Published online Jan 27, 2012. doi: 10.4240/wjgs.v4.i1.23
Revised: November 13, 2011
Accepted: November 20, 2011
Published online: January 27, 2012
We focus on the diagnostic and therapeutic problems of duodenal adenocarcinoma, reporting a case and reviewing the literature. A 65-year old man with adenocarcinoma in the third duodenal portion was successfully treated with a segmental resection of the third part of the duodenum, avoiding a duodeno-cephalo-pancreatectomy. This tumor is very rare and frequently affects the III and IV duodenal portion. A precocious diagnosis and the exact localization of this neoplasia are crucial factors in order to decide the surgical strategy. Given a non-specificity of symptoms, endoscopy with biopsy is the diagnostic gold standard. Duodeno-cephalo-pancreatectomy (DCP) and segmental resection of the duodenum (SRD) are the two surgical options, with overlapping morbidity (27% vs 18%) and post operative mortality (3% vs 1%). The average incidence of postoperative long-term survival is 100%, 73.3% and 31.6% of cases after 1, 3 and 5 years from surgery, respectively. Long-term survival is made worse by two factors: the presence of metastatic lymph nodes and tumor localization in the proximal duodenum. The two surgical options are radical: DCP should be used only for proximal localizations while SRD should be chosen for distal localizations.
- Citation: Sista F, Santis GD, Giuliani A, Cecilia EM, Piccione F, Lancione L, Leardi S, Amicucci G. Adenocarcinoma of the third duodenal portion: Case report and review of literature. World J Gastrointest Surg 2012; 4(1): 23-26
- URL: https://www.wjgnet.com/1948-9366/full/v4/i1/23.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i1.23
Duodenal carcinoma is very rare. It represents 33%-45% of all tumors of the small bowel[1], which are 4%-5% of all tumors of the gastrointestinal bowel[2]. Moreover, it causes only 1% of the deaths due to gastrointestinal neoplasias[3].
The clinical picture is nonspecific (postprandial abdominal pains with cramps) and the diagnosis is often accidental[1,4,5]. Therefore, duodenal carcinoma is difficult to diagnose due to its rarity and clinical picture[1,2].
The aim of the present work is to offer a further contribution to the resolution of therapeutic and diagnostic problems, reporting a new case of adenocarcinoma of the third duodenal portion and reviewing the relevant literature.
A 65-year old man recently came to our unit, the General Surgery Department of the University of L’Aquila, Italy, with a 3-mo history of abdominal pains. They usually started 3-4 h after meals and were intermittently associated with dyspepsia. This patient had a history of a duodenal ulcer 20 years earlier. Gastric and biliary vomit was associated with abdominal pains without hematemesis and melena. The alvus was open to gas and feces.
At admission, biohumoral findings and serum levels of tumor markers were normal; only carcinoembryonic antigen was slightly elevated (6.1 ng/mL).
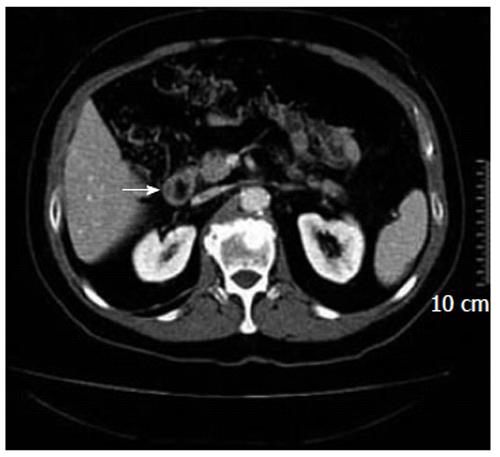
Because of his symptoms, the patient underwent esophagogastroduodenoscopy (EGDS), which showed a “mild degree of esophagitis, normal gastric mucosa, bleeding ulceration in the III portion of the duodenum with irregular edge”. Bioptic samples were taken and showed a “tubule and villus adenocarcinoma in situ”. The abdominal computed tomography (CAT), taken to determine the staging of the neoplasia, showed “an irregularity and thickening of the wall of the III portion of the duodenum without clear hepatic, pulmonary, lymph nodal and peritoneal metastases” (Figure 1). Thus, the patient underwent surgery. During the operation, after an extended Kocherization of the duodenum, a 2-cm wide neoplasia was found in the third portion of the duodenum. It did not involve the sphincter of Oddi and had no hepatic metastases. The absence of lymphatic locoregional metastases was confirmed by an intraoperative lymphatic frozen section. Taking all this into account, the surgeon decided to avoid a duodeno-cephalo-pancreatectomy (DCP) and opted for a segmental resection of the duodenum (SRD) with a termino-terminal anastomosis.
The duodenum was separated from the pancreatic head using an ultrasonic dissector which, compared to electric scalpels, has a lower heat propagation on the surrounding tissues, avoiding injury to the pancreatic parenchyma. Preserving the anterior and posterior duodeno-pancreatic vascular arcades, a termino-terminal anastomosis was made using silk points. No mechanical staplers were used.
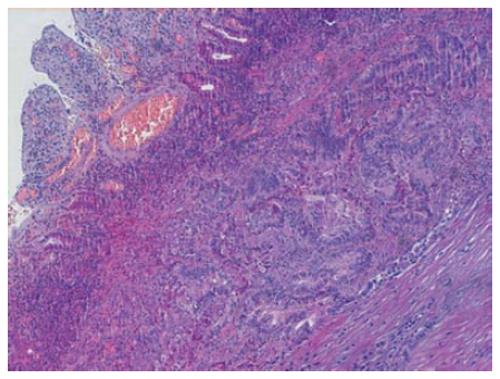
The histopathological findings reported “tubulo-papillary adenocarcinoma, rather differentiated, infiltrating two third of the muscular wall and the negative margins of resection; pT2 pN0 pM0” (Figure 2). Postoperative period was normal and presented no complications. The patient resumed oral feeding in the 7th postoperative day after a radiological control of anastomosis and was discharged on the 9th postoperative day, excluding adjuvant chemotherapy after oncological advice. A 2-year clinical-instrumental follow-up showed no locoregional recurrence of the disease.
Primitive neoplasia of the duodenum is very rare[1,2]. The III and the IV duodenal portions are the most common areas[4,6], with the localization of 45% of tumors, 40% in the II and only 15% in the first[6]. Thus, our case can be included in the first group.
The symptoms are not specific[1]; 65% of cases are characterized by the association of intermittent abdominal pains with cramps and biliary vomit[7], as in our case.
EGDS with biopsy is the diagnostic gold standard, even though it is not unusual to obtain false negative cases in the III and IV duodenal portions[1,8,9].
If diagnostic doubts are present, CAT may be essential to show the neoplasia in the duodenal wall and determine the tumor staging. Nevertheless, it is important to take into account that CAT diagnostic accuracy for a duodenal neoplasia smaller than 2 cm is not optimal, with a sensitivity of 94% and a specificity of 82%[4,7,10].
The surgical treatment is not yet well defined and codified.
DCP associated with “en bloc” locoregional lymph nodal exeresis (hepatic artery and aortocaval district) would seem to be the chosen option[11,12]. Nevertheless, this therapeutic strategy has recently been questioned in favor of a SRD[7,11,13-15].
DCP would be recommended only in proximally localized tumors, while a SRD would be appropriate for distal localizations[13], as in our case.
However, morbidity rate is almost the same after the two types of surgery (27% after DCP, 18% after SRD)[14], even if some authors[15,16] remark on a higher morbidity, although not significant, after SRD. Peripancreatic fistulae (pancreatic or duodenal ones) (9%-25%), intra-abdominal abscesses (16%-20%), peritonitis (8%-9%), endoabdominal bleeding (3%-13%) and delayed gastric emptying (7%-22%)[7,10,14,15] are frequent complications. Pancreatic leakage is present in 33%-66% of cases after DCP, while it is absent after SRD. As reported in the literature, our case also had a postoperative low-flow duodenal fistula which cleared up after a 3-wk medical treatment.
The mortality rate after DCP is not particularly different from the one after SRD (3% vs 1% of cases)[15,16]. However, we need to take into account that DCP, also considering the same procedure for pancreatic tumors, has a higher mortality rate if it is not carried out in centers of excellence. In fact, mortality would increase from 3% to 13.8%-16.5% in those centers performing less than 5 DCP per year[7], constituting a significant statistical difference.
The average incidence of postoperative long-term survival is 100%, 73.3% and 31.6% of cases after 1, 3 and 5 years after surgery, respectively[7,10,13], even if conflicting results are shown in literature[1,3,12,14,17].
The incidence of survival after time changes if we analyse some factors strictly correlated with it. Three years after surgery, survival is found in 72% of cases without any affected lymph nodes vs 30% if lymph node metastases are present[14]. Five years after surgery, survival decreases to 53.3%-68%[3,18] of cases without any lymph nodes diseases, vs 0% if an illness is present. Moreover, other authors[12,17] have not found significantly different incidences of survival regarding the invasion of metastases of lymph nodes.
The localization of the duodenal neoplasia seems to be another point that affects survival[19]. In fact, tumors of the proximal duodenum seem to have a worse long-term prognosis as the tumors act as retroperitoneal neoformation[6,8,9,12,20]. With no affected lymph nodes, 5 years after surgery, tumors with a proximal localization have a survival rate from 0%[21] to 25%[22], but the same rate reaches 62% in distal localizations.
The data in the literature are not exhaustive regarding the relationship between survival and type of adopted surgery (DCP vs SRD). There are not many studies that compare the two surgical techniques.
At post-operative 5-year follow-up, patients who underwent DCP had a better incidence of survival than those who underwent SRD (27.8% vs 16.9% of cases)[7,10]. On the other hand, other authors[20] have observed the same incidence of survival (31.6% of cases), upholding radicality; with this perspective, the kind of operation would not be a factor that may improve survival.
As there are no studies comparing the two surgical procedures within the same localization in the literature, some authors[9,20] have shown that in cases presenting the same stage and surgical risk, there are ontogenetic motivations to perform a different operation according to the place of the neoplasia.
The worst prognosis of neoplasia placed in the first duodenal portion seems to be due to the close topographic relationship with the surrounding organs that may be affected soon. The same does not happen for neoplasias in the III and IV duodenal portion that have an independent embryological development from the surrounding organs[9].
Moreover, the drainage of the lymph nodes seems different in the I and II duodenal portion compared to the III or IV. The first ones seem to rush to anterior and posterior pancreatic-duodenal chains of lymph nodes; the second ones seem to drain into the chains of lymph nodes of the upper mesenteric[20]. Therefore, DCP and SRD would have the same clearance of the lymph nodes[9,20,23] for tumors of the III and IV duodenal portion, while DCP seems more radical for proximal localization.
Thus, as SRD has less complications, an easier postoperative management and almost the same survival[8,9,20], in the literature and also without any firm evidence, DCP seems to be indicated for proximal localizations while SRD is preferable for distal localizations, along with careful and methodical functional lymphectomy[6,8,9,13,20,23].
The role of adjuvant chemotherapy is still unclear[5,9,13,19]. Some studies show important statistical benefits for medium- and long-term survival[5,13]. In the light of this evidence, the treatment which our patient underwent seems to be appropriate.
In conclusion, having examined our case and the data from the literature, taking into account the rarity of the adenocarcinoma of the duodenum, a precocious diagnosis of this neoplasia and its exact localization are crucial points. In fact, the staging and localization play the most important role for long-term survival and affect the subsequent surgical strategy.
Thus, taking into account that both surgical treatments are radical, DCP, even though it is valid for all duodenal localizations, should be used only for the proximal ones and SRD should be the choice for the distal ones.
Peer reviewer: Khosro Ayazi, MD, Associate Professor and Chairman, Division of General and Laparoscopic Surgery, Imam Hossein University Hospital,Shahid Madnai St, Tehran 16179, Iran
S- Editor Wang JL L- Editor Roemmele A E- Editor Xiong L
| 1. | Chung WC, Paik CN, Jung SH, Lee KM, Kim SW, Chang UI, Yang JM. Prognostic factors associated with survival in patients with primary duodenal adenocarcinoma. Korean J Intern Med. 2011;26:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Bal A, Joshi K, Vaiphei K, Wig JD. Primary duodenal neoplasms: a retrospective clinico-pathological analysis. World J Gastroenterol. 2007;13:1108-1111. [PubMed] |
| 3. | Vuilleumier H, Cuttat JF, Blum AL, Chapuis G. [Adenocarcinoma of the duodenum. Contribution to the study of a rare pathology]. Helv Chir Acta. 1994;60:557-567. [PubMed] |
| 4. | Stock C, Keutgen XM, Pisapia D, Crawford C, Zarnegar R. Heterotopic pancreatic neoplasm presenting as an obstructing mass at the fourth portion of the duodenum. JOP. 2011;12:241-243. [PubMed] |
| 5. | Onkendi EO, Boostrom SY, Sarr MG, Farnell MB, Nagorney DM, Donohue JH, Kendrick ML, Lombardo KM, Haddock MG, Que FG. Neoadjuvant Treatment of Duodenal Adenocarcinoma: A Rescue Strategy. J Gastrointest Surg. 2011;[Epub ahead of print]. [PubMed] |
| 6. | Coit DG. Cancer of the small intestine. Principles and practice of oncology. Philadelphia (PA): Lippincott Williams & Wilkins 2001; 1204-1206. |
| 7. | Han SL, Cheng J, Zhou HZ, Zeng QQ, Lan SH. The surgical treatment and outcome for primary duodenal adenocarcinoma. J Gastrointest Cancer. 2010;41:243-247. [PubMed] |
| 8. | Tocchi A, Mazzoni G, Puma F, Miccini M, Cassini D, Bettelli E, Tagliacozzo S. Adenocarcinoma of the third and fourth portions of the duodenum: results of surgical treatment. Arch Surg. 2003;138:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Pozzetto B, Guarino G, Tonello C, Liguori G. [Treatment of adenocarcinoma of the duodenum: presentation of 4 clinical cases and review of the literature]. Chir Ital. 2002;54:195-201. [PubMed] |
| 10. | Han SL, Cheng J, Zhou HZ, Guo SC, Jia ZR, Wang PF. Surgically treated primary malignant tumor of small bowel: a clinical analysis. World J Gastroenterol. 2010;16:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 11. | Ito H, Perez A, Brooks DC, Osteen RT, Zinner MJ, Moore FD, Ashley SW, Whang EE. Surgical treatment of small bowel cancer: a 20-year single institution experience. J Gastrointest Surg. 2003;7:925-930. [PubMed] |
| 12. | Cheng XD, Du YA, Xu ZY, Huang L, Yang LT, Wang B, Zhou YM, Yu PF, Yu QM. A Modified Pancreaticojejunostomy: Kissing Pancreaticojejunostomy. Hepatogastroenterology. 2011;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Czaykowski P, Hui D. Chemotherapy in small bowel adenocarcinoma: 10-year experience of the British Columbia Cancer Agency. Clin Oncol (R Coll Radiol). 2007;19:143-149. [PubMed] |
| 14. | Kaklamanos IG, Bathe OF, Franceschi D, Camarda C, Levi J, Livingstone AS. Extent of resection in the management of duodenal adenocarcinoma. Am J Surg. 2000;179:37-41. [PubMed] |
| 15. | Chung RS, Church JM, vanStolk R. Pancreas-sparing duodenectomy: indications, surgical technique, and results. Surgery. 1995;117:254-259. [PubMed] |
| 16. | de Castro SM, van Eijck CH, Rutten JP, Dejong CH, van Goor H, Busch OR, Gouma DJ. Pancreas-preserving total duodenectomy versus standard pancreatoduodenectomy for patients with familial adenomatous polyposis and polyps in the duodenum. Br J Surg. 2008;95:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 17. | Bucher P, Gervaz P, Morel P. Long-term results of radical resection for locally advanced duodenal adenocarcinoma. Hepatogastroenterology. 2005;52:1727-1729. [PubMed] |
| 18. | Lang H, Nadalin S, Raab R, Jähne J. [Results of surgical therapy of primary adenocarcinoma of the duodenum]. Chirurg. 1999;70:571-577. [PubMed] |
| 19. | Bakaeen FG, Murr MM, Sarr MG, Thompson GB, Farnell MB, Nagorney DM, Farley DR, van Heerden JA, Wiersema LM, Schleck CD. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. 2000;135:635-641; discussion 641-642. [PubMed] |
| 20. | Barnes G, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Delcore R, Thomas JH, Forster J, Hermreck AS. Improving resectability and survival in patients with primary duodenal carcinoma. Am J Surg. 1993;166:626-630; discussion 630-631. [PubMed] |
| 22. | Carloni A, Perri S, Gola P, Lotti R, Caterino G, Altilia F, Schietroma M, Citone G. [Adenocarcinoma of the duodenojejunal flexure. A report of 2 clinical cases and a review of the literature]. Ann Ital Chir. 2000;71:133-138. [PubMed] |
| 23. | Albagli RO, Carvalho GS, Mali Junior J, Eulálio JM, de Melo EL. Comparative study of the radical and standard lymphadenectomy in the surgical treatment of adenocarcinoma of the ampula of Vater. Rev Col Bras Cir. 2010;37:420-425. [PubMed] |