TME has been shown to be safe with acceptable short-term clinical and oncological outcomes[5,17-19]. The 2 most recent trials, ALaCaRT and the ACOSOG Z6051, have not been able to demonstrate the non-inferiority of laparoscopic resections compared to open resections in terms “completeness of excision” using a composite scoring system[20,21]. However, they are still accruing data on long term oncological outcomes. Laparoscopic TME can be technically challenging and should be undertaken by experienced surgeons[12,20-22]. Caution should therefore be exercised when evaluating results of laparoscopic TME when the expertise of the surgeons has not been defined. The senior surgeons have had a mean experience of 6 years between them with over 100 laparoscopic resections each prior to the commencement of this study. From this study, we see that acceptable long-term oncological results can be safely achieved when laparoscopic approach is pragmatically applied by appropriately trained surgeons in the context of multimodal therapy overseen by MDT.
The few RCTs reporting 5 year survival were not specifically designed or powered for long term outcomes[3]. More recently several meta-analyses published have not come up with any strong conclusions either way with respect to long-term survival[3,4,19,23,24]. However, laparoscopic resection seems to be associated with a lower local recurrence rate[24]. This lack of clarity has been the cause for variable uptake of Lap TME ranging from 0%-100%[2,25].
We believe that this study is one of the first to report on outcomes of laparoscopic rectal resections outside of RCTs or case control studies. Tables 4 and 5 show our results which compare favourably to other published studies. Figure 2 shows the Kaplan Meier curves for our cohort which shows an overall survival of 81% and disease free survival of 90% at median follow-up. This compares favourably with other series with similar follow-up which have reported a predicted overall survival of 81% and disease free survival of 70%[26]. Our survival figures show that our cohort of patients were more likely to die from other causes than from disease recurrence, in keeping with the high co-morbidity of our catchment population[27], most of which falls within the highest quintile of the deprivation index in the United Kingdom.
A 12-year follow-up of Dutch TME trial cohort showed local recurrence of 6.5% (68 patients) in 1082 patients who had an R0 resection[28]. In comparison, we observed a local recurrence rate of 2.4% (3 patients) in 124 patients having an R0 resection. All recurrences were in patients with URC with no recurrences in LRC. We observed only 1 local recurrence in 9 patients who had an R1 resection (11.1%). However extrapolating similar data from the Dutch TME trial would give a figure of 20.8% patients with involved margins developing a local recurrence. This comparison however, may be misleading as the follow up in our study (53 mo) is shorter than the Dutch TME trial (12 years).
Traditionally, local recurrence after rectal cancer resection usually presents within 2 years[2,28]. In our series, we have had a median follow up of 53 mo and have not noticed any local recurrence in the LRC group. The follow-up of the Dutch TME trial cohort confirmed that pre-operative radiotherapy not only reduced local recurrence but was especially effective in preventing anastomotic recurrences[28]. The same effect probably accounts for the absence of local recurrence noted in our study for the low rectal cancers in spite of 10% (6 of 61 LRC) CRM positivity. Another hypothesis worth considering could be that CRM positivity due to lymph nodes may carry a lesser risk of local recurrence when compared with cases where the CRM was involved by the primary tumour.
We believe this observed low rate of local recurrence is due to effective working within a well-established specialist MDT, resulting in appropriate use of NAT for our cohort of patients.
In conclusion, this study demonstrates that good long term oncological outcomes can be achieved for patients with rectal cancer when appropriate multi-disciplinary expertise is combined with surgery being performed by adequately trained surgeons. Neo-adjuvant chemo-radiotherapy improves the oncological outcomes without precluding the routine use of the laparoscopic approach to rectal resection.
The authors would like to thank all members of the MDT for their commitment to providing high quality care to these patients. In particular, we would like to acknowledge Mr Shah PR (surgeon), Mrs Lewis M, Mrs Williams D, Mrs Howells S (specialist nurses) for their contribution to maintaining the database and Mr Brown C (trainee) for help in updating the follow-up data.
Rectal cancer accounts for a third of patients with large bowel cancer. Historically, management of rectal cancers has been of variable standard with significant differences in local recurrence rates. The Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the National Institute for Health and Care Excellence (NICE) have both recommend that rectal cancer should be managed by a multi-disciplinary team (MDT). This has led to initiatives to standardize MDT practises across the country. The authors undertook this retrospective analysis of a prospectively maintained database to assess the effectiveness of the MDT rectal cancer management outcomes.
Providing evidence to the concept of multidisciplinary management of rectal cancer; optimizing outcomes following laparoscopic rectal resection.
This study adds evidence to the increasing evidence in the evolving management of rectal cancers
MDT consists of Colorectal Surgeons, Specialist GI Clinical Oncologist, Specialist GI Radiologists, Specialist GI pathologist, Colorectal Specialist Nurse, Enhanced Recovery co-ordinator, Enterostomal Therapists, Palliative care specialists and Gastroenterologists.
This is a good paper, showing that excellent results can be achieved by dedicate teams. This retrospective analysis focus on the MDT (several related specialities coming together every week) on rectal cancer management and they suggest MDT for better early and late outcomes and for laparoscopy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fiori E, Kayaalp C, Tiberio GA S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Cancer Research UK. Bowel Cancer Incidence Statistics. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/uk-bowel-cancer-incidence-statistics. |
| 2. | Association of Coloproctology of Great Britain & Ireland. National Bowel Cancer Audit Annual Report 2013. ACPGBI Website;. 2013;. |
| 3. | Ng SS, Lee JF, Yiu RY, Li JC, Hon SS, Mak TW, Leung WW, Leung KL. Long-term oncologic outcomes of laparoscopic versus open surgery for rectal cancer: a pooled analysis of 3 randomized controlled trials. Ann Surg. 2014;259:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Trastulli S, Cirocchi R, Listorti C, Cavaliere D, Avenia N, Gullà N, Giustozzi G, Sciannameo F, Noya G, Boselli C. Laparoscopic vs open resection for rectal cancer: a meta-analysis of randomized clinical trials. Colorectal Dis. 2012;14:e277-e296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2292] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 6. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3109] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 7. | Association of Coloproctology of Great Britain & Ireland. Guidelines for Management of Colorectal Cancer. Available from: http://acpgbi.mixd.co.uk/content/uploads/2007-CC-Management-Guidelines.pdf. |
| 8. | National Institute of Clinical Excellence. Colorectal Cancer: The diagnosis and management of colorectal cancer. NICE Clinical Guidelines 131. Available from: http://www.nice.org.uk/guidance/cg131/resources/guidance-colorectal-cancer-pdf. |
| 9. | Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 747] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 10. | National Institute of Clincal Excellence. Laparoscopic surgery for colorectal cancer - NICE Technology Appraisals [TA105]. United Kingdom: NICE 2009; . |
| 11. | Shah PR, Haray PN. Colorectal Cancer Information DVD - The Ultimate Development In Patient Empowerment! Colorectal Dis. 2010;12:46. |
| 12. | Shah PR, Haray PN. A tool-kit for the quantitative assessment of proficiency in laparoscopic colorectal surgery. Colorectal Dis. 2011;13:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Shah PR, Joseph A, Haray PN. Laparoscopic colorectal surgery: learning curve and training implications. Postgrad Med J. 2005;81:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Dhruva Rao PK, Shah PR, Haray PN. The Stepwise Approach to laparoscopic colorectal resection - making training safer. LTP21. Colorectal Dis. 2013;15:31. |
| 15. | Shah PR, Haray P. Laparoscopic rectal excision made easy: A stepwise approach - Video Presentation V078. Surg Endosc. 2011;25:S167. |
| 16. | Boutros M, Hippalgaonkar N, Silva E, Allende D, Wexner SD, Berho M. Laparoscopic resection of rectal cancer results in higher lymph node yield and better short-term outcomes than open surgery: a large single-center comparative study. Dis Colon Rectum. 2013;56:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc. 2004;18:1211-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 750] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 19. | Dhruva Rao PK, Nair MS, Haray PN. Feasibility and oncological outcomes of laparoscopic rectal resection following neo-adjuvant chemo-radiotherapy: A systematic review. World J Surg Proced. 2015;5:147-154. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 761] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 21. | Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR, Maun D, Chang G, Herline A. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 821] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 22. | Shah PR, Gupta V, Haray PN. A unique approach to quantifying the changing workload and case mix in laparoscopic colorectal surgery. Colorectal Dis. 2011;13:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Huang MJ, Liang JL, Wang H, Kang L, Deng YH, Wang JP. Laparoscopic-assisted versus open surgery for rectal cancer: a meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Colorectal Dis. 2011;26:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Ahmad NZ, Racheva G, Elmusharaf H. A systematic review and meta-analysis of randomized and non-randomized studies comparing laparoscopic and open abdominoperineal resection for rectal cancer. Colorectal Dis. 2013;15:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Penninckx F, Kartheuser A, Van de Stadt J, Pattyn P, Mansvelt B, Bertrand C, Van Eycken E, Jegou D, Fieuws S. Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br J Surg. 2013;100:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Staudacher C, Di Palo S, Tamburini A, Vignali A, Orsenigo E. Total mesorectal excision (TME) with laparoscopic approach: 226 consecutive cases. Surg Oncol. 2007;16 Suppl 1:S113-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Welsh Government. Deprivation and Health: Merthyr Tydfil. Welsh: Welsh Government Website 2006; . |
| 28. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1338] [Article Influence: 95.6] [Reference Citation Analysis (0)] |