Published online Jul 27, 2013. doi: 10.4240/wjgs.v5.i7.224
Revised: May 22, 2013
Accepted: June 5, 2013
Published online: July 27, 2013
Processing time: 132 Days and 20.3 Hours
Despite the advance of diagnostic modalities, carcinoma in the body and tail of the pancreas are commonly presented at a late stage. With unresectable lesions, long-term survival is extremely rare, and surgery remains the only curative option for pancreatic cancer. An aggressive approach by applying extended distal pancreatectomy with the resection of the celiac axis may increase the resectability and analgesic effect but great care must be taken with the arterial blood supply to the liver and stomach. Sometimes, accidental injury to the pancreatoduodenal artery compromises collateral blood flow and leads to fatal complications. Therefore, knowledge of any alternative restoration of the compromised collateral flow before surgery is essential. The present case report shows a patient with a pancreatic body cancer in whom the splenic, celiac, and common hepatic arteries were involved with the tumor, which extended almost to the root of the gastroduodenal artery. We modified the procedure by reanastomosis between the proper hepatic artery and middle colic artery without vascular graft. The postoperative course was uneventful, and the patient was discharged on postoperative day 19. The patient was immediately free of epigastric and back pain.
Core tip: The present case report shows a patient with a pancreatic body cancer in whom the splenic, celiac, and common hepatic arteries were involved with the tumor, which extended almost to the root of the gastroduodenal artery. We modified the procedure by reanastomosis between the proper hepatic artery and middle colic artery without vascular graft.
- Citation: Suzuki H, Hosouchi Y, Sasaki S, Araki K, Kubo N, Watanabe A, Kuwano H. Reconstruction of the hepatic artery with the middle colic artery is feasible in distal pancreatectomy with celiac axis resection: A case report. World J Gastrointest Surg 2013; 5(7): 224-228
- URL: https://www.wjgnet.com/1948-9366/full/v5/i7/224.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i7.224
Carcinoma of the body of the pancreas is often discovered at an advanced stage by reason of lack of symptoms. Because the only long-term survivors of cancer of the pancreatic body have been those who have undergone resection, complete surgical resection is the only treatment that may lead to a prolonged survival[1]. However, the resection rate is low, and chances for resection are often lost to distant metastasis, regional invasion into adjacent organs, or the involvement of major vessels. Tumor involvement of the common hepatic artery (CHA) and/or celiac axis (CA) is a primary reason for this. Several high-volume pancreatic centers have reported that advanced pancreatic body/tail carcinoma can be safely treated by left-sided pancreatectomy with en bloc resection of infiltrated major vascular structures[2]. These methods were supported by the reported prognosis of the patients, which seems to be similar to that in patients without resection of major vascular structures[3].
Hirano et al[4] reported that, in patients who underwent distal pancreatectomy with en bloc celiac axis resection (DP-CAR), the surgical margins were histologically clear in 95% of patients and the postoperative mortality rate was 0%, despite a high morbidity rate (48%). The estimated overall 1- and 5-year survival rates were 72% and 17%, respectively, and the median survival was 21 mo. Moreover, radical distal pancreatectomy with en bloc resection of the celiac artery, celiac plexus, and celiac ganglions is reported to provide patients with locally advanced cancer of the pancreatic body with complete and enduring pain relief[5]. Therefore, radical surgery is justifiable in patients with advanced cancer of the pancreatic body, even when it has invaded adjacent major vessels, such as the celiac artery and/or the portal vein. When performing DP-CAR surgery, it is essential to preserve the arterial blood supply to the stomach and liver. The arterial blood supply during this procedure is maintained by the collateral pathways via the pancreatoduodenal arcades from the superior mesenteric artery to the gastroduodenal and hepatic arteries, which develop immediately after ligation of the celiac artery[6]. However, there are some cases in which the gastroduodenal artery arises from the area close to the CA. In such a situation, it would be difficult to preserve the gastroduodenal artery during DP-CAR surgery. In addition, the effort to preserve the gastroduodenal artery during surgery introduces risk of injury. In this case, we performed DP-CAR with reconstruction of the common hepatic artery by use of the middle colic artery in the patient with gastroduodenal artery (GDA) arising from the area close to the CA. We here report a technique used for reconstruction that was feasible in the case of shutoff of the arterial blood supply to the liver in DP-CAR.
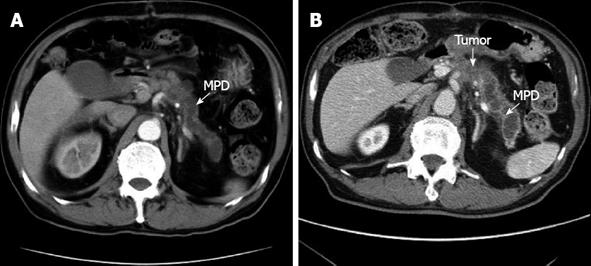
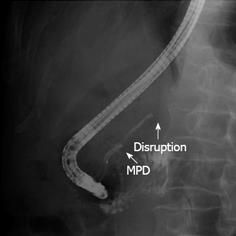
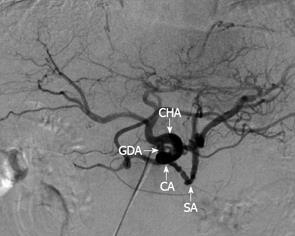
A 78-year-old male was referred to the emergency ward of the Gunma Prefectural Cardiovascular Center with acute onset abdominal pain. A computed tomography (CT) scan of the thorax and abdomen confirmed an infrarenal abdominal aortic aneurysm (AAA) and evidence of a contained acute rupture to the left retroperitoneum. Therefore, emergency surgery for AAA was performed. A follow-up CT revealed dilatation of the main pancreatic duct from the body to the tail. However, a tumor at the body of the pancreas was not identified (Figure 1A). Four months after the operation, the patient had severe epigastric and back pain, and his serum level of carbohydrate antigen 19-9 (CA19-9) was elevated to 652 U/mL. Therefore, he was referred to the Department of General Surgical Science, Graduate School of Medicine, Gunma University 6 mo after the AAA surgery. Dilatation of the main pancreatic duct was evident in CT, and the tumor, identified at the body of the pancreas, had invaded the CA and CHA (Figure 1B). Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a stricture of the main pancreatic duct in the body of the pancreas (Figure 2). Abdominal angiography showed that the CA and splenic artery (SA) were involved with a tumor of the pancreatic body (Figure 3). These findings were consistent with a diagnosis of locally advanced cancer of the body of the pancreas that invaded the CA and SA. Preoperative evaluation of angiography showed that the GDA arose near the CA (Figure 3).
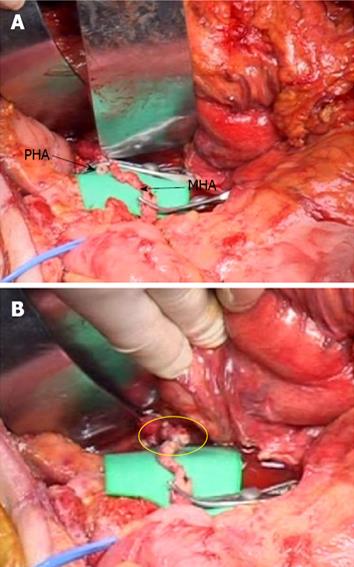
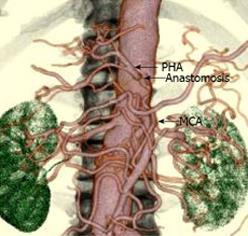
The patient then underwent a DP-CAR. An extended Kocher maneuver was performed. Because of the previous surgery, there was severe adhesion at the retroperitoneum. The origin of the superior mesenteric artery (SMA) and CA was palpated and confirmed to be uninvolved. The greater sac was then entered adjacent to the colon, and the splenocolic ligament was divided, allowing the inferior border of the body and the tail of the pancreas to be incised. The tumor originated from the body of the pancreas, and the celiac and common hepatic arteries were involved. Following the common hepatic artery, it was found to be densely encased by a tumor extending almost to the root of GDA. The GDA was exposed and encircled with a proximal vessel loop. By dissecting the right celiac ganglion and celiac nerve plexus, the origin of the CA was exposed. The body and the tail of the pancreas were freed from the posterior abdomen in this inflamed adhesion retroperitoneal plane. The dissection was continued from the hilum of the spleen to the superior mesenteric vein. The spleen was released from its attachment to the diaphragm, allowing it to be lifted and retracted medially. A portion of Gerota’s fascia was lifted with the specimen to expose the left kidney and renal vessels. Gerota’s fascia was then excised in continuity. The inferior mesenteric vein was ligated inferiorly. At that time, we completely excised the GDA, which was involved with the tumor under the pancreas. Despite the presence of tumor involvement at the GDA, curative pancreatic resection with arterial resection was considered to be possible. Therefore, the GDA was ligated and divided proximal to the artery. After cutting the proper hepatic artery at the root of the GDA, the stump of the proper hepatic artery (PHA) and the middle colic artery (MCA) were anastomosed using an end-to-end technique with 7-0 prolene interrupted sutures and surgical loupes at × 2.5 magnification (Figure 4). The pulsation of the PHA recovered after completion of the anastomosis and was also identified by Doppler ultrasonography. The neck of the pancreas was transected over the right side of the portal vein (PV). The left side of the SMA was dissected with the surrounding lymph node. Care was taken to preserve the inferior pancreaticoduodenal artery (IPDA) arising from the SMA or the first jejunal artery. The left gastric artery was then divided, and, thereafter, the short gastric vessels along the greater curvature were ligated. Finally, the celiac trunk was divided at its origin with a transfixing suture, and the specimen was removed.
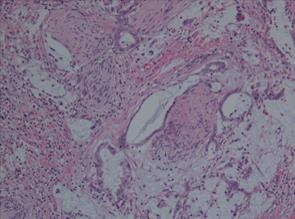
Histopathological examination of surgical specimens revealed mucinous carcinoma of the pancreas. There was prominent formation of mucinous nodules and a mucinous carcinoma including a large quantity of mucus (Figure 5). The peak aspartate/alanine aminotransferase (AST/ALT) was 1844/1265 U/L on the first postoperative day and gradually returned to a normal level over two weeks. The postoperative course was uneventful, and the patient was discharged on postoperative day 19. Postoperative 3D-CT angiography revealed that patency was maintained in the anastomosis between the GDA and the MCA (Figure 6). The patient was free from epigastric and back pain immediately and doing well after the operation. Five months after the operation, metastatic lesions were noted in the hilar of the liver by CT scan but without local recurrence. The patient was diagnosed with cholangitis and died of hepatic failure about 6 mo after the surgery.
Adenocarcinoma of the body and tail of the pancreas often presents in the advanced stage and is considered unresectable in the majority of patients. The traditional determinants of unresectability with such cancers are the presence of hepatic metastases, peritoneal dissemination of the tumor, and local invasion of major vascular structures, even in the absence of distant disease. With unresectable lesions, long-term survival is extremely rare. Surgery is the only curative option for pancreatic cancer[1]. Improved operative techniques have led to improved results and long-term survival outcomes for patients with pancreatic cancer following surgery[7]. Through cumulated experience in the operative and perioperative management of patients undergoing pancreatic surgery, the criteria for resectability have gradually expanded.
Hirano et al[4] reported that they performed DP-CAR on 37 patients to treat locally advanced cancer of the pancreatic body involving the common hepatic artery and/or celiac axis. The estimated 1- and 5-year survival rates were 72% and 17%, respectively, and the median survival was 21 mo. Moreover, radical distal pancreatectomy with en bloc resection of the celiac artery, celiac plexus, and celiac ganglions is reported to provide patients with locally advanced cancer of the pancreatic body with complete and enduring pain relief[5]. Since the perineural and neural invasion of the nerve plexus is one reason for the high recurrence rate of pancreatic cancer, dissection of the surrounding neural plexus is necessary in curative surgery. Concomitant resection of the celiac artery with a distal pancreatectomy facilitates lymph node and neural plexus dissection around the celiac artery.
Great care was taken to maintain the arterial blood supply to the liver and stomach during the surgery before the transection of the CA. It is essential to protect the blood flow from the SMA via the pancreaticoduodenal arcades to the GDA. Arterial blood flow through the GDA can nourish the liver and stomach via the PHA, right gastric artery, and right gastroepiploic arteries (GEA). However, vascular anomalies in the peripancreatic region, including the hepatic artery and SMA as well as the CA, have been reported elsewhere[8]. Because collateral circulation is significantly important in DP-CAR, a careful preoperative work-up is necessary. Even with vascular anomalies, it is justifiable to perform extended surgery with arterial resection for pancreatic cancer, given that surgery is the only curative option. Sometimes the dorsal pancreatic artery, which arises from the SMA, leads to the GDA. At times, an SMA dorsal artery plays an important role in the collateral circulation in patients with celiac axis stenosis[9]. In such cases, considerable attention must be paid when dissecting around the GDA. However, definitive assessment or recognition of the risk of ischemia of the liver and the stomach can only be made intraoperatively. In our case, the GDA was intact in the preoperative examination but arose from the area close to the CA and was involved with a tumor under the pancreas. In that situation, it is difficult to preserve the GDA for curative surgery. We decided to anastomose the PHA with the MCA during the curative operation. A specimen of the pancreas revealed that microscopic curative resection was possible. In addition, there were no serious complications following surgery. Therefore we believe that the techniques used for this reconstruction, which was a feasible and safe procedure, required shutoff of the arterial blood supply to the liver in DP-CAR.
DP-CAR surgery may facilitate resection in patients with tumors that are classified as T4 and require total clearance of the pancreatic and celiac lymph nodes[2-4]. The clinical benefit of extended pancreatic resections must, however, be balanced with the risk of the procedure. Therefore, knowledge of the reconstruction artery during DP-CAR surgery is essential. Konishi et al[10] reported that they reconstructed the hepatic artery using a graft of the splenic artery from the resected specimen because of weak pulsation of the proper hepatic artery after occlusion of the celiac axis. No complications related to hepatic ischemia were observed. Kondo et al[11] reported that, in compromised collateral flow via the pancreatoduodenal arcades, the MCA and GEA bypass is one of the procedures of choice to reestablish collateral flow.
Immediately after the AAA operation, dilatation of the main pancreatic duct from the body to the tail of the pancreas is the most likely indicator of a main duct type intraductal papillary mucinous tumor (IPMN). However, in this case the tumor grew rapidly after the AAA operation. The final pathological diagnosis showed mucinous carcinoma. Mucinous carcinoma of the pancreas is a rare type of tumor that is sometimes difficult to diagnose. Sakamoto et al[12] reported that endoscopic ultrasonography (EUS) and contrast-enhanced harmonic EUS are useful for the correct diagnosis of small pancreatic tumors, including synchronous and metachronous occurrence of IPMN and ductal adenocarcinoma. If EUS is performed at the earliest possible time, an accurate differential diagnosis between main pancreatic duct type IPMN and mucinous carcinoma can be made.
In the case reported here, the results remain unsatisfactory, although the patient is doing well and the resection resulted in the elimination of epigastric and back pain immediately after surgery. To improve the possibility of survival, a search for effective systemic chemotherapeutic agents and their testing in trials with neoadjuvant and/or adjuvant therapy with radiotherapy is essential to complement the oncologic benefit achieved after a complete surgical resection.
P- Reviewers Konishi T, Sergeant GP S- Editor Zhai HH L- Editor Hughes D E- Editor Lu YJ
| 1. | Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316-3322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Boggi U, Del Chiaro M, Croce C, Vistoli F, Signori S, Moretto C, Amorese G, Mazzeo S, Cappelli C, Campani D. Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery. 2009;146:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, Weitz J. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. 2011;254:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 4. | Hirano S, Kondo S, Hara T, Ambo Y, Tanaka E, Shichinohe T, Suzuki O, Hazama K. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg. 2007;246:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Kondo S, Katoh H, Omi M, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, Kanai M, Yano T. Radical distal pancreatectomy with en bloc resection of the celiac artery, plexus, and ganglions for advanced cancer of the pancreatic body: a preliminary report on perfect pain relief. JOP. 2001;2:93-97. [PubMed] |
| 6. | Mayumi T, Nimura Y, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Hamaguchi K, Hayakawa N. Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas. Int J Pancreatol. 1997;22:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Yekebas EF, Bogoevski D, Cataldegirmen G, Kunze C, Marx A, Vashist YK, Schurr PG, Liebl L, Thieltges S, Gawad KA. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg. 2008;247:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, Cho BH, So YH, Park JH. Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology. 2010;255:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Song SY, Chung JW, Kwon JW, Joh JH, Shin SJ, Kim HB, Park JH. Collateral pathways in patients with celiac axis stenosis: angiographic-spiral CT correlation. Radiographics. 2002;22:881-893. [PubMed] |
| 10. | Konishi M, Kinoshita T, Nakagori T, Inoue K, Oda T, Kimata T, Kikuchi H, Ryu M. Distal pancreatectomy with resection of the celiac axis and reconstruction of the hepatic artery for carcinoma of the body and tail of the pancreas. J Hepatobiliary Pancreat Surg. 2000;7:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Kondo S, Ambo Y, Katoh H, Hirano S, Tanaka E, Okushiba S, Morikawa T, Igawa H, Yamamoto Y, Sugihara T. Middle colic artery-gastroepiploic artery bypass for compromised collateral flow in distal pancreatectomy with celiac artery resection. Hepatogastroenterology. 2003;50:305-307. [PubMed] |
| 12. | Sakamoto H, Kitano M, Komaki T, Imai H, Kamata K, Kimura M, Takeyama Y, Kudo M. Small invasive ductal carcinoma of the pancreas distinct from branch duct intraductal papillary mucinous neoplasm. World J Gastroenterol. 2009;15:5489-5492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |