Published online May 27, 2013. doi: 10.4240/wjgs.v5.i5.146
Revised: February 20, 2013
Accepted: March 28, 2013
Published online: May 27, 2013
Processing time: 144 Days and 4.3 Hours
Outcomes in hepatic resectional surgery (HRS) have improved as a result of advances in the understanding of hepatic anatomy, improved surgical techniques, and enhanced peri-operative management. Patients are generally cared for in specialist higher-level ward settings with multidisciplinary input during the initial post-operative period, however, greater acceptance and understanding of HRS has meant that care is transferred, usually after 24-48 h, to a standard ward environment. Surgical trainees will be presented with such patients either electively as part of a hepatobiliary firm or whilst covering the service on-call, and it is therefore important to acknowledge the key points in managing HRS patients. Understanding the applied anatomy of the liver is the key to determining the extent of resection to be undertaken. Increasingly, enhanced patient pathways exist in the post-operative setting requiring focus on the delivery of high quality analgesia, careful fluid balance, nutrition and thromboprophlaxis. Complications can occur including liver, renal and respiratory failure, hemorrhage, and sepsis, all of which require prompt recognition and management. We provide an overview of the relevant terminology applied to hepatic surgery, an approach to the post-operative management, and an aid to developing an awareness of complications so as to facilitate better confidence in this complex subgroup of general surgical patients.
Core tip: Applied anatomy as used in hepatic surgery is different to the traditional morphological teaching. Applied hepatic anatomy is complex but trainees require an understanding of the basic principles to allow an appreciation of the operations performed. Complications require a low threshold of suspicion as they often have important consequences in relation to patient outcome. Recognition of such with rapid alerting of senior staff can facilitate timely and effective management. To date, no universal protocol exists for management of the post-operative period and varies from centre to centre. We provide a practical overview of the terminology, post-operative management, and complications associated with hepatic surgery.
- Citation: Farid SG, Prasad KR, Morris-Stiff G. Operative terminology and post-operative management approaches applied to hepatic surgery: Trainee perspectives. World J Gastrointest Surg 2013; 5(5): 146-155
- URL: https://www.wjgnet.com/1948-9366/full/v5/i5/146.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i5.146
The structural design and unique innate property of the liver to regenerate functioning parenchyma after tissue loss forms an important basis of hepatic resection surgery (HRS). Early experience was associated with significant mortality and morbidity but these are now reported at 1%-4% and 15%-35% respectively in high volume centres[1-5].
Outcomes have improved as a result of advances in the understanding of hepatic anatomy, improved surgical techniques, and enhanced peri-operative management. Patients are generally cared for in specialist higher-level ward settings with multidisciplinary input during the initial post-operative period but greater acceptance and understanding of HRS has meant that care is transferred, usually after 24-48 h to a standard ward environment. The surgical trainee will be presented with such patients either electively as part of a hepatobiliary firm or whilst on-call, and it is therefore important to understand the key points in managing HRS patients.
Herein we provide an overview of the relevant terminology of hepatic surgery, an approach to the post-operative management, and provide hints to highten awareness of complications so as to facilitate better confidence in this complex subgroup of general surgical patients.
In the United Kingdom and Europe the commonest indication for HRS remains colorectal liver metastasis (CRLM). Resection is also performed for other benign and primary malignant hepatobiliary tumours [cholangiocarcinoma (CCA) and hepatocellular carcinoma (HCC)], donation for transplantation and trauma[6-8]. Most resections performed for CRLM are on liver with otherwise normal or mildly diseased parenchyma such as post-chemotherapy fatty livers. Less frequently in the United Kingdom, HRS is performed for HCCs arising in cirrhotic patients, and such resections are associated with a higher complication rate[9,10].
Unlike other general surgical operations where the nature of the procedure is readily grasped, HRS requires some knowledge of hepatic anatomy, and specific nomenclature is applied to such resections[11]. The surgically applied anatomy of the liver is different to the traditional (morphological) teaching in undergraduate medical school. The core principle relates to the Couinaud classification of liver anatomy[12].
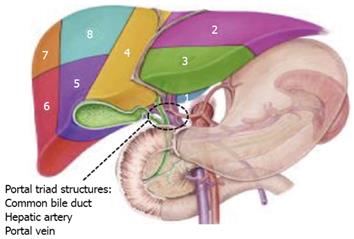
In this system the liver is divided into eight functionally independent segments (Figure 1). Each segment has its own vascular inflow, outflow and biliary drainage. In the centre of each segment there is a branch of the portal vein, hepatic artery and bile duct. In the periphery of each segment is the vascular outflow via the hepatic veins which link to form the right, middle and left hepatic veins. These in turn drain into the inferior vena cava. Crucially, the segmental portal and hepatic blood supply, together with the biliary drainage are unique, and allow for contiguous segments to be resected without compromising the vascular supply to the adjacent tissue.
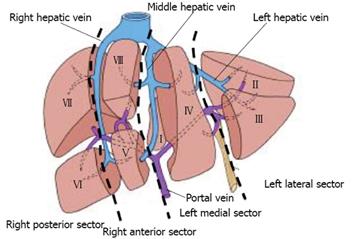
In addition, the liver is separated into four sectors by the hepatic veins (Figure 2). Briefly, the right hepatic vein divides the right lobe into anterior and posterior segments; the middle hepatic vein divides the liver into right and left lobes (hemi-livers) and the left hepatic vein divides the left lobe into medial and lateral sectors.
This knowledge forms the basis of the consensus nomenclature outlined in the Brisbane 2000 terminology guidelines for hepatic resections[13]. In Table 1 the operation titles and number of segments are illustrated. While complex, it is more important perhaps for the trainee to be aware as to what constitutes a minor and major hepatic resection, as the extent of resection is associated with mortality and morbidity. A major resection was traditionally defined as ≥ 3 segments but more recently established as ≥ 4 segments[14].
| Anatomical term | Couinaud segments | Term for HRS | Major or minor resection |
| Right hemi liver | 5, 6, 7, 8 | Right hemihepatectomy or right hemihepatectomy | Major |
| Left hemi liver | 2, 3, 4 (+/- 1) | Left hemihepatectomy or left hemihepatectomy | Major |
| Right anterior section | 5, 8 | Right anterior sectionectomy | Minor |
| Right posterior section | 6, 7 | Right posterior sectionectomy | Minor |
| Left medial section | 4 | Left medial sectionectomy or resection segment 4 or segmentectomy 4 | Minor |
| Left lateral section | 2, 3 | Left lateral sectionectomy or bisegmentectomy 2, 3 | Minor |
| - | 4, 5, 6, 7, 8 , (+/- 1) | Right trisectionectomy or extended right hemihepatectomy or extended right hepatectomy | Major |
| - | 2, 3, 4, 5, 8 , (+/- 1) | Left trisectionectomy or extended left hemihepatectomy or extended left hepatectomy | Major |
In the case of CRLM, the extent of resection that can be safely performed is now governed by two factors: the ability to resect all malignant tissue, and an adequate predicted volume of hepatic tissue remaining, the so-called functional liver remnant (FLR)[15,16]. As such during the pre-operative work-up it is important that surgeons work as part of a multi-disciplinary team with radiologists, oncologists and gastroenterologists to plan HRS to assess these factors[17].
The primary investigations used in determining the extent of resection are cross-sectional imaging studies with computed tomography (CT) ± magnetic resonance imaging (MRI) and if there is concern regards extra-hepatic disease, positron emission tomography (PET) scans are useful[18]. If there is concern regards the FLR then portal vein embolization of the diseased portion of the liver can be performed to induce hypertrophy of the remaining parenchyma. For otherwise normal parenchyma the ratio of FLR to total estimated liver volume should be in the order of 25% but 40% may be required in the presence of cirrhosis or other liver disease[19-24].
When proposing operating on cirrhotic livers it is also useful to perform a quantitative assessment of liver function, and in the Far East where HRS is more frequently performed for HCC, indocyanine green clearance (ICG) is carried out in all such patients to confirm the presence of an adequate volume of functioning parenchyma[25-30]. In the setting of CRLM, most patients have traditionally been observed to have normal parenchyma. However the widespread use of chemotherapy and its associated risk of liver injury such as steatohepatitis and sinusoidal obstruction syndrome may increase morbidity and potentially mortality associated with resection[31-33]. As a consequence such parenchyma may no longer be considered “normal” in this subgroup.
Biopsies of CRLM are not performed pre-operatively if a curative resection is planned because of concerns of needle track seeding[34]. In cases of HCC, biopsies are sometimes performed if imaging is inconclusive and may be indicated to assess the surrounding parenchyma[35].
There are now a wide range of devices and pharmaceutical agents available to the hepatic surgeon. Their collective aim is to reduce blood loss during surgery as blood loss and the need for blood transfusion are regarded as important prognostic indicators for outcome[36-38]. The most widely used device is the cavitron ultrasonic surgical aspirator (CUSA) that dissects liver tissue utilizing ultrasound.
A number of clamping maneuvers can also be employed to reduced bleeding during the phase in which the liver parenchyma is transected[39,40]. The most commonly performed procedure is the Pringle maneuver in which inflow to the liver is controlled by compressing the hepatic artery and portal vein at the level of the hepatic pedicle. A number of different protocols exist in which the vessels are intermittently clamped and released, usually at 15 min intervals.
Many units are now incorporating HRS patients into enhanced recovery programs with early targets for introduction of enteral diet, mobilization, prompt removal of invasive monitoring devices, reduction in the use of opiate analgesia, and judicious use of intravenous fluids[41-43]. These measures mean that most patients will expect to stay less than a week following their surgery. The increasing use of laparoscopic techniques has also contributed to the reduction in hospital stay especially for minor resections[44-46].
Perhaps one of the most challenging aspects for the junior trainee in the post-operative period is making sense of liver function tests. A transient early rise in serum hepatic transaminase levels as a result of hepatocellular damage is common, usually peaking at 24-48 h with the extent of derangement being related to the extent of resection[47]. A persistent rise should alert the surgeon to the presence of ongoing hepatic ischeamia. Such a problem is more likely in those in whom a vascular reconstruction has been performed or if there has been prolonged clamping of the hepatic pedicle. This is an indication for urgent notification of senior staff and a Doppler study is useful in looking at the patency of the hepatic artery and portal veins. Early intervention by means of re-operation or interventional radiological techniques may be appropriate.
An isolated rise in alkaline phosphatase or an elevation of this enzyme in association with gamma-glutamyltransferase may indicate normal hepatic regeneration rather than a pathological process, with levels of the enzyme peaking at around 14 d[48].
A sustained rise in bilirubin coupled with elevation in alkaline phosphatase should prompt a search for a cause of biliary obstruction. This is uncommon after a minor liver resection and is usually seen after a major resection in which a biliary reconstruction has been performed[49-52]. An ultrasound scan is the first line investigation to look for evidence of dilated biliary radicles. Further investigations and management can be arranged depending upon the findings of initial studies.
Changes in platelet count, prothrombin International normalized ratio (INR) and activated partial thromboplastin times (aPPT), which are markers of coagulation status, may be deranged and reflect the magnitude of resection. Specifically, a post-operative rise in INR between days 1-5 as well as a decrease in platelet count and fibrinogen levels are common and thought to be due to a combination of decreased synthetic function of the remnant liver and a consumptive coagulopathy[53-55]. This is usually self-limiting particularly in the setting of normal liver parenchyma and does not need correction with fresh frozen plasma (FFP) or platelet infusions. While there are no established guidelines for the use of FFP to prevent coagulopathy, some centers do use prophylactic FFP if the INR is > 2, in particular in cirrhotic patients[56]. This can be administered in combination with other products including vitamin K and human recombinant factor VIIa to treat clinically significant coagulopathy.
Changes in liver function are coupled with fluid and electrolytes imbalances in the post-operative setting. The principles of goal-directed therapy in maintaining adequate fluid balance, haemodynamics and renal function (urine output > 0.5 mL/kg per hour) as outlined in the British Consensus Guidelines on intravenous fluid therapy for adult surgical patients should be followed (http://www.bapen.org.uk/pdfs/bapen_pubs/giftasup.pdf). However, there are some important caveats following HRS. In the setting of cirrhosis, colloids or human albumin solutions are preferred rather than crystalloids. In addition, sodium restriction, judicious use of diuretics, and selective paracentesis are additional important measures to be considered. Under normal circumstances liver gluconeogenesis consumes a large proportion of body lactate but in the post HRS setting serum lactate can rise, as it is not efficiently metabolised. There are a number of reports implicating the negative impact of elevated lactate and base excess on outcomes after HRS, and some centers advocate the use of non-lactate containing solutions[57].
Hypo/hyperglycemia, hypocalcaemia and hypophoshataemia particularly after major resection should not be ignored and require correction. Strict control of glucose levels has been shown to improve outcomes using a variety of techniques and most intensive/high dependency care units have dedicated protocols. Phosphate is an important component of efficient cell energy metabolism. A decreased level can affect many systems and functions including respiratory failure, cardiac and neurological dysfunction, and insulin resistance[58]. Replacement can be with phosphate infusions, potassium phosphate solutions and oral and paraenteral replacement. The exact mechanism behind the pathogenesis of hypophosphataemia is likely to be increased renal excretion[59]. Hypocalcaemia should be corrected with calcium gluconate or calcium chloride to optimize coagulation status since calcium is critically important in the coagulation cascade and in liver regeneration[60].
The prevalence of venous thromboembolism (VTE) after surgery particularly in oncological patients cannot be overemphasised. In HRS there has been reluctance in the past to prescribe pharmacologic thrombo-prophylaxis due to concerns regarding bleeding and so-called ‘auto-anticoagulation’. However, VTE can still occur even in the presence of elevated INR and aPPT following HRS[61]. Indeed, evidence now confirms patients are more hypercoagulable and the use of pharmacologic thrombo-prophylaxis lowers the incidence of symptomatic VTE after major HRS without increasing the rate of blood transfusion[62,63]. The majority of patients undergoing HRS will undergo placement of an epidural catheter and so low molecular weight heparins should be started on the day of surgery unless explicit instructions from the operating team regarding increased risk of bleeding. During the surgery, pneumatic compression devices are employed to reduce the risk of thrombosis and mechanical should be continued with compression stockings post-operatively.
It is crucial for the junior doctor reviewing a patient to insure they have adequate analgesia as poor pain control leads to prolonged recovery time, inefficient respiratory effort, a poor appetite and a general slowing down of recovery. There are many options available that and can be tailored to the patient, the two most commonly used being patient-controlled analgesia with intravenous agents (opioids or paracetamol), and epidural analgesia[64]. Local anaesthetic techniques such as transversus abdominis plane (TAP) blocks and infusion catheters are also useful techniques to spare the use of opioids[65,66]. Patients can then be switched to regular and as required oral analgesics according to the world health organization analgesic ladder[67].
As the liver is an important organ for drug metabolism and detoxification it is important to realise potential risks of each modality in the context of liver parenchyma status, magnitude of resection, and concomitant liver or renal failure. Opiates have traditionally been the main stay of analgesia but can be associated with respiratory depression, excessive sedation, and exacerbation of hepatic encephalopathy[68]. As such patients on opiates require close observation in particular after major resections, HRS carried out in the presence of cirrhosis or renal impairment. Better alternatives to simple morphine in cirrhotics include hydromorphone and fentanyl as they are less affected by renal impairment, and are better secreted by the kidney[69]. Intramuscular routes should be avoided, as bioavailability is variable. Non-steroidal anti-inflammatory agents are generally avoided post hepatectomy due to concerns in relation to coagulation and renal impairment[69].
Unit guidelines will dictate when drains are used and when they should be removed, as there are no published guidelines. In reality, the decision to remove drains is dependent on the reason the drain was inserted, the type of fluid draining and the volume of that fluid. If bile is observed then senior colleagues should be informed as imaging studies may be indicated especially if drainage persists or volume increases. Some have advocated the “3×3” rule (drain-fluid bilirubin level below 3 mg/dL on day 3 after operation) as criterion for removal of prophylactically placed abdominal drains after hepatic resection[70]. Interestingly, a Cochrane review has shown that routine abdominal drainage for uncomplicated liver resection is not needed and if used a closed drain system is associated with less infectious complication and hospital stay than open systems[71].
Following major HRS, patients enter a catabolic state and so require early nutritional support to optimise liver regeneration, prevent infections, and promote general recovery. Those undergoing minor resection with normal parenchyma will often only require re-introduction of normal diet the first post-opertaive day. A systematic review of nutrition following HRS confirmed that early nutrition by enteral route is associated with a lower incidence of wound infections and complications as compared to parenteral, and therefore remains the favoured route of nutritional support[72].
In addition to early feeding, data is now emerging to encourage the use of pre- and pro-biotics (known as symbiotic therapy) in an attempt to address gut barrier dysfunction and microbial flora to reduce the gut-mediated systemic inflammatory response syndrome and encourage liver regeneration[73,74]. This therapy is yet to be validated in large randomised controlled trials and not used routinely in current United Kingdom clinical practice.
The mortality rates in the majority of published series are now in the order of 0%-2%, however, with reported morbidity rates of 25% to 45% it is important to be alert to potential complications following HRS in all patients. Risk factors for complications include: age > 65 years; ASA score ≥ 3; larger extent of resection (multiple tumours, bilobar disease); requirement for blood transfusion; and involved resection margins[75]. Up to 30% can suffer “major” complications; specifically bleeding, liver/kidney/respiratory failure and sepsis and account for the majority of deaths post surgery[75]. In an attempt to allow comparison across series, the Clavien-Dindo classification of post-operative complication is now frequently reported[76].
Around 3%-5% of patients may develop liver failure following their resection and will usually show signs and symptoms from 48-72 h after their surgery[2]. These are usually patients undergoing major resections, or resections carried out in the presence of cirrhosis. The International Study Group of Liver Surgery recently developed a consensus definition for post-hepatectomy liver failure namely ‘the impaired ability of the liver to maintain its synthetic, excretory, and detoxifying functions, which are characterized by an increased international normalized ratio and concomitant hyperbilirubinemia (according to the normal limits of the local laboratory) on or after postoperative day 5[77]. They graded the severity of post-hepatectomy liver failure on the basis to its impact on clinical management: Grade A post-hepatectomy liver failure requires no change of the patient's clinical management. The clinical management of patients with grade B post-hepatectomy liver failure deviates from the regular course but does not require invasive therapy. The need for invasive treatment defines grade C post-hepatectomy liver failure.
In our own practice, the following indices are used in the monitoring of hepatic function and identifying dysfunction: (1) persistent hyperbilirubinemia [serum bilirubin level > 4.1 mg/dL (to convert to micromoles per liter, multiply by 17.104)]; (2) coagulopathy with anINR > 2.5, despite early attempted correction with clotting factors; (3) abdominal ascites (drainage volumes > 500 mL/d); and (4) encephalopathy with hyperbilirubinemia and exclusion of other acute confusional states[36].
Another practical definition of post-hepatectomy liver failure is indicated by a prothrombin time < 50% and serum bilirubin > 50 mmol/L (the "50-50" criteria) and been shown to predictive factor of mortality when measured at days 3 and 5[78].
Patients with significantly impaired hepatic function may exhibit hepatic encephalopathy (HE). The West Haven criteria (Table 2) grades HE from I to IV according to severity and is widely used[79]. It is based on changes of consciousness, intellectual function, behavior, and is useful in monitoring patient progress. Ammonia levels should be measured if HE is suspected and lactulose and systemic antibiotcs prescribed to alter gut flora and reduce the production and absorption of ammonia[80].
| HE grade | Mental state |
| 1 | Mild confusion, slowing of ability to do mental tasks, e.g., serial 7’s |
| 2 | Drowsiness, inappropriate behaviour |
| 3 | Somnolent but rousable, marked confusion |
| 4 | Coma |
A number of risk factors have been identified for the development of post-hepatectomy liver failure and have been summarised in a recent review[81]. When confronted with a picture of liver failure, it is important to attempt to determine the underlying cause, as some elements are correctable. Causes of liver failure are usually multifactorial and include: bleeding; sepsis; hepatic ischeamia; portal vein thrombosis; venous outflow obstruction; and a poorly functioning liver remnant. There hepatotoxic effects of pre-operative chemotherapy on the parenchyma, and the presence of steatosis may also contribute to insufficiency.
Intensivists, senior surgeons and hepatologists lead the management of this most feared complication. The mainstay of treatment is supportive with blood products administered to support synthetic function, aggressive investigation and treatment of infection, and radiological investigation to ensure patency of major vascular and biliary structures. The use of exogenous antioxidants such as N-acetylcysteine (Parvolex®) has been used by some including our own unit in attempting to reduce the damage by oxygen free radical associated ischaemic reperfusion injury of the liver[82]. However this remains to be accepted as universal practice and currently lacks a strong evidence base[83,84].
Intra- and post-operative bleeding, and the requirement for blood transfusion are associated with increased morbidity, mortality and poorer long-term disease-specific outcomes in CRLM and HCC[85,86]. Kooby et al[37] in a study of 1351 liver resections noted a variation in operative mortality between 1.2% for no transfusion to 11.1% when more than 2 units of blood were transfused. A recent review by Dixon et al[38] highlighted the negative effects of blood loss on outcome in surgical oncology patients, and suggested that the need for transfusion may be an indicator of the quality of surgery performed.
The operating surgeon and anaesthetist incorporate multiple techniques including: low intra-operative central venous pressure; dynamic intra-operative coagulation monitoring; drugs (aprotinin, tranexamic acid); and haemostatic products on the cut surface of the liver to reduce the occurrence of this complication. As a result median blood loss in overall HRS has significantly reduced and reported to be less than 700-800 mL[87]. Indeed, the median transfusion rate in the majority of contemporary series is zero.
Blood loss during surgery should be clearly documented on the operative note. Unit protocols drive the specific haemoglobin criteria for transfusion and should be referred to when assessing the patient in this early stage. During the post-operative phase, patients will have haemoglobin and haematocrit measurements determined regularly. It would be expected that patients would stabilise during the initial 24-48 h and any deterioration following this should trigger referral to senior colleagues and a request for imaging studies. Patients actively haemorrhaging may require re-exploration or radiological embolisation of bleeding vessels.
As evidence grows implicating post-operative complications, in particular infection, in poorer disease-free survival, an important aim must be to pro-actively attempt to minimise infections, and when present to identify and implement treatment in an expedient manner[75]. Risk factors known to be associated with infection include: obesity; major resections requiring blood transfusions; presence of co-morbidities (diabetes, chronic obstructive pulmonary disease); and post-operative bile leaks[88]. Standard effective interventions to minimise infections include ensuring adequate chest physiotherapy, early patient mobilisation, prompt removal of indwelling devices, and institution of broad-spectrum antibiotics therapy where indicated.
Bile leakage is an important complication occurring after liver surgery and its reported incidence ranges between 4.8%-7.6% in large series[89-95] and is less common in surgery for CRLM than for HCC or CCA. The International Study Group of Liver Surgery has recently proposed a uniform definition of bile leakage and a grading system according to severity, which is based on drain fluid bilirubin concentration of greater than three times the serum bilirubin concentration on day 3 after surgery or the need for additional interventions[96]. Management of bile leaks includes treatment of associated infection, defining the location of leak, externalizing the bile with a radiologically placed drain, and the consideration of insertion of biliary stents and/or reconstructive surgery[97].
No consensus protocol exists for the post-operative management of HRS, as each centre will have different guidelines reflecting preferences of senior staff with regards to the finer points of management. It is important to deliver early nutrition, effective analgesia, and promote good respiratory function. Furthermore close observation in the early post-operative period is required to identify and aggressively manage bleeding, infection and prevent the development of liver failure. The surgical trainee is required to have a basic grounding and have the ability to appreciate exactly what resection has been performed in a patient to allow for meaningful assessment. Such knowledge will provide insight into being able to alert senior staff appropriately and expediently in this challenging dynamic subgroup of patients.
P- Reviewers Llado, L, Wakai T S- Editor Song XX L- Editor A E- Editor Lu YJ
| 1. | Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, Del Gaudio M, Pinna AD. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 810] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 3. | Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, Lodge JP, Toogood GJ. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozzetti F, Bonalumi MG. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg. 1995;82:377-381. [PubMed] |
| 5. | Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 625] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 6. | Yasui K, Shimizu Y. Surgical treatment for metastatic malignancies. Anatomical resection of liver metastasis: indications and outcomes. Int J Clin Oncol. 2005;10:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Li Petri S, Gruttadauria S, Pagano D, Echeverri GJ, Di Francesco F, Cintorino D, Spada M, Gridelli B. Surgical management of complex liver trauma: a single liver transplant center experience. Am Surg. 2012;78:20-25. [PubMed] |
| 8. | Kim SJ, Na GH, Choi HJ, Yoo YK, Kim DG. Surgical outcome of right liver donors in living donor liver transplantation: single-center experience with 500 cases. J Gastrointest Surg. 2012;16:1160-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Paugam-Burtz C, Wendon J, Belghiti J, Mantz J. Case scenario: postoperative liver failure after liver resection in a cirrhotic patient. Anesthesiology. 2012;116:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207-115; discussion 1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Celinski SA, Gamblin TC. Hepatic resection nomenclature and techniques. Surg Clin North Am. 2010;90:737-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg. 1999;16:459-467. [PubMed] |
| 13. | Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 682] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 14. | Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW, Geller DA, Clary BM. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford). 2011;13:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Gazzaniga GM, Cappato S, Belli FE, Bagarolo C, Filauro M. Assessment of hepatic reserve for the indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005;12:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 17. | Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 18. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Mullin EJ, Metcalfe MS, Maddern GJ. How much liver resection is too much? Am J Surg. 2005;190:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [PubMed] |
| 21. | Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness--study in 26 patients. Radiology. 2003;227:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [PubMed] |
| 24. | de Baere T, Robinson JM, Deschamps F, Rao P, Teriitheau C, Goere D, Elias D. Preoperative portal vein embolization tailored to prepare the liver for complex resections: initial experience. Cardiovasc Intervent Radiol. 2010;33:976-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | de Liguori Carino N, O’Reilly DA, Dajani K, Ghaneh P, Poston GJ, Wu AV. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol. 2009;35:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Greco E, Nanji S, Bromberg IL, Shah S, Wei AC, Moulton CA, Greig PD, Gallinger S, Cleary SP. Predictors of peri-opertative morbidity and liver dysfunction after hepatic resection in patients with chronic liver disease. HPB (Oxford). 2011;13:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Lam CM, Fan ST, Lo CM, Wong J. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255-1259. [PubMed] |
| 29. | Lee CF, Yu MC, Kuo LM, Chan KM, Jan YY, Chen MF, Lee WC. Using indocyanine green test to avoid post-hepatectomy liver dysfunction. Chang Gung Med J. 2007;30:333-338. [PubMed] |
| 30. | Ren Z, Xu Y, Zhu S. Indocyanine green retention test avoiding liver failure after hepatectomy for hepatolithiasis. Hepatogastroenterology. 2012;59:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, Prasad KR. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Baumgaertner I, Ratziu V, Vaillant JC, Hannoun L, Poynard T, André T. [Hepatotoxicity of metastatic colorectal cancer chemotherapy: systematic review]. Bull Cancer. 2010;97:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Wolf PS, Park JO, Bao F, Allen PJ, DeMatteo RP, Fong Y, Jarnagin WR, Kingham TP, Gönen M, Kemeny N. Preoperative chemotherapy and the risk of hepatotoxicity and morbidity after liver resection for metastatic colorectal cancer: a single institution experience. J Am Coll Surg. 2013;216:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Cresswell AB, Welsh FK, Rees M. A diagnostic paradigm for resectable liver lesions: to biopsy or not to biopsy? HPB (Oxford). 2009;11:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 36. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 37. | Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860-869; discussion 860-869;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 369] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 38. | Dixon E, Datta I, Sutherland FR, Vauthey JN. Blood loss in surgical oncology: neglected quality indicator? J Surg Oncol. 2009;99:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Hoekstra LT, van Trigt JD, Reiniers MJ, Busch OR, Gouma DJ, van Gulik TM. Vascular occlusion or not during liver resection: the continuing story. Dig Surg. 2012;29:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Chouillard EK, Gumbs AA, Cherqui D. Vascular clamping in liver surgery: physiology, indications and techniques. Ann Surg Innov Res. 2010;4:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhaug A, Fearon KC, Garden OJ, Dejong CH. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Schultz NA, Larsen PN, Klarskov B, Plum LM, Frederiksen HJ, Christensen BM, Kehlet H, Hillingsø JG. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg. 2013;100:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Lin DX, Li X, Ye QW, Lin F, Li LL, Zhang QY. Implementation of a fast-track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys. 2011;61:413-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [PubMed] |
| 45. | Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg. 2013;257:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 879] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 47. | Pelton JJ, Hoffman JP, Eisenberg BL. Comparison of liver function tests after hepatic lobectomy and hepatic wedge resection. Am Surg. 1998;64:408-414. [PubMed] |
| 48. | Muraoka I, Soyama A, Takatsuki M, Tomonaga T, Hidaka M, Kanematsu T, Eguchi S. Transition of serum alkaline phosphatase isoenzymes during liver regeneration in humans. Hepatogastroenterology. 2011;58:1436-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Boonstra EA, de Boer MT, Sieders E, Peeters PM, de Jong KP, Slooff MJ, Porte RJ. Risk factors for central bile duct injury complicating partial liver resection. Br J Surg. 2012;99:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Ferrero A, Russolillo N, Viganò L, Sgotto E, Lo Tesoriere R, Amisano M, Capussotti L. Safety of conservative management of bile leakage after hepatectomy with biliary reconstruction. J Gastrointest Surg. 2008;12:2204-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Fragulidis G, Marinis A, Polydorou A, Konstantinidis C, Anastasopoulos G, Contis J, Voros D, Smyrniotis V. Managing injuries of hepatic duct confluence variants after major hepatobiliary surgery: an algorithmic approach. World J Gastroenterol. 2008;14:3049-3053. [PubMed] |
| 52. | Reed DN, Vitale GC, Wrightson WR, Edwards M, McMasters K. Decreasing mortality of bile leaks after elective hepatic surgery. Am J Surg. 2003;185:316-318. [PubMed] |
| 53. | De Pietri L, Montalti R, Begliomini B, Scaglioni G, Marconi G, Reggiani A, Di Benedetto F, Aiello S, Pasetto A, Rompianesi G. Thromboelastographic changes in liver and pancreatic cancer surgery: hypercoagulability, hypocoagulability or normocoagulability? Eur J Anaesthesiol. 2010;27:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Bezeaud A, Denninger MH, Dondero F, Saada V, Venisse L, Huisse MG, Belghiti J, Guillin MC. Hypercoagulability after partial liver resection. Thromb Haemost. 2007;98:1252-1256. [PubMed] |
| 55. | Weinberg L, Scurrah N, Parker EC, Dauer R, Marshall J, McCall P, Story D, Smith C, McNicol L. Markers of coagulation activation after hepatic resection for cancer: evidence of sustained upregulation of coagulation. Anaesth Intensive Care. 2011;39:847-853. [PubMed] |
| 56. | Martin RC, Jarnagin WR, Fong Y, Biernacki P, Blumgart LH, DeMatteo RP. The use of fresh frozen plasma after major hepatic resection for colorectal metastasis: is there a standard for transfusion? J Am Coll Surg. 2003;196:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Watanabe I, Mayumi T, Arishima T, Takahashi H, Shikano T, Nakao A, Nagino M, Nimura Y, Takezawa J. Hyperlactemia can predict the prognosis of liver resection. Shock. 2007;28:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. 2010;14:R147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 59. | Nafidi O, Lapointe RW, Lepage R, Kumar R, D’Amour P. Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg. 2009;249:824-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Garcin I, Tordjmann T. Calcium signalling and liver regeneration. Int J Hepatol. 2012;2012:630670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Senzolo M, Sartori MT, Lisman T. Should we give thromboprophylaxis to patients with liver cirrhosis and coagulopathy? HPB (Oxford). 2009;11:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Reddy SK, Turley RS, Barbas AS, Steel JL, Tsung A, Marsh JW, Clary BM, Geller DA. Post-operative pharmacologic thromboprophylaxis after major hepatectomy: does peripheral venous thromboembolism prevention outweigh bleeding risks? J Gastrointest Surg. 2011;15:1602-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Morris-Stiff G, White A, Gomez D, Toogood G, Lodge JP, Prasad KR. Thrombotic complications following liver resection for colorectal metastases are preventable. HPB (Oxford). 2008;10:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Ritchey RM. Optimizing postoperative pain management. Cleve Clin J Med. 2006;73 Suppl 1:S72-S76. [PubMed] |
| 65. | Petersen PL, Mathiesen O, Torup H, Dahl JB. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 66. | Chan SK, Lai PB, Li PT, Wong J, Karmakar MK, Lee KF, Gin T. The analgesic efficacy of continuous wound instillation with ropivacaine after open hepatic surgery. Anaesthesia. 2010;65:1180-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Mercadante S, Fulfaro F. World Health Organization guidelines for cancer pain: a reappraisal. Ann Oncol. 2005;16 Suppl 4:iv132-iv135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Rudin A, Lundberg JF, Hammarlund-Udenaes M, Flisberg P, Werner MU. Morphine metabolism after major liver surgery. Anesth Analg. 2007;104:1409-114, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 70. | Yamazaki S, Takayama T, Moriguchi M, Mitsuka Y, Okada S, Midorikawa Y, Nakayama H, Higaki T. Criteria for drain removal following liver resection. Br J Surg. 2012;99:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev. 2007;CD006232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Richter B, Schmandra TC, Golling M, Bechstein WO. Nutritional support after open liver resection: a systematic review. Dig Surg. 2006;23:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Usami M, Miyoshi M, Kanbara Y, Aoyama M, Sakaki H, Shuno K, Hirata K, Takahashi M, Ueno K, Tabata S. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J Parenter Enteral Nutr. 2011;35:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Rayes N, Pilarski T, Stockmann M, Bengmark S, Neuhaus P, Seehofer D. Effect of pre- and probiotics on liver regeneration after resection: a randomised, double-blind pilot study. Benef Microbes. 2012;3:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP, Prasad KR. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 76. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 77. | Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, Fan ST, Nimura Y, Figueras J, Vauthey JN. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford). 2011;13:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 78. | Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, Belghiti J. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 79. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1411] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 80. | Toris GT, Bikis CN, Tsourouflis GS, Theocharis SE. Hepatic encephalopathy: an updated approach from pathogenesis to treatment. Med Sci Monit. 2011;17:RA53-RA63. [PubMed] |
| 81. | Golse N, Bucur PO, Adam R, Castaing D, Sa Cunha A, Vibert E. New paradigms in post-hepatectomy liver failure. J Gastrointest Surg. 2013;17:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | Lee EJ, Silva SM, Simões Mde J, Montero EF. Effect of N-acetylcysteine in liver ischemia-reperfusion injury after 30% hepatectomy in mice. Acta Cir Bras. 2012;27:346-349. [PubMed] |
| 83. | Jegatheeswaran S, Siriwardena AK. Experimental and clinical evidence for modification of hepatic ischaemia-reperfusion injury by N-acetylcysteine during major liver surgery. HPB (Oxford). 2011;13:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | McKay A, Cassidy D, Sutherland F, Dixon E. Clinical results of N-acetylcysteine after major hepatic surgery: a review. J Hepatobiliary Pancreat Surg. 2008;15:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Shiba H, Ishida Y, Wakiyama S, Iida T, Matsumoto M, Sakamoto T, Ito R, Gocho T, Furukawa K, Fujiwara Y. Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg. 2009;13:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 87. | McNally SJ, Revie EJ, Massie LJ, McKeown DW, Parks RW, Garden OJ, Wigmore SJ. Factors in perioperative care that determine blood loss in liver surgery. HPB (Oxford). 2012;14:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 88. | Garwood RA, Sawyer RG, Thompson L, Adams RB. Infectious complications after hepatic resection. Am Surg. 2004;70:787-792. [PubMed] |
| 89. | Capussotti L, Ferrero A, Viganò L, Sgotto E, Muratore A, Polastri R. Bile leakage and liver resection: Where is the risk? Arch Surg. 2006;141:690-694; discussion 695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 90. | Erdogan D, Busch OR, van Delden OM, Rauws EA, Gouma DJ, van Gulik TM. Incidence and management of bile leakage after partial liver resection. Dig Surg. 2008;25:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Hayashi M, Hirokawa F, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Arisaka Y, Masuda D, Tanigawa N. Clinical risk factors for postoperative bile leakage after liver resection. Int Surg. 2010;95:232-238. [PubMed] |
| 92. | Ishii H, Ochiai T, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Fujiwara H. Risk factors and management of postoperative bile leakage after hepatectomy without bilioenteric anastomosis. Dig Surg. 2011;28:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Lo CM, Fan ST, Liu CL, Lai EC, Wong J. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg. 1998;133:156-161. [PubMed] |
| 94. | Nagano Y, Togo S, Tanaka K, Masui H, Endo I, Sekido H, Nagahori K, Shimada H. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, Sugimachi K. Bile leakage after hepatic resection. Ann Surg. 2001;233:45-50. [PubMed] |
| 96. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1414] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 97. | Hoekstra LT, van Gulik TM, Gouma DJ, Busch OR. Posthepatectomy bile leakage: how to manage. Dig Surg. 2012;29:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |