Published online Dec 27, 2013. doi: 10.4240/wjgs.v5.i12.337
Revised: October 22, 2013
Accepted: November 15, 2013
Published online: December 27, 2013
Processing time: 42 Days and 20.3 Hours
Bariatric surgeries have been used in an effort to curtail the obesity epidemic. The type of surgery used has changed over time, with sleeve gastrectomies being one of the preferred options. This has been associated with some complications, including staple line leaks. We report a 43-year old female who had undergone a laparoscopic sleeve gastrectomy that was complicated by a proximal gastric pouch leak at the gastroesophageal junction. We used self-expandable stents (SEMS) in the management of the leak. Seven weeks after the insertion of the initial SEMS, the patient presented with a massive gastrointestinal bleed that could not be localized due to profuse bleeding. The patient underwent a computerized tomography angiogram and then an angiogram that could not localize the site of the bleed. An emergency laparotomy was performed and identified the source of bleeding to be an aortoesophageal fistula. A graft of the diseased area was attempted but the patient unfortunately did not survive the procedure. An aortoesophageal fistula after an esophageal SEMS insertion for a benign disease has rarely been reported and only in cases where there was a thoracic neoplasm, thoracic aortic aneurism, endovascular stent repair, foreign body or esophageal surgery. To our knowledge, this is the first case that reports an aortoesophageal fistula as a result of a SEMS for the management of a gastric pouch leak after a laparoscopic sleeve gastrectomy.
Core tip: One modality for managing staple line leaks after laparoscopic sleeve gastrectomies depends on a non-surgical approach, including elimination of oral intake, parenteral nutrition, use of broad spectrum antimicrobial therapy, drainage procedures and the use of esophageal self-expandable metal stents for sealing these leaks and the induction of tissue hyperplasia that would close these defects. Although this seems as a less invasive procedure when compared to a repeated surgical procedure and there is a body of evidence in the literature that supports such an approach, it is not void of complications. Here we report a fatal aortoesophageal fistula as a complication.
- Citation: Almadi MA, Bamihriz F, Aljebreen AM. Fatal aortoesophageal fistula bleeding after stenting for a leak post sleeve gastrectomy. World J Gastrointest Surg 2013; 5(12): 337-340
- URL: https://www.wjgnet.com/1948-9366/full/v5/i12/337.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i12.337
Obesity has become a major public health challenge, associated with a significant morbidity, mortality as well as decreased quality of life. Bariatric surgeries have been used as a modality to treat obesity, preferably after a multidisciplinary assessment. Although such an intervention has been proven to be effective in decreasing the excess weight of patients, it is associated with some complications of which surgical leaks are one of the most unfavorable with a considerable morbidity and mortality[1]. The management of staple line leaks post sleeve gastrectomy has evolved from surgical reinterventions to a less invasive approach with elimination of oral intake, parenteral nutrition, broad spectrum antimicrobial therapy, as well as percutaneous drainage procedures[1]. More recently, the use of esophageal self-expandable metal stents (SEMS) or self-expandable plastic stents (SEPS)[1-3] as method of occluding these leaks has become a more acceptable form of management.
We present an unusual case where a SEMS resulted in a massive gastrointestinal bleed secondary to an aortoesophageal fistula.
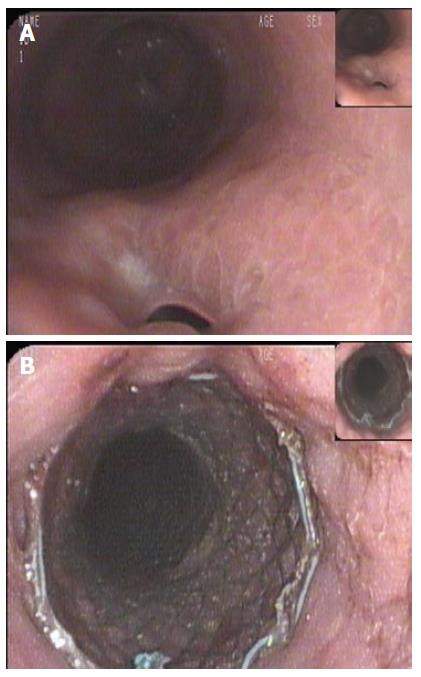
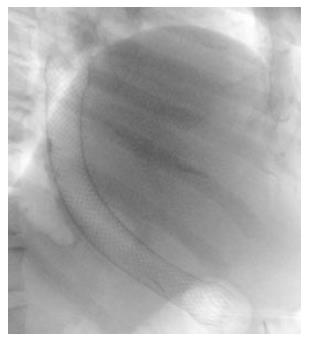
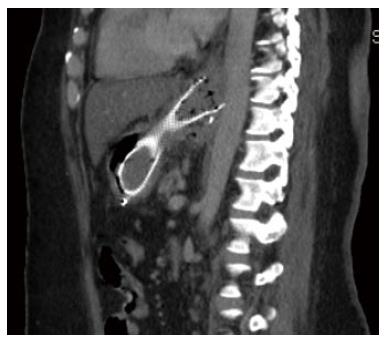
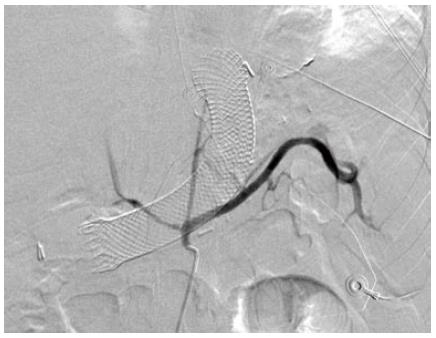
A 43-year old female was referred to our institution after the development of a proximal gastric pouch staple line leak at the gastroesophageal junction three weeks after a laparoscopic sleeve gastrectomy, which was confirmed by a contrast swallow study. The patient was started on broad-spectrum antibiotics and a percutaneous drainage tube was inserted to treat a subdiaphragmatic fluid collection seen on computer tomography (CT). An esophagogastroduodenoscopy (EGD) showed an opening at the area of the staple line at the gastroesophageal junction (Figure 1A). As one of the preferable modalities to treat staple line leaks post sleeve gastrectomy, a 12 cm fully covered SEMS was inserted (Figure 1B). The patient presented with nausea and vomiting three weeks later. An EGD found the distal end of the SEMS to be narrowed at the antrum angulation, it was removed and a second 15 cm partially covered SEMS was inserted as the staple line leak was still present. Four weeks later, the patient presented with hematemesis, hypotension and tachycardia. She was resuscitated and an EGD showed blood in the stomach but the source of bleeding could not be identified. The patient underwent a CT angiogram (Figure 2) and then an angiogram (Figure 3), but no clear source was found apart from doubtful areas along the left gastric (Figure 4) and gastroduodenal arteries that were coiled, but the patient continued to bleed.
An emergency laparotomy was performed. During exploration, the gastric pouch was opened distally and the stent was removed but the patient continued to bleed proximally, necessitating opening the gastric pouch completely up to the level of gastroesophageal junction where the source of the fresh blood was identified. A small pinpoint hole at the base of a small ulcer in the distal esophagus was bleeding profusely. The diagnosis of an aortoesophageal fistula was made and was confirmed by a left thoracoabdominal incisional approach by a cardiovascular surgeon. A short segment of tense fibrosis between the distal esophagus and aorta with a 2-3 mm opening communicating between them was seen. A graft of the diseased area was attempted but the patient unfortunately did not survive the procedure.
In two systematic reviews, the incidence of leaks after laparoscopic sleeve gastrectomy was found to be 2.2%[3] to 2.4%[4] and the leaks are usually in the proximal third of the stomach near the gastroesophageal junction in 85%-89%[1,4] of cases, with an associated mortality rate of 6%-14.7%[1]. The use of enteric SEMSs has evolved from the management of malignant diseases to benign strictures as well as leaks. A systematic review incorporating 25 studies with a total of 267 patients demonstrated a success rate of 85% with the use of SEMSs for the management of enteric leaks with no difference in the rate of clinical success between the type of SEMS used, whether partially or fully-covered SEMS or SEPS (P = 0.97)[5]. In these studies, the patient population included cases who had esophageal anastomotic leaks or a benign rupture of the esophagus[5]. A second meta-analysis for patients who exclusively had leaks post bariatric surgery and were managed by SEMSs found a success rate of 87.8% (95%CI: 79.4%-94.2%)[6].
An aortoesophageal fistula[7,8] is a rarely reported complication of esophageal SEMS and the majority of reported cases are secondary to thoracic aortic aneurisms, endovascular stent repairs, thoracic neoplasms, foreign bodies, radiofrequency ablation for atrial fibrillation or esophageal surgery. An aortoesophageal fistula after an esophageal SEMS insertion for an esophageal benign disease has rarely been reported and only in cases where there was an esophageal stricture[9,10]. More recently, the management of aortoenteric fistulas has been via thoracic endovascular aortic repair to control bleeding in the acute setting, either as a stand alone procedure or combined with a more definite management in an elective setting[11]. Other management strategies include endovascular aortic repair and subtotal esophageal resection followed by gastroesophageal reconstruction or open thoracic surgery[11]. The advantage of the former approach compared to the stand alone endovascular aortic repair is that, although it controls bleeding acutely, there is a higher probability of graft infection and mediastinitis given that the esophageal defect is not corrected[12]. Even when a diagnosis of an aortoesophageal fistula is reached, the morbidity and mortality is high, reaching up to 40%[13].
In a case series of 52 patients who required SEMSs for enteric leaks, there was a report of a death from severe hemorrhage after the insertion of a fully covered SEMS; the patient refused any intervention and thus the cause of the bleeding was unknown[2]. Also, there was a report of 4 deaths in a series of patients who had stents inserted for leaks after various bariatric surgeries but none were related to the stents[14].
Although the patient had adequate initial resuscitation that permitted the performance of an EGD as well as two radiological procedures, the diagnosis was not easily reached, an emergency surgery was required and even after reaching a definite diagnosis, the patient did not survive the surgery.
This case demonstrates that although the success rate with the use of SEMSs for the management of staple line leaks after laparoscopic sleeve gastrectomies is high, they still have potential complications and a high index of suspicion is required in order to pursue timely management of such complications.
To our knowledge, this is the first case that reports an aortoesophageal fistula as a result of a SEMS for the management of a gastric pouch leak after a laparoscopic sleeve gastrectomy.
This is a case of fatal aortoesophageal fistula bleeding after stenting for a leak post sleeve gastrectomy.
This is the first case that reports an aortoesophageal fistula as a result of a self-expandable stents (SEMS) for the management of a gastric pouch leak after a laparoscopic sleeve gastrectomy.
Although the success rate with the use of SEMSs for the management of staple line leaks after laparoscopic sleeve gastrectomies is high, they still have potential complications and a high index of suspicion is required in order to pursue timely management of such complications.
The authors have reported an interesting case of sleeve gastrectomy complication and an alarming finding on metal stent use for treatment of gastroesophageal leak.
P- Reviewers: Leuratti L, Maleki AR S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Kumar N, Thompson CC. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol. 2013;11:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | van Boeckel PG, Dua KS, Weusten BL, Schmits RJ, Surapaneni N, Timmer R, Vleggaar FP, Siersema PD. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 4. | Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 5. | van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Um SJ, Park BH, Son C. An aortoesophageal fistula in patient with lung cancer after chemo-irradiation and subsequent esophageal stent implantation. J Thorac Oncol. 2009;4:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 9. | Unosawa S, Hata M, Sezai A, Niino T, Yoda M, Shimura K, Furukawa N, Minami K. Surgical treatment of an aortoesophageal fistula caused by stent implantation for esophageal stenosis: report of a case. Surg Today. 2008;38:62-64. [PubMed] |
| 10. | Rogart J, Greenwald A, Rossi F, Barrett P, Aslanian H. Aortoesophageal fistula following Polyflex stent placement for refractory benign esophageal stricture. Endoscopy. 2007;39 Suppl 1:E321-E322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Göbölös L, Miskolczi S, Pousios D, Tsang GM, Livesey SA, Barlow CW, Kaarne M, Shambrook J, Lipnevicius A, Ohri SK. Management options for aorto-oesophageal fistula: case histories and review of the literature. Perfusion. 2013;28:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Chiesa R, Melissano G, Marone EM, Kahlberg A, Marrocco-Trischitta MM, Tshomba Y. Endovascular treatment of aortoesophageal and aortobronchial fistulae. J Vasc Surg. 2010;51:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |