Published online Sep 27, 2014. doi: 10.4240/wjgs.v6.i9.175
Revised: June 21, 2014
Accepted: July 17, 2014
Published online: September 27, 2014
Processing time: 125 Days and 11.6 Hours
AIM: To provide an overview of the literature on pancreatic extragastrointestinal stromal tumors (EGISTs).
METHODS: We report a case of pancreatic EGIST and review published studies on pancreatic EGIST accessed via the PubMed, MEDLINE, Google Scholar, and Google databases. The keywords used were “pancreas and GIST”, “pancreas and extra GIST”, “pancreas and gastrointestinal stromal tumor”, and “pancreas and extragastrointestinal stromal tumor”. Literature reviews and/or duplicate studies were excluded. The search included articles published in the English language between January 1, 2000 and May 15, 2014.
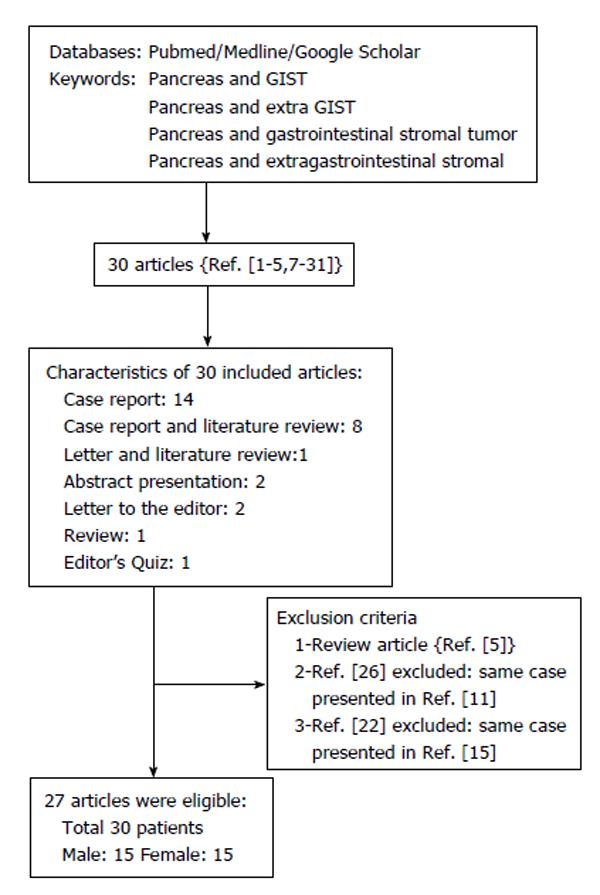
RESULTS: From our literature survey, 30 manuscripts on pancreatic EGISTs were considered, of which 27 met the search criteria and three were excluded. The studies involved 30 patients (15 men, 15 women) with a mean age of 55.3 ± 14.3 years (range 30-84 years). The mean age of the male patients was 50.8 ± 13.7 years (range 30-84 years); that of the female patients was 59.9 ± 13.3 years (range 38-81 years). Tumor dimensions were obtained for 28 cases (mean 114.4 ± 78.6 mm; range 20-350 mm). Tumors were diagnosed incidentally in 23.3% of patients; abdominal discomfort and weight loss were the major complaints in symptomatic patients. Risk of aggressive behavior according to Fletcher criteria was determined in 25 of 30 patients (68%: high risk, 28%: intermediate risk, 4%: low risk). Histopathological examination revealed the presence of spindle cells in 96.1% of cases; CD117 and CD34 were present immunohistochemically in 96.6% and 84% of patients, respectively. The most common surgical procedures were distal pancreatectomy with splenectomy (n = 9) and pancreaticoduodenectomy (n = 7). The total follow-up period for the 28 patients ranged from 3-66 mo, during which locoregional or distant metastases were diagnosed in six patients and two patients died.
CONCLUSION: Studies on EGISTs have only been published in the last decade. The lack of studies with large patient cohorts and long-term follow-up limits evidence-based commentary. In theory, each case should be assessed individually and further genetic and immunohistochemical studies are needed.
Core tip: Gastrointestinal stromal tumors are the most common gastrointestinal (GI) tract tumors of mesenchymal origin. Stromal tumors of extragastrointestinal origin are termed extragastrointestinal stromal tumors (EGISTs) and are not associated with the walls of GI tubular organs or the serosal walls. The pancreas is among the organs that are rarely the site of origin, and according our knowledge, about 30 cases of pancreatic EGISTs have been reported to date. In this study, we reviewed studies on pancreatic EGISTs and report a case of pancreatic head EGIST.
- Citation: Akbulut S, Yavuz R, Otan E, Hatipoglu S. Pancreatic extragastrointestinal stromal tumor: A case report and comprehensive literature review. World J Gastrointest Surg 2014; 6(9): 175-182
- URL: https://www.wjgnet.com/1948-9366/full/v6/i9/175.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i9.175
Gastrointestinal stromal tumors (GISTs) are the most common tumors of mesenchymal origin in the gastrointestinal (GI) tract[1-3]. The disease originates from neoplastic transformation of the interstitial cells of Cajal or their precursors in the GI tract. Although GISTs can be diagnosed in all sites of the GI tract, i.e., from the esophagus to the anus, they are most commonly diagnosed in the stomach and intestines[1-6]. Stromal tumors of extragastrointestinal origin are termed extragastrointestinal stromal tumors (EGISTs) and are not associated with the walls of GI tubular organs or serosal surfaces[3,7,8]. The morphological, histopathological, immunohistochemical, and molecular profiles of EGISTs are similar to those of GISTs[2,9,10]. Although EGISTS potentially originate from a variety of sites in the abdominal cavity, the majority of initial tumor progression sites include the omentum, retroperitoneum, mesentery, and the liver[1,2,11,12]. The pancreas is rarely the site of origin, and according our knowledge, 30 cases of pancreatic EGISTs have been reported to date[1-5,7-31]. We report a case of pancreatic EGIST and review the literature on pancreatic EGISTs.
Our primary aim was to report the rare case of a 61-year-old patient who underwent surgical treatment for pancreatic head EGIST. The secondary aim was to analyze previously published articles related to pancreatic GIST. We searched for published studies on pancreatic GIST using different keyword combinations, including “pancreas and GIST”, “pancreas and extra-GIST”, “pancreas and gastrointestinal stromal tumor”, and “pancreas and extra-gastrointestinal stromal tumor” in the PubMed, MEDLINE, Google Scholar, and Google databases. Studies for which full-text versions were available and that contained adequate patient details for comparison were included; literature reviews and duplicate reports were excluded. The publication language was not an exclusion criterion, and studies published before May 15, 2014 were included. Tables 1 and 2 lists the year of publication, country, patient age and sex, clinical presentation, physical examination, radiological tests, tumor size (mm), cell type (spindle, epithelioid, mixed), mitotic count [per high-power field (HPF)], immunohistochemical staining (CD117, CD34), surgical procedure, recurrence, outcome, and follow-up obtained from the studies.
| Ref. | Year | Country | Age (yr) | Sex | Clinical presentation | Examination | Radiologic tools | Tumor location | Tumor size (cm) |
| Tian et al[4] | 2014 | China | 61 | M | Incidental finding | Abdominal mass | CT | Tail | 60 × 80 |
| 60 | M | Incidental finding | NS | CT | Head | 60 × 50 | |||
| Paklina et al[11] | 2013 | Russia | 38 | F | Abdominal discomfort | NS | CT | Head | 90 |
| Serin et al[1] | 2013 | Turkey | 30 | M | Abdominal distension | NS | US + CT | Tail | 130 |
| Soufi et al[16] | 2013 | Morocco | 39 | M | Weight loss + abd pain + constipation | Distension | CT + endoscopy | Head | 90 × 70 × 50 |
| Wegge et al[2] | 2012 | USA | 55 | M | Haematemesis + haematochezia | Non-specific | CT + MRCP + endoscopy | Head | 46 × 45 × 44 |
| Babu et al[13] | 2012 | China | 55 | F | Upper abdominal pain | Non-specific | CT + US | Head | 50 × 40 × 30 |
| Kim et al[3] | 2012 | Korea | 55 | M | Abdominal discomfort | Non-specific | CT + MR | Tail | 130 × 90 × 85 |
| Čečka et al[9] | 2011 | Czech | 74 | F | Abdominal mass | Palpable mass | US + CT | Tail | 110 × 80 × 40 |
| Vij et al[14] | 2011 | India | 35 | M | Weight loss + abdominal discomfort | Non-specific | US + CT | Head | 80 × 60 |
| Rao et al[7] | 2011 | India | 40 | M | Weight loss + abdominal pain + anemia | Non-specific | US + CT | Head + Body | 65 × 60 |
| Yang et al[15] | 2011 | China | 55 | M | Abdominal discomfort | Abdominal mass | CT + MR | Body + Tail | 178 × 196 |
| Barros et al[12] | 2011 | Brasil | 63 | F | Abdominal pain + ponderal loss | NS | NS | NS | NS |
| 81 | F | Difficult gastric emptying + ponderal loss | NS | NS | NS | 100 | |||
| Joshi et al[17] | 2010 | USA | 84 | M | Weight loss + abdominal distension | Distension | CT | Entire pancreatic tissue | 340 × 240 × 270 |
| Crisan et al[18] | 2010 | Romania | 61 | M | Weight loss + fever + intense sweating | Diffuse tenderness | CT X | Tail + Body | 140 |
| Saif et al[19] | 2010 | USA | 31 | M | Weight loss + abdominal pain + anemia | NS | CT + MR + endoscopy | Head | 56 × 51 × 42 |
| Padhi et al[8] | 2010 | India | 42 | F | Weight loss + abdominal pain | Palpable mass | CT + MR | Body + Tail | 350 × 300 × 250 |
| Harindhanavudhi et al[20] | 2009 | USA | 63 | F | Fatigue + weakness+anemia | Non-specific | CT + EUS | Body | 160 × 110 |
| Trabelsi et al[21] | 2009 | Tunisia | 52 | F | Epigastric pain | Palpable mass | US + CT | Head | 105 × 80 × 30 |
| Goh et al[10] | 2009 | Singapore | 58 | M | Incidental finding | NS | NS | Head | 90 |
| Showalter et al[23] | 2008 | USA | 72 | F | Incidental finding | NA | MR | Tail | 70 |
| Yan et al[24] | 2008 | USA | 47 | M | Nausea + vomiting + (hepatitis B) | Splenomegaly | CT + EUS | Uncinate process | 24 × 21 |
| Ganesh et al[25] | 2008 | UK | 76 | F | Weight loss + abdominal pain | Diffuse tenderness | CT + endoscopy | Tail + body | NS |
| Daum et al[27] | 2005 | Czech | 70 | F | Incidental finding | Palpable mass | CT | Head | 100 × 80 × 60 |
| Krska et al[28] | 2005 | Czech | 38 | F | Abdominal pain + fatigue | Tenderness | CT + US + EUS + CT + endoscopy | Head + Body | 170 × 120 |
| Pauser et al[29] | 2005 | USA | 51 | M | Incidental finding | NS | US + CT + endoscopy | Tail | 30 |
| 54 | F | Abdominal discomfort | NS | US | Body | 20 | |||
| Neto et al[30] | 2004 | Brasil | 67 | F | Weight loss + abd pain + gastric bloating | NS | NS | Body + Tail | 200 × 190 × 120 |
| Yamaura et al[31] | 2004 | Japan | 54 | F | Incidental finding | Palpable mass | US + CT + MR + angiography | Tail | 140 × 120 × 80 |
| Ref. | Cell type | Mitotic count (/50 HPF) | CD117 | CD34 | Surgical procedures | Recurrence (after surgery) | Outcome (follow-up) | Medical treatment |
| Tian et al[4] | Spindle | < 5 (intermediate risk) | (+) | (+) | Distal pancreatectomy + splenectomy | No | Alive (36 mo) | No |
| Spindle | > 5 (high risk) | (+) | NS | Tumor resection | Yes (liver, 12 mo) | Alive (36 mo) | Gleevec + TACE | |
| Paklina et al[11] | Spindle | 1-2 (intermediate risk) | (+) | NS | NS | NS | NS | NS |
| Serin et al[1] | NS | NS (high risk) | (+) | NS | Distal pancreatectomy + splenectomy | No | Alive (21 mo) | No |
| Soufi et al[16] | Spindle | < 5 (intermediate risk) | (+) | (+) | Whipple + segmental colectomy | No | Alive (24 mo) | Gleevec |
| Wegge et al[2] | Spindle | 6 (intermediate risk) | (+) | (+) | Whipple | No | Alive (5 mo) | Gleevec |
| Babu et al[13] | Spindle | 6-8 (high risk) | (+) | (+) | Pancreatic head resection | No | Alive (11 mo) | No |
| Kim et al[3] | Spindle | 7 (high risk) | (+) | (+) | Distal pancreatectomy + splenectomy | No | Alive (4 mo) | Gleevec |
| Čečka et al[9] | Spindle | 5 (high risk) | (+) | (+) | Distal pancreatectomy + splenectomy | No | Alive (66 mo) | No |
| Vij et al[14] | Spindle | 12-15 (high risk) | (+) | (-) | Whipple | Yes (liver, 24 mo)a | Alive (48 mo) | Gleevec |
| Rao et al[7] | Spindleb | 8-10 (high risk) | (+) | (+) | Whipple | Yes (liver, 24 mo) | Alive (30 mo) | Gleevec |
| Yang et al[15] | Spindle | > 30/10 HPF (high risk) | (+) | (+) | Distal pancreatectomy + splenectomy | Yes (intraperitoneal, 24 mo)c | Alive (41 mo) | Gleevec |
| Barros et al[12] | NS | < 5 | (+) | (+) | No | NS | Death (8 mo) | No |
| NS | < 5 (intermediate risk) | (+) | (+) | Laparotomy + biopsy | Surgery not performed | Alive (12 mo) | Gleevec | |
| Joshi et al[17] | Spindle | NS | (+) | (+) | None performedd | Surgery not performed | Death (5 d) | No |
| Crisan et al[18] | Spindle | (high risk) | (+) | (+) | Distal pancreatectomy + splenectomy + partial colectomy + duodenojejunal resection | NS | Alive (3 mo) | NS |
| Saif et al[19] | Spindlee | 48 (high risk) | (+) | (-) | Whipple, pylorus preserving | Yes (liver, 9 mo) | Alive (NS) | Gleevec |
| Padhi et al[8] | Spindle | 6-8 (high risk) | (+) | (+) | Distal pancreatectomy + splenectomy + left hemicolectomy | No | Alive (10 mo) | No |
| Harindhanavudhi et al[20] | Spindle | < 5 (high risk) | (+) | (+) | Cystojejunostomyf | NS | Alive (NS) | Gleevec |
| Trabelsi et al[21] | Spindle | 6 (high risk) | (+) | (+) | Whipple + partial colectomy | No | Alive (10 mo) | No |
| Goh et al[10] | Spindle | > 10 (high risk) | (+) | NS | Whipple | No | Alive (58 mo) | NS |
| Showalter et al[23] | NA | 3 (intermediate risk) | (+) | (-) | Distal pancreatectomy + splenectomy - laparoscopic | No | Alive (27 mo) | NS |
| Yan et al[24] | Spindleg | 3 (low risk) | (+) | NS | NS | NS | NS | NS |
| Ganesh et al[25] | Spindleh | NS | (+) | (+) | No (inoperable) | Surgery no performed | Alive (30 mo) | Gleevec |
| Daum et al[27] | Spindle | 2 (intermediate risk) | (+) | (-) | Whipple | No | Alive (6 mo) | Gleevec |
| Krska et al[28] | Spindlei | 1 (high risk) | (-) | (+) | Partial pancreatectomy | No | Alive (30 mo) | NS |
| Pauser et al[29] | Spindle | NS | (+) | (+) | Resection | No | Alive (24 mo) | NS |
| Spindle | NS | (+) | (+) | Resection | No | Alive (48 mo) | NS | |
| Neto et al[30] | Mixed | 120 (high risk) | (+) | (+) | Distal pancreatectomy | Yes (peritoneum) | Alive (NS) | Gleevec |
| Yamaura et al[31] | Spindle | Few (high risk) | (+) | (+) | Distal pancreatectomy + splenectomy + partial gastric resection | NS | Alive (30 mo) | NS |
Based on the above-mentioned search criteria, 30 manuscripts were identified[1-5,7-31]: 27 met the criteria and three were excluded[5,22,26]. The criteria are detailed in the flow chart in Figure 1. The studies involved 30 patients with pancreatic GIST: 15 were male and 15 were female; mean age was 55 ± 14.3 years (range 30-84 years). The mean ages of male and female patients were 50.8 ± 13.7 years (range 38-81 years) and 59.9 ± 13.3 years (range 38-81 years), respectively. Information regarding tumor size was obtained from 28 cases (mean 114.4 ± 78.6 mm; range 20-350 mm). The demographic and clinical data of the 30 patients are presented in Table 1. Table 2 summarizes the morphological characteristics, treatments, and outcomes of the 30 patients.
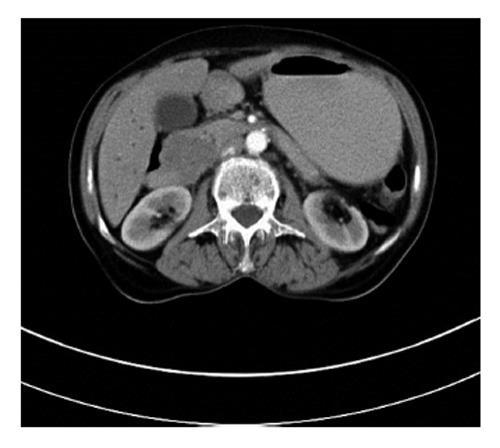
A 61-year-old woman was admitted to our clinic for a routine check-up. One year previously, she had visited another clinic complaining of loss of appetite, weight loss, and jaundice. Blood tests showed elevated liver enzymes and leucocyte count. Abdominal ultrasonography (USG) revealed bile duct dilatation, multiple metastatic liver lesions, and a pancreatic head mass. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a 97 mm × 63 mm heterogeneous mass with well-defined margins in the pancreatic head, which had resulted in the bile duct dilatation. Perihilar gross lymphadenopathy was also detected. Following bile duct decompression by percutaneous transhepatic cholangiography, percutaneous biopsy samples were collected from the liver lesions and portal lymph nodes under USG guidance. The specimens were evaluated histopathologically and immunohistochemically [CD117(+); CD34(−); smooth muscle actin (SMA)(−)], and GIST was diagnosed. As the primary tumor was metastatic prior to surgery, 400 mg/d imatinib mesylate (Gleveec®, Novartis) was started and administered for four months. MRI subsequently showed a reduction in tumor size to 15 × 15 mm. CT performed during the same period showed that the tumor had shrunk to 15 × 20 mm and that the liver lesions had disappeared. Based on these findings, surgical treatment was advised, but the patient refused surgery; therefore, she was discharged and prescribed imatinib. When admitted to our clinic, she had no significant physical findings except cachexia. Laboratory test parameters, including tumor markers, were within the normal limits. Control abdominal CT scan showed that the tumor measured 45 mm × 40 mm (Figure 2). The common bile duct and major pancreatic duct diameter was 17 mm and 7 mm, respectively. No metastatic liver lesions were detected. F-18 fluorodeoxyglucose positron emission tomography-CT (PET-CT) detected a mass with increased glucose consumption at the duodenal site, consistent with a malignant lesion. Given the increased tumor size and the current complaints of the patient, surgical treatment was recommended. We detected a well-demarcated, 50 × 40 mm, semi-solid, visually heterogeneous pancreatic head mass without invasion to the surrounding tissues. Metastatic liver lesions were not observed, and lymphadenopathy was detected in the peripancreatic site and hepatoduodenal ligament. Standard pancreaticoduodenectomy with lymph node dissection was performed. The postoperative course was uneventful; she was discharged on day 13. Pathologically, the specimen contained tumor cells with low mitotic activity, severe pleomorphism, and cellularity (spindle cells); we diagnosed GIST. Postoperative imatinib mesylate was started, and there was neither locoregional nor distant metastases at the last follow-up 48 mo later.
In 1892, Cajal first observed interstitial cells of Cajal in the intestinal wall under a light microscope, which were termed “interstitial neural cells”. Approximately 80 years later, Faussone-Pellegrini et al[32] viewed the same cells under an electron microscope and renamed them interstitial cells of Cajal[5,32]. Studies conducted during the 1970s showed that pathological changes to interstitial cells of Cajal may result in GI motility disorders and GISTs[5]. Since they were first described histologically, physiological testing has proven that interstitial cells of Cajal function as GI pacemakers[5,20,32,33].
Defined by Mazur and Clark in 1983, GISTs are the most common non-epithelial mesenchymal tumors of the GI tract[5]. Genetic studies have revealed that 90% and 5%-7% of GISTs have tyrosine kinase gene mutations in c-KIT and platelet-derived growth factor receptor alpha (PDGFRA), respectively[1,5]. The incidence of GIST varies between 10 and 20 cases per million people annually[5,9]. GISTs represent 0.1%-3% of all GI tumors and 80% of GI mesenchymal tumors, and may present at any site in the GI tract where there are interstitial cells of Cajal. The most frequently affected GI organs are the stomach (40%-70%), intestines (20%-40%), rectum and colon (< 10%), and the esophagus (rare)[5].
“EGIST” was initially used by Reith et al[33] in 2000 to define stromal tumors originating from outside the GI tract. EGISTs represent 5%-10% of all GISTs[1,4,5,9,12]. Although the locations from which EGISTs originate do not contain interstitial cells of Cajal, cells with the same clinical, pathological, immunohistochemical, transmission electron microscopy morphology, and biological behavior patterns as interstitial cells of Cajal have been detected[2,5,6]. Experimental and clinical studies have detected cells with biological and histopathological features similar to interstitial cells of Cajal in pancreatic tissue (interstitial Cajal-like cells = telocytes)[5,34]. The pancreas and GI tubular organs have a common embryological origin, suggesting that EGIST and GIST cells originate from multipotent mesenchymal stem cells (intestinal mesenchymal precursors)[1,5,21]. Several EGIST studies have suggested that most EGISTs are likely mural GISTs with diffuse extramural invasion resulting in loss of communication with the intestinal muscularis propria. This may occur during operative or postoperative manipulation. Furthermore, true EGISTs may be extramurally growing GISTs that lose communication with the muscularis propria after reaching this layer[2,10,16]. This is known as extensive extramural growth and requires further study.
More than 80% of EGISTs originate from EGI abdominal wall structures, including the intestinal mesentery, mesocolon, omentum, retroperitoneum, abdominal wall, liver, and pancreas[10,13]. Pancreatic EGISTs represent less than 1% of malignant pancreatic tumors, and less than 5% of EGISTs originate from the pancreas[16].
The majority of EGISTs are well demarcated and unencapsulated. Due to their slow growth rate, they may exist without any clinical signs until the majority of the abdominal cavity is invaded. Among the reported cases, tumors are 100-120 mm in diameter (range 10-400 mm)[4]. EGISTs are usually diagnosed in adults, predominantly in females[14]. Our literature review determined near equal rates of occurrence between females and males.
Pancreatic EGISTs are usually asymptomatic or minimally symptomatic and diagnosed incidentally by radiological examination[7,9]. When present, the severity of symptoms is related to tumor dimensions and location in the pancreatic tissue[2,4,7,9,16]. The most common symptoms and findings are nonspecific abdominal pain, weight loss, fatigue, abdominal mass and distention, fever of unknown origin, obstruction, GI bleeding, anemia, portal vein thrombosis, jaundice, and hepatic encephalopathy (rare)[4,16,18]. Of the cases we reviewed, 23.3% were diagnosed incidentally. The most common symptoms were weight loss and abdominal discomfort.
The most common diagnostic studies for pancreatic masses involve biochemical [carbohydrate antigen 19-9, carcinoembryonic antigen (CEA)], radiological, histopathological, immunohistochemical, and genetic testing[3-5,21]. However, the diagnostic value of tumor markers such as CA 19-9 and CEA for pancreatic EGIST is limited, and are rarely used[4]. Abdominal CT, MRI, USG, endoscopic USG (Endo-USG), and PET-CT are the most frequently used radiological techniques, and aid in determining tumor localization, dimensions, margin irregularity, invasion of surrounding tissues, distant metastases, and resectability; however, most of them are non-diagnostic. USG and CT are often used in fine needle biopsies[5,7,17,20,24,25,28]. Endo-USG is a valuable diagnostic tool, allowing simultaneous diagnosis and biopsy of solid or cystic pancreatic masses[4,5,16,19,20,24]. PET-CT is used more frequently for both diagnosing and monitoring GIST and is very efficient in cases where CT and MRI are inconclusive[35].
Histopathologically, GISTs are classified into spindle (70%), epithelioid (20%), or mixed (< 10%) type. Most pancreatic EGISTs consist of spindle cells[4]. Therefore, leiomyoma, leiomyosarcoma, liposarcoma, rhabdomyosarcoma, schwannoma, fibromatosis, inflammatory fibroid polyps, solitary fibrous tumor, and malignant fibrous histiocytoma should be considered in the differential diagnoses[3,8,11,24,27]. Of the cases presented here, 26 had detailed histopathological data and 25 (96.1%) had spindle cells.
EGISTs have typical immunohistological staining features, among which CD117 is the most well known. KIT is a transmembrane receptor for binding tyrosine kinase enzymes, and c-KIT is a newly discovered member of this receptor family, on whose receptor CD117 is an epitope that can be stained immunohistochemically. The introduction of CD117 staining in the 1990s changed the terminology for connective tissue tumors; since then, 95% of tumors defined as GIST or EGIST stain CD117-positive. For the 5% of tumors with negative staining, another tyrosine kinase receptor family member, PDGFRA, was investigated in immunohistochemical studies, with 33.3% positive staining[5]. Additionally, GISTs stain positive for CD34 (60%-70%), heavy caldesmon (80%), SMA (30%-40%), S100 (5%), and desmin (< 5%)[2-4,8,9]. Of the 30 cases presented, 29 (96.6%) stained CD117-positive and 21 (84%) of 25 cases stained CD34-positive.
Predicting GIST clinical and biological behavior is difficult. Fletcher defined the criteria of the National Institutes of Health (Fletcher criteria) to estimate the risks of GIST aggressive behavior and metastasis (locoregional and/or distant) using tumor dimensions (cm) and mitotic counts (per 50 HPF)[2,9]. According to the criteria, GISTs are classified based on their risk of aggressive behavior: very low (< 2 cm, < 5/50 HPF), low (2-5 cm, < 5/50 HPF), intermediate (< 5 cm, 6-10/50 HPF or 5-10 cm, < 5/50 HPF), and high (> 5 cm, > 5/50 HPF or > 10 cm, any mitotic count)[3,4,9,21]. This classification aids in surgical treatment selection or neoadjuvant and/or adjuvant treatment planning. The risk of aggressive behavior according to the Fletcher criteria was determined in 25 of the 30 patients in our literature review: risk of pancreatic EGIST aggressive behavior was high in 17 cases. The remaining 8 cases were intermediate risk (n = 7; 28%) and low risk (n = 1; 4%).
The goal of surgical treatment, which is the most desirable treatment option for primary pancreatic EGISTs, is complete resection with microscopically clean (R0) margins[4,5,36]. Generally, primary surgery, surgical treatment following neoadjuvant chemotherapy, and debulking surgery for metastatic and/or advanced disease are considered in the surgical treatment of GISTs[2,5]. Surgical treatment selection depends on pancreatic EGIST localization. Standard or pylorus-preserving pancreaticoduodenectomy is the optimal treatment for pancreatic head tumors[4]. Duodenum-preserving pancreatic head resection may be performed for small tumors, low-grade tumors, or patients who cannot tolerate the Whipple procedure[4,36]. Conversely, radical surgical treatment may be the best option for preventing locoregional and/or distant metastases[13,15]. Regional lymph node metastases are rare in pancreatic EGIST cases, and routine systematic regional lymph node dissection is not indicated[4,13,16,18]. In our patient, EGIST was diagnosed after lymph node biopsy. Therefore, we suggest lymphadenectomy for cases of pathological lymphadenopathy observed during surgical exploration and for lymph node metastasis-positive cases based on intraoperative frozen section analysis. Depending on intraoperative findings and the surgeon’s experience, pancreaticoduodenectomy, distal pancreatectomy with splenectomy, or partial pancreatic resection may be used for treating tumors in the pancreatic tail and corpus[13]. Nine and seven of the 30 patients underwent distal pancreatectomy with splenectomy, and the Whipple procedure, respectively.
The responses of GISTs to conventional chemotherapy and radiotherapy were very limited, being 10% and 5%, respectively[9,21]. These response rates changed when imatinib mesylate, an agent used for treating chronic myelogenous leukemia, was administered to a GIST case in the early 2000 s. Philadelphia chromosome-positive leukemia patients carry a mutation in the BCR-ABL gene, which is a KIT receptor family member. Additionally, the mutated c-KIT and PDGFRA genes seen in GISTs are members of the same family. Consequently, tyrosine kinase transmembrane receptors have been targeted in GIST treatment using two agents: imatinib mesylate and sunitinib malate. Imatinib was the first c-KIT tyrosine kinase inhibitor used for treating GISTs, specifically metastatic and unresectable GISTs, and was approved by the US Food and Drug Administration. Sunitinib was subsequently introduced for patients who could not tolerate imatinib or who were imatinib-resistant[2,23]. Recently, new tyrosine kinase inhibitors, such as nilotinib, sorafenib, dovitinib, and dasatinib, were introduced[5]. Despite the controversial approach of “which tyrosine kinase inhibitor, which patient and when”, there is consensus for initiating imatinib treatment in patients with high mitotic activity, gross dimensions, necrosis, and locoregional and/or distant metastasis[2,15]. Imatinib may be used as a neoadjuvant agent to downstage gross tumor volume for R0 resection and contributes to good prognosis[4]. Imatinib may be used as adjuvant treatment in cases with R1 (positive microscopic margin) or R2 (residual gross visible tumor) resection, risk of aggressive behavior, or poor prognostic features[4,5]. Similarly, imatinib treatment may be used as a primary modality in metastatic or unresectable cases to reduce tumor size, resulting in better prognosis[4]. Metastatic pancreatic EGIST cases benefit from debulking surgery, which increases the efficacy of imatinib[2]. The positive response to imatinib in patients with GISTs is 60%-70%, which can extend overall survival up to 5 years[4].
In conclusion, the term EGIST was introduced into the literature in the last decade. Debates on the similarities and differences between EGISTs and GISTs are ongoing. Despite their behavioral similarities, the initial asymptomatic period accounts for the gross tumor size of EGISTs. The lack of comprehensive case reports on EGISTs, including pancreatic EGISTs, limited our evidence-based review. Long-term follow-up studies of EGISTs are currently unavailable, limiting the amount of available information on tumor behavior. We are limited to the case reports that have been published to date and further immunohistochemical and genetic studies regarding EGIST behavior and response to treatment are needed.
Gastrointestinal stromal tumors (GISTs) are the most common tumors of mesenchymal origin in the gastrointestinal (GI) tract. The disease originates from neoplastic transformation of interstitial cells of Cajal or their precursors in the GI tract. Stromal tumors of extragastrointestinal origin are termed extragastrointestinal stromal tumors (EGISTs) and are not associated with the walls of GI tubular organs or serosal surfaces. The morphological, histopathological, immunohistochemical and molecular profiles of EGISTs are similar to those of GISTs.
The primary aim was to report the rare case of a 61-year-old patient who underwent surgical treatment for pancreatic head EGIST. The secondary aim was to analyze previously published articles related to pancreatic GIST. To this end, the authors searched for studies on pancreatic GIST using different keyword combinations in the PubMed, MEDLINE, Google Scholar, and Google databases.
GISTs are the most common mesenchymal tumors of the GI tract. EGISTs are defined as tumors originating from outside the GI tract. Imatinib mesylate was the first c-KIT tyrosine kinase inhibitor used to treat GISTs. The Fletcher criteria are used to estimate the risk of GIST aggressive behavior and metastasis using tumor size and mitotic counts.
This paper comprises a case history, and a comprehensive review of the literature on pancreas GIST. The strength of the paper is that the authors have tried to collect available literature of the limited articles published on this topic.
P- Reviewer: Fabre JM, Kapoor S, Soreide JA, Sumi S S- Editor: Ji FF L- Editor: Webster JR E- Editor: Liu SQ
| 1. | Serin KR, Keskin M, Gulluoglu M, Emre A. Atypical localisation of a gastrointestinal stromal tumor: A case report of pancreas gastrointestinal stromal tumor. Ulusal Cer Derg. 2013;29:42-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Wegge J, Bartholomew DM, Burke LH, Miller LA. Pancreatic extra-gastrointestinal stromal tumour masquerading as a bleeding duodenal mass. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Kim HH, Koh YS, Park EK, Seoung JS, Hur YH, Kim JC, Cho CK, Kim HJ. Primary extragastrointestinal stromal tumor arising in the pancreas: report of a case. Surg Today. 2012;42:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Tian YT, Liu H, Shi SS, Xie YB, Xu Q, Zhang JW, Zhao DB, Wang CF, Chen YT. Malignant extra-gastrointestinal stromal tumor of the pancreas: report of two cases and review of the literature. World J Gastroenterol. 2014;20:863-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Padhi S, Sarangi R, Mallick S. Pancreatic extragastrointestinal stromal tumors, interstitial Cajal like cells, and telocytes. JOP. 2013;14:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142. [PubMed] |
| 7. | Rao RN, Vij M, Singla N, Kumar A. Malignant pancreatic extra-gastrointestinal stromal tumor diagnosed by ultrasound guided fine needle aspiration cytology. A case report with a review of the literature. JOP. 2011;12:283-286. [PubMed] |
| 8. | Padhi S, Kongara R, Uppin SG, Uppin MS, Prayaga AK, Challa S, Nagari B, Regulagadda SA. Extragastrointestinal stromal tumor arising in the pancreas: a case report with a review of the literature. JOP. 2010;11:244-248. [PubMed] |
| 9. | Čečka F, Jon B, Ferko A, Šubrt Z, Nikolov DH, Tyčová V. Long-term survival of a patient after resection of a gastrointestinal stromal tumor arising from the pancreas. Hepatobiliary Pancreat Dis Int. 2011;10:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Goh BK, Chow PK, Kesavan SM, Yap WM, Chung YF, Wong WK. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: critical appraisal and a comparison with their gastrointestinal counterparts. J Gastrointest Surg. 2009;13:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Paklina OV, Setdikova GR, Voskanyan SE. Extragastrointestinal stromal tumor of the pancreas: A case report. 25 th European congress of pathology Lisbon. Poster No: 14 2013; . |
| 12. | Barros A, Linhares E, Valadão M, Gonçalves R, Vilhena B, Gil C, Ramos C. Extragastrointestinal stromal tumors (EGIST): a series of case reports. Hepatogastroenterology. 2011;58:865-868. [PubMed] |
| 13. | Babu SR, Kumari S, Zhang Y, Su A, Wang W, Tian B. Extra gastrointestinal stromal tumor arising in the pancreas: a case report and literature review. J GHR. 2012;1:80-83. |
| 14. | Vij M, Agrawal V, Pandey R. Malignant extra-gastrointestinal stromal tumor of the pancreas. A case report and review of literature. JOP. 2011;12:200-204. [PubMed] |
| 15. | Yang F, Jin C, Fu D, Ni Q. Extra-gastrointestinal stromal tumor of the pancreas: clinical characteristics, diagnosis, treatment, and outcome. J Surg Oncol. 2011;103:739-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Soufi M, Bouziane M, Massrouri R, Chad B. Pancreatic GIST with pancreas divisum: A new entity. Int J Surg Case Rep. 2013;4:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Joshi J, Rustagi T. Pancreatic Extra-Gastrointestinal Stromal Tumor: An Unusual Presentation of a Rare Diagnosis. Gastrointest Cancer Res. 2010;S29-S30. |
| 18. | Crisan A, Nicoara E, Cucui V, Cornea G, Laza R. Prolonged fever associated with gastrointestinal stromal tumor-case report. J Exp Med Surg Res. 2010;17:219-224. |
| 19. | Saif MW, Hotchkiss S, Kaley K. Gastrointestinal stromal tumors of the pancreas. JOP. 2010;11:405-406; author reply 412. [PubMed] |
| 20. | Harindhanavudhi T, Tanawuttiwat T, Pyle J, Silva R. Extra-gastrointestinal stromal tumor presenting as hemorrhagic pancreatic cyst diagnosed by EUS-FNA. JOP. 2009;10:189-191. [PubMed] |
| 21. | Trabelsi A, Yacoub-Abid LB, Mtimet A, Abdelkrim SB, Hammedi F, Ali AB, Mokni M. Gastrointestinal stromal tumor of the pancreas: A case report and review of the literature. N Am J Med Sci. 2009;1:324-326. [PubMed] |
| 22. | Yang F, Long J, Di Y, Fu DL, Jin C, Ni QX, Zhu HG. A giant cystic lesion in the epigastric region. Pancreatic malignant gastrointestinal stromal tumour (GIST). Gut. 2008;57:1494, 1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Showalter SL, Lloyd JM, Glassman DT, Berger AC. Extra-gastrointestinal stromal tumor of the pancreas: case report and a review of the literature. Arch Surg. 2008;143:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Yan BM, Pai RK, Van Dam J. Diagnosis of pancreatic gastrointestinal stromal tumor by EUS guided FNA. JOP. 2008;9:192-196. [PubMed] |
| 25. | Ganesh M, Kumar S, Krishnamoorthy R, Ang Y. Rare cause of pancreatic mass responding to imatinib treatment. Gastroenterology Today. 2008;18:50-51. |
| 26. | Paklina OV, Setdikova GR, Voskanyan SE. Gastrointestinal Stromal Tumor of a Pancreas: Case Report and literature review. Медицинская визуализация. 2013;2:122. |
| 27. | Daum O, Klecka J, Ferda J, Treska V, Vanecek T, Sima R, Mukensnabl P, Michal M. Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation. Virchows Arch. 2005;446:470-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Krska Z, Pesková M, Povýsil C, Horejs J, Sedlácková E, Kudrnová Z. GIST of pancreas. Prague Med Rep. 2005;106:201-208. [PubMed] |
| 29. | Pauser U, da Silva MT, Placke J, Klimstra DS, Klöppel G. Cellular hamartoma resembling gastrointestinal stromal tumor: a solid tumor of the pancreas expressing c-kit (CD117). Mod Pathol. 2005;18:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Neto MR, Machuca TN, Pinho RV, Yuasa LD, Bleggi-Torres LF. Gastrointestinal stromal tumor: report of two unusual cases. Virchows Arch. 2004;444:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Yamaura K, Kato K, Miyazawa M, Haba Y, Muramatsu A, Miyata K, Koide N. Stromal tumor of the pancreas with expression of c-kit protein: report of a case. J Gastroenterol Hepatol. 2004;19:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999;47:248-266. [PubMed] |
| 33. | Reith JD, Goldblum JR, Lyles RH, Weiss SW. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13:577-585. [PubMed] |
| 34. | Popescu LM, Hinescu ME, Ionescu N, Ciontea SM, Cretoiu D, Ardelean C. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9:169-190. [PubMed] |
| 35. | Williams A, Gutzeit A, Germer M, Pless M. PET-Negative Gastrointestinal Stromal Tumors. Case Rep Oncol. 2013;6:508-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Yamashita S, Sakamoto Y, Saiura A, Yamamoto J, Kosuge T, Aoki T, Sugawara Y, Hasegawa K, Kokudo N. Pancreas-sparing duodenectomy for gastrointestinal stromal tumor. Am J Surg. 2014;207:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |