Published online Jul 27, 2011. doi: 10.4240/wjgs.v3.i7.110
Revised: June 25, 2011
Accepted: July 4, 2011
Published online: July 27, 2011
Split liver transplantation for two adults offers a valuable opportunity to expand the donor pool for adult recipients. However, its application is mainly hampered by the physiological limits of these partial grafts. Small for size syndrome is a major concern during transplantation with partial graft and different techniques have been developed in living donor liver transplantation to prevent the graft dysfunction. Herein, we report the first application of synergic approaches to optimise the hepatic hemodynamic in a split liver graft for two adults. A Caucasian woman underwent liver transplantation for alcoholic cirrhosis (MELD 21) with a full right liver graft (S5-S8) without middle hepatic vein. Minor and accessory inferior hepatic veins were preserved by splitting the vena cava; V5 and V8 were anastomosed with a donor venous iliac patch. After implantation, a 16G catheter was advanced in the main portal trunk. Inflow modulation was achieved by splenic artery ligation. Intraportal infusion of PGE1 was started intraoperatively and discontinued after 5 d. Graft function was immediate with normalization of liver test after 7 d. Nineteen months after transplantation, liver function is normal and graft volume is 110% of the recipient standard liver volume. Optimisation of the venous outflow, inflow modulation and intraportal infusion of PGE1 may represent a valuable synergic strategy to prevent the graft dysfunction and it may increase the safety of split liver graft for two adults.
- Citation: Domenico SD, Andorno E, Varotti G, Valente U. Hepatic flow optimization in full right split liver transplantation. World J Gastrointest Surg 2011; 3(7): 110-102
- URL: https://www.wjgnet.com/1948-9366/full/v3/i7/110.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v3.i7.110
We read with great interest the editorial article the paper by Gonzalez et al[1], published in the December 2010 issue of World Journal of Gastrointestinal Surgery, reviewing the current strategies to prevent the small-for-size-syndrome (SFSS) following partial liver graft transplantation.
Given the lack of data on this topic in adult-to-adult split liver transplantation (A/A-SLT), we consider it interesting to report the first case of synergic use of different techniques of flow optimization applied to an A/A-SLT.
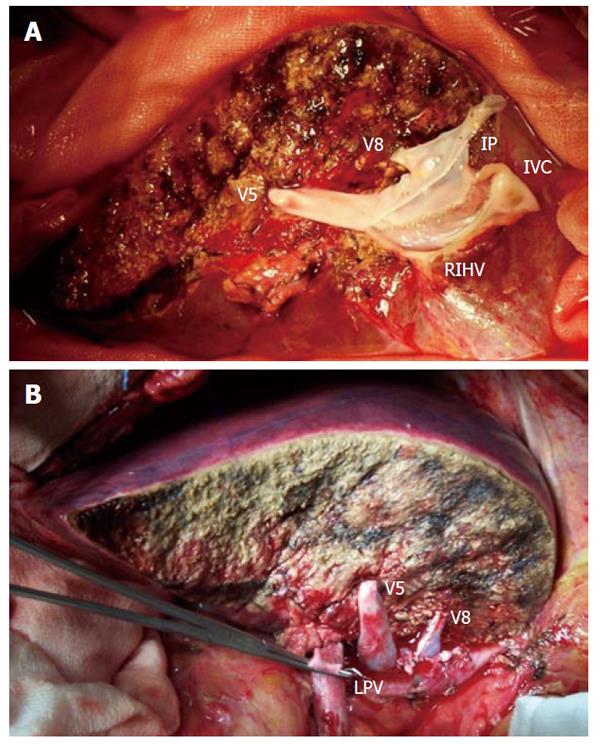
A 44-year-old woman (162 cm × 66 kg) with hepatocellular carcinoma on alcoholic cirrhosis (Meld 21) underwent liver transplantation receiving an in-situ full-right graft (FRG, including segments 5-8) procured from a cadaveric donor. During splitting procedure, parenchymal transection was performed leaving the middle hepatic vein to the left side. One major segment 5 and one segment 8 tributary veins (V5, V8) were isolated and temporarily ligated. Hilar structures were divided, assigning right bile duct, right hepatic artery and right portal vein to the right hemiliver and leaving the main elements to the left hemiliver. The retrohepatic venacava (IVC) was longitudinally split leaving a half hemicava patch to each hemiliver. At back table surgery, a wide venous patch was created among V5, V8 and the hemi-right IVC, using an interposing venous iliac patch (Figure 1A).
The final FRG weight was 680 g, leading to a graft-to-recipient weight ratio (GRWR) of 1.03. Recipient hepatectomy was performed preserving the retrohepatic IVC. Implantation of the graft was carried out with a side-to-side anastomosis between the venous patch of the graft and the recipient’s IVC. Portal vein anastomosis was performed between the right portal branch of the recipient and the right portal branch of the graft, preserving the left portal branch for a possible hemiporto-caval shunt (Figure 1B).
Cold and warm ischemia time resulted in 8 h 15 min and 1 h 11 min respectively. Inflow measurement was calculated both indirectly by Doppler portal flow (PVF) calculation and directly by portal pressure (PVP) measurement using a 16 G catheter previously inserted through the abdominal wall into the left colic vein and progressed until the main portal trunk and then secured with elastic ligatures. Although the graft did not appear macroscopically congested, PVP and PVF were 29.5 mmHg and 3360 mL/min (494 mL/min per 100 g) respectively, demonstrating a picture of portal overflow. Thus, we firstly ligated the splenic artery, with consequent reduction of both PVP and PVF to 18 mmHg and 2430 mm/min (357 mL/min per 100 g) respectively. Because we considered the achieved values within safe limits, we sutured the left portal vein, avoiding the creation of a hemiporto-caval shunt.
Intra-portal continuous infusion of PGE1 (Alprostadil®) was started intraoperatively with a rate of 500 mcg/d. Postoperatively graft perfusion was monitored with continuous measurement of PVP through the catheter and daily Doppler examination. On POD5, PGE1 infusion was discontinued and the intraportal catheter was removed uneventfully. On POD 23, patient was discharged with normal liver function and she remains in a healthy condition after 24 mo of follow-up.
At 1-year CT scan showed a homogenous graft; calculated graft liver volume was 1525 mL, resulting in 110% of the standard liver volume and 225% of the original volume. Because of reduced liver mass, partial grafts, including A/A-SLT and living donor liver transplantation (LDLT), result in an increased risk of SFSS[2,3]. Although the pathogenesis of SFSS is still unclear, different studies have suggested that intrahepatic hemodynamics may play a central role[4]. In LDLT especially, different strategies of flow modulation have been proposed to enhance venous outflow and to optimize portal inflow[5-7]. Besides graft size, different characteristics of donor and recipient as well as intraoperative factors can contribute to SFSS; especially in SLT, SFSS can occur in grafts with GRWR > 0.8 because of elevated ischemia time and donor death-related hemodynamic instability[3]. Since the role of intraparenchymal graft perfusion was highlighted, different surgical solutions have been proposed to optimize the venous outflow and to modulate the portal inflow. To provide an optimal venous drainage of the right lateral sector, we adopted the split-cava technique[8]. To optimize the venous drainage of the paramedian sector, we created a wide venous patch, applying the so-called “blanket technique” firstly proposed in LDLT[9]. Different authors have reported that LDLT grafts with PVP > 20 mmHg or PVF > 250 mL/min per 100 g have an higher risk of graft failure[2,3]. Based on PVF and PVP measurement, different strategies of inflow modulation have been reported in LDLT, including splenic artery ligation, hemiporto-caval shunt construction, porto-systemic spontaneous shunt ligation and intraportal drug infusion[5-7,10].
We applied some of these techniques in our FRG, achieving a significant portal inflow reduction. We think that these synergic techniques of hepatic flow optimization should be systematically adopted in A/A-SLT and could lead to a dramatic improvement of the results of this challenging technique, as already demonstrated in LDLT.
Peer reviewer: Mehmet Fatih Can, Assistant Professor, Gulhane School of Medicine, Department of Surgery, Etlik 06018, Ankara, Turkey
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Gonzalez HD, Liu ZW, Cashman S, Fusai GK. Small for size syndrome following living donor and split liver transplantation. World J Gastrointest Surg. 2010;2:389-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Colledan M, Andorno E, Valente U, Gridelli B. A new splitting technique for liver grafts. Lancet. 1999;353:1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Tucker ON, Heaton N. The 'small for size' liver syndrome. Curr Opin Crit Care. 2005;11:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, Van Vlierberghe H, de Hemptinne B. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005;5:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Yagi S, Iida T, Taniguchi K, Hori T, Hamada T, Fujii K, Mizuno S, Uemoto S. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005;11:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, Hashimoto K, Mochida Y, Hashimoto S, Maehara Y. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl Int. 2005;18:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Gundlach M, Broering D, Topp S, Sterneck M, Rogiers X. Split-cava technique: liver splitting for two adult recipients. Liver Transpl. 2000;6:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Nadalin S, Bockhorn M, Malagó M, Valentin-Gamazo C, Frilling A, Broelsch CE. Living donor liver transplantation. HPB (Oxford). 2006;8:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yamada T, Tanaka K, Uryuhara K, Ito K, Takada Y, Uemoto S. Selective hemi-portocaval shunt based on portal vein pressure for small-for-size graft in adult living donor liver transplantation. Am J Transplant. 2008;8:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |