Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.106813
Revised: March 31, 2025
Accepted: May 6, 2025
Published online: June 27, 2025
Processing time: 84 Days and 3.5 Hours
An efficient index holds the potential to predict rectal cancer prognosis.
To investigate the impact of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) on rectal cancer prognosis.
This retrospective study involved 180 patients with rectal cancer from the Changzhi People’s Hospital of Shanxi Province. A 2-mL blood sample was collected at 24 h preoperatively and 72 h postoperatively to measure neutrophils, lymphocytes, platelets, and monocytes using an automatic blood analyzer. Preoperative and postoperative NLR, PLR, and MLR were compared. Patients were followed up for 12 months and categorized into good and poor prognosis groups. A receiver operating characteristic curve was constructed to analyze their predictive values.
The NLR, PLR, and MLR values were significantly lower post-surgery (P < 0.05). A total of 152 and 28 patients were categorized in the good and poor prognosis groups, respectively. Patients with poor prognoses exhibited slightly higher postoperative NLR, PLR, and MLR values than those with good prognoses (P < 0.05). Receiver operating characteristic analysis showed that the area under the curve for NLR, PLR, and MLR was 0.828 with a sensitivity and specificity of 89.29% and 90.79%, respectively. These values were higher than individual NLR (area under the curve: 0.660, sensitivity: 67.86%, specificity: 54.61%), PLR (0.668, 75.00%, 55.30%), and MLR (0.635, 60.71%, 48.03%), all showing statistically significant differences (P < 0.05), effectively predicting patient outcomes.
The findings of this study indicated that NLR, PLR, and MLR values of patients with rectal cancer can be used to effectively predict the outcome of patients.
Core Tip: This retrospective study of 180 patients with rectal cancer revealed that combined assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio provided superior prognostic value compared with individual ratios. Post-surgery neutrophil-to-lymphocyte/platelet-to-lymphocyte/monocyte-to-lymphocyte ratio levels showed significant discriminative power (area under the curve = 0.828) with 89.29% sensitivity and 90.79% specificity for predicting poor outcomes. The dynamic reduction of inflammatory ratios in responders highlighted their clinical utility for postoperative monitoring and risk stratification.
- Citation: Shao LL, Li X, Wang LF. Role of neutrophil-to-lymphocyte, platelet-to-lymphocyte, and monocyte-to-lymphocyte ratios in rectal cancer prognosis. World J Gastrointest Surg 2025; 17(6): 106813
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/106813.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.106813
Colorectal cancer, a prevalent malignant tumor of the digestive system, is associated with global cancer incidence and mortality. Rectal cancer, which constitutes approximately 30% of newly diagnosed cases, poses a substantial threat to human health. Although surgical resection remains the primary approach for extending long-term survival, posto
Previous studies showed a strong connection between inflammation and the development, progression, and prognosis of cancer. Inflammatory responses in patients with tumors are primarily reflected in alterations in peripheral blood cell counts, with neutrophils, lymphocytes, monocytes, and platelets playing crucial roles in tumor biology[2]. Among these, the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) have emerged as significant indicators of the immune-inflammatory response, showing considerable potential for predicting tumor prognosis. However, existing research has certain limitations. Some studies were conducted with small sample sizes, potentially compromising the stability and reliability of the results. Additionally, inconsistencies in testing methodologies and time points for inflammatory markers across studies have led to varying results. Although numerous studies have focused on individual inflammatory markers, few have comprehensively analyzed the combined predictive value of multiple indicators[3].
This study aimed to evaluate the prognostic utility of NLR, PLR, and MLR in patients following rectal cancer surgery. A retrospective study was conducted on 180 patients with rectal cancer to compare the preoperative and postoperative values of inflammatory markers and to analyze their predictive significance for patient outcomes. The study contributed to the existing literature by confirming the association between inflammatory markers and cancer prognosis while introducing several innovations. First, in terms of methodology, a relatively large sample size with rigorous inclusion and exclusion criteria was applied to enhance the reliability of the findings[4]. Second, the study focused on patients with rectal cancer who had undergone surgery, representing a specific and clinically relevant population. Third, the analysis included the simultaneous assessment of the NLR, PLR, and MLR, providing a more comprehensive assessment of the immune-inflammatory status[5,6]. The conclusions derived offer practical guidance for clinical practice, highlighting the potential use of these markers in personalized treatment planning and monitoring disease progression in patients with rectal cancer.
A retrospective study was conducted on 180 patients with rectal cancer (102 males and 78 females) selected between April 2021 and June 2023. The average age of patients was 58.73 ± 5.16 years, ranging from 35 years to 75 years. Clinical characteristics included 84 patients in stage I of the tumor, node, metastasis classification, 28 patients with lymph node metastases, 31 patients with perineural invasion, and 12 patients with vascular cancer. Based on tissue classification tumors were poorly differentiated in 54 patients, moderately differentiated in 67 patients, and highly differentiated in 59 patients.
The inclusion criteria were as follows: (1) Included patients who met the criteria for rectal cancer[7] and had pathological diagnostic confirmation; (2) Indications for radical rectal cancer surgery were present, and (3) Complete clinical and follow-up data were available. The exclusion criteria were as follows: (1) Patients with tumors present on other sites; (2) Patients with immune system defects or blood or coagulation disorders; (3) Patients who received anti-tumor therapy before enrollment; (4) Patients with acute or chronic infections before surgery; (5) Death due to unrelated causes after surgery; (6) Severe anemia prior to surgery; (7) Tumor recurrence; and (8) Incomplete clinical data.
Peripheral venous blood (2 mL) was collected 1 day prior to surgery and 72 h after surgery. Samples were collected in ethylenediamine tetraacetate dipotassium anticoagulation tubes and processed using the XN-9000 blood cell analyzer to measure neutrophil, lymphocyte, platelet, and monocyte counts. All procedures were performed in strict accordance with the manufacturer’s instructions. After the blood cell counts were obtained, NLR, PLR, and MLR values were calculated. All patients underwent follow-up for 12 months post-surgery, with postoperative prognoses assessed through telephone interviews and outpatient reviews.
Patients were divided into two groups based on their outcomes: The good prognosis group (no tumor recurrence, metastasis, or death within 12 months after surgery) and the poor prognosis group (recurrence and metastasis were experienced by the patients within 12 months after surgery). A 12-month postoperative follow-up period was chosen for two primary reasons. First, the peak recurrence and metastasis of rectal cancer typically occur within 1-2 years after surgery, making this 12-month follow-up effective for observing disease progression during this critical timeframe. Second, considering the postoperative recovery of the patient, treatment duration, feasibility, and cost-effectiveness, a 12-month follow-up cycle is reasonable, as it allows for the collection of valid data while minimizing the burden on patients and medical resources.
The observational indicators were as follows: (1) Comparison of preoperative and postoperative NLR, PLR, and MLR values; (2) Comparison of postoperative NLR, PLR, and MLR values between the good and poor prognosis groups; and (3) Analysis of the predictive value of NLR, PLR, and MLR on patient outcomes.
Statistical analysis of the data was performed using the SPSS 26.0 statistical software. Measurement data followed a normal distribution. Comparisons of data before and after surgery and between the two groups were conducted using paired and sample t-tests. Count data were expressed as rates (%) and analyzed using the χ2 test. Receiver operating characteristic (ROC) curves were constructed to calculate the maximum area under the curve (AUC) when P < 0.05.
The postoperative NLR, PLR, and MLR values were significantly lower (P < 0.05) (Table 1).
| Group | Case | NLR | PLR | MLR |
| Preoperative | 180 | 4.58 ± 1.01 | 182.84 ± 52.19 | 0.34 ± 0.18 |
| Postoperative | 180 | 2.86 ± 0.82 | 108.49 ± 41.03 | 0.21 ± 0.14 |
| t | 17.738 | 15.026 | 7.649 | |
| P value | < 0.001 | < 0.001 | < 0.001 |
A total of 180 patients were followed up for 12 months after surgery, with 152 patients and 28 patients in the good and poor prognosis groups, respectively. Postoperative NLR, PLR, and MLR values were higher for patients with poor prognoses than for patients with good prognoses (P < 0.05) (Table 2).
| Group | Case | NLR | PLR | MLR |
| Good prognosis | 152 | 2.48 ± 0.48 | 92.48 ± 38.18 | 0.17 ± 0.10 |
| Poor prognosis | 28 | 3.84 ± 0.51 | 121.07 ± 41.96 | 0.25 ± 0.16 |
| t | 13.644 | 3.585 | 3.498 | |
| P value | < 0.001 | < 0.001 | 0.001 |
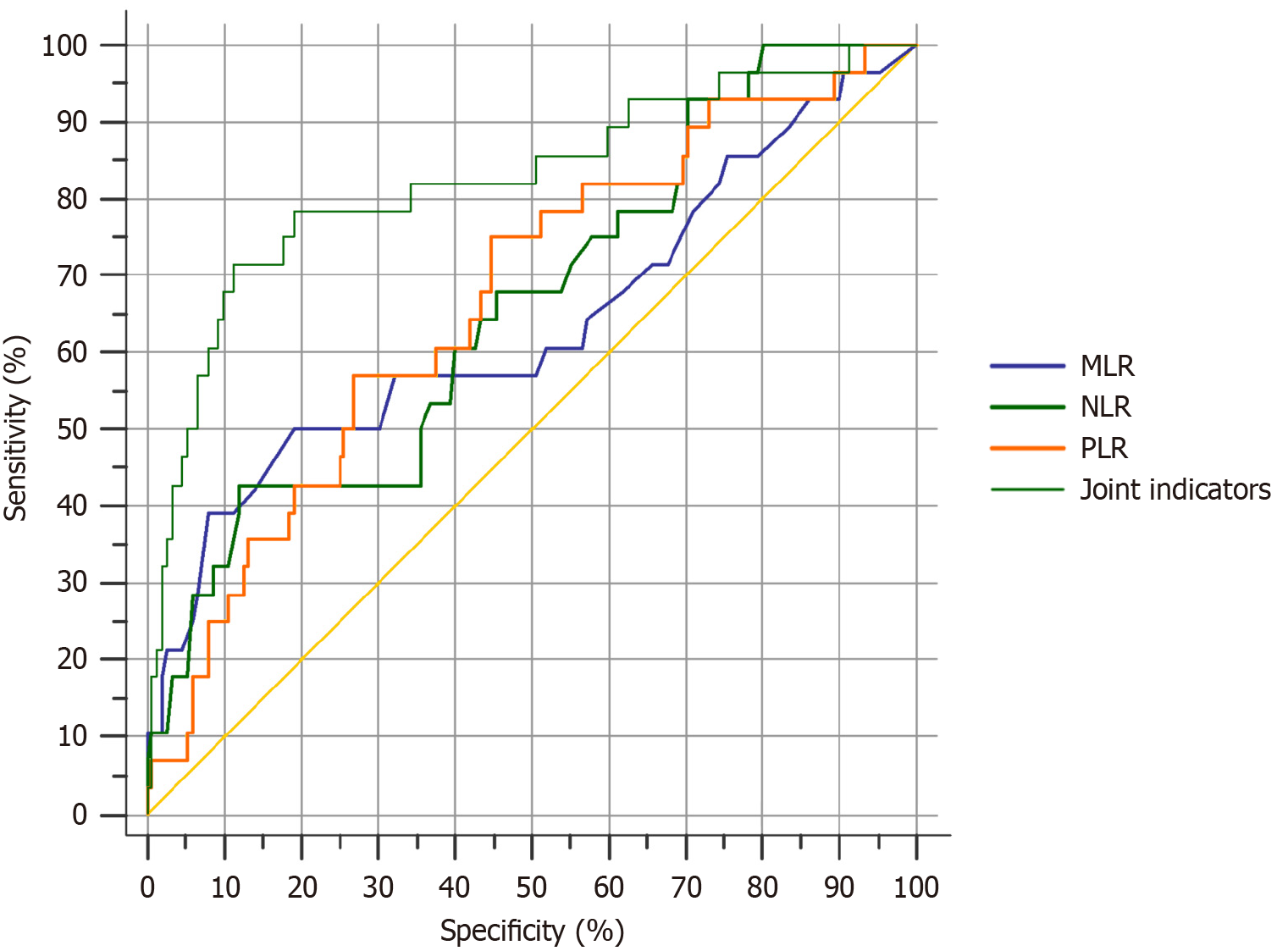
According to the ROC analysis, the AUC, sensitivity, and specificity of NLR, PLR, and MLR were higher than that of the joint NLR, PLR, and MLR (P < 0.05) (Table 3 and Figure 1).
| Index | AUC | Sensitivity, % | Specificity, % | Youden index | SE | 95%CI |
| NLR | 0.660 | 67.86 | 54.61 | 0.310 | 0.0563 | 0.586-0.729 |
| PLR | 0.668 | 75.00 | 55.30 | 0.303 | 0.0553 | 0.595-0.737 |
| MLR | 0.635 | 60.71 | 48.03 | 0.314 | 0.0657 | 0.560-0.706 |
| Joint indicators | 0.825a | 89.29 | 90.79 | 0.602 | 0.0496 | 0.761-0.877 |
The current standard treatment for rectal cancer involves radical surgical resection combined with radiotherapy and chemotherapy, which can significantly extend postoperative survival and improve long-term survival rates. However, postoperative outcomes are influenced by multiple factors, and some patients may experience tumor recurrence or metastasis after surgery. Early screening and evaluation of disease progression are crucial for selecting an appropriate postoperative anti-cancer treatment plan and improving patient prognosis. Previous studies have identified inflammation, genetic instability, and mutation as key pathological factors driving tumor initiation and progression[8]. During prolonged inflammatory reactions the production of various inflammatory cells and chemokines can facilitate the initiation, progression, invasion, and metastasis of tumor cells. In turn tumor cells can destroy tissue and cellular DNA, inducing specific inflammatory responses and further elevating inflammation levels[9]. Therefore, the inflammation index of the body plays a crucial role in evaluating characteristics, efficacy, evaluation, and prognosis of the tumor. Among these indicators NLR, PLR, and MLR are key indicators that reflect the immune-inflammation response of the body.
This study showed that postoperative NLR, PLR, and MLR values were significantly lower (P < 0.05), indicating a substantial decrease in the immune-inflammatory response in patients with rectal cancer following surgery. Generally, inflammatory indicators can promote the occurrence and progression of malignant tumors. Activated neutrophils can facilitate the release of cytokines, chemokines, and granule proteins, thereby creating a favorable microenvironment for tumor initiation and progression. At the same time they can suppress peripheral leukocyte activation and promote immune suppression and tumor progression[10].
Lymphocytes, as key components of the human immune system, play a crucial role in inhibiting tumor occurrence and progression[11]. Platelets regulate angiogenesis, enabling tumor cells to evade immune surveillance[12] and facilitating the differentiation of tumor-associated macrophages, which release growth and angiogenic factors that support tumor angiogenesis and invasion[13]. Therefore, in patients with rectal cancer NLR, PLR, and MLR values are significantly elevated. The removal of tumor lesions following radical resection disrupts the influence of tumors on the inflammatory response of the body, leading to a reduction in NLR, PLR, and MLR values.
The study showed that postoperative NLR, PLR, and MLR values were higher in the poor prognosis group than in the good prognosis group (P < 0.05). These results suggested that NLR, PLR, and MLR values are closely related to patient prognosis. Portale et al[14] reported that high NLR and PLR levels were closely related to the less favorable overall survival rates and shorter disease-free survival in patients with rectal cancer. Colloca et al[15] noted that a baseline NLR > 3 was associated with both reduced overall survival and disease-free survival. Lisanti et al[16] investigated the relationship between gender and MLR in patients with rectal cancer and noted that the MLR cutoff in females (0.27) than in males (0.49). Furthermore, MLR values exceeding 0.27 in females and 0.49 in males were associated with poorer survival outcomes. Therefore, postoperative NLR, PLR, and MLR values can be used to predict patient prognosis to some extent.
In this study, the AUC of NLR, PLR, and MLR was 0.828 with a sensitivity and specificity of 89.29% and 90.79%, respectively, which were higher than those of individual NLR, PLR, and MLR values (P < 0.05). The ROC analysis revealed that the combined test of NLR, PLR, and MLR achieved higher AUC, sensitivity, and specificity than those of the single indices (P < 0.05), indicating that the combined test provides a more robust predictive value for the patient prognosis. In clinical practice personalized treatment options can be developed based on these indicators. For example, patients with elevated postoperative NLR, PLR, and MLR, indicating a higher risk of tumor recurrence and metastasis, may benefit from intensive adjuvant therapy, such as extending the chemotherapy cycle or adopting more efficient chemotherapeutic drugs combined with radiotherapy to enhance local control rates. Additionally, these indicators can be used to dynamically monitor changes in the condition of the patient. During follow-up the NLR, PLR, and MLR can be tested regularly. Persistent increases in these values should raise suspicion of tumor recurrence or metastasis. In such cases further diagnostic measures, such as imaging examinations, should be promptly undertaken to enable early detection of lesions and timely adjustment to the treatment strategy.
Recent studies have examined the role of inflammatory markers in predicting rectal cancer prognosis. Consistent with the current findings, earlier studies suggested that indicators such as NLR, PLR, and MLR are associated with patient outcomes and potentially serve as prognostic tools. However, differences in sample characteristics and testing methods are evident. Some studies employed smaller sample sizes, potentially compromising the stability of their results. Additionally, variations in testing time points and methodologies could have led to numerical discrepancies. The lower predictive efficacy of MLR compared with NLR and PLR observed in this study may be attributed to its higher susceptibility to confounding factors, such as baseline immune status and comorbidities of the patient. Furthermore, the underlying mechanism of action of MLR in rectal cancer development appears to be indirect, making its significance less pronounced compared to NLR and PLR.
These results support the above conclusions. Inflammatory and immune cells play critical roles within the tumor microenvironment. They can induce inflammation during the initial stages of tumor formation, and by suppressing the anti-tumor immune response, they promote tumor cell migration, proliferation, and metastasis of tumor cells. With the continuous progression of tumors, the inflammatory response intensifies, leading to the suppression of the anti-tumor immunity of the body and promoting tumor metastasis[12]. Higher values of NLR, PLR, and MLR indicate an increased inflammatory response and diminished anti-tumor effects, predicting tumor recurrence and metastasis. Among inflammatory cells neutrophils play a pivotal role in regulating the inflammatory response of the body. Upon inflammation activation they release various active substances to counteract inflammation. Tumor cell formation and necrosis can induce inflammation, and inflammatory chemokines stimulate neutrophil migration to the tumor microenvironment. This promotes the release of large amounts of inflammatory factors, enabling tumor cells to evade immune destruction and induce metastasis[17]. Lymphocytes are essential components of the immune system and release active substances such as perforin and granzyme, which induce apoptosis of cytotoxic T cells, thereby preventing tumor cell proliferation and invasion. However, a reduced lymphocyte count allows tumor cells to evade immune surveillance, fostering tumor growth, metastasis, and invasion, thereby affecting patient prognosis and outcomes[18].
Higher postoperative NLR values are associated with an increased risk of tumor cell proliferation, metastasis, and invasion and poorer prognosis. Platelet activation can facilitate tumor cell metastasis, enhance angiogenesis, protect tumor cells from immune destruction, promote tumor cell proliferation, and ultimately lead to metastasis. Consequently, elevated postoperative PLR values indicate a higher risk of tumor recurrence and metastasis. Similarly, an increase in monocyte count can inhibit anti-tumor T cell activity and induce programmed death-ligand 1 expression, driving tumor cell proliferation and metastasis[19]. Persistently high MLR levels post-surgery may predispose patients to tumor recurrence and metastasis. Relying on a single index for prognosis evaluation often introduces bias, making it essential to consider multiple indicators. When NLR, PLR, and MLR are highly expressed in vivo, they collectively contribute to tumor cell invasion, metastasis, and immune evasion, followed by tumor recurrence and progression.
The NLR, PLR, and MLR values of patients with rectal cancer were significantly lower than preoperative values, and the postoperative NLR, PLR, and MLR values of patients with poor prognosis were slightly higher than those with good prognosis. When NLR, PLR, and MLR values increase after surgery, it indicates the invasion, metastasis, and angio
| 1. | Wilkinson N. Management of Rectal Cancer. Surg Clin North Am. 2020;100:615-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol. 2020;17:414-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 3. | Dietz S, Fritzmann J, Weidlich A, Schaser KD, Weitz J, Kirchberg J. [Treatment strategies for recurrent rectal cancer]. Chirurgie (Heidelb). 2024;95:495-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Sawada R, Akiyoshi T, Kitagawa Y, Hiyoshi Y, Mukai T, Nagasaki T, Yamaguchi T, Konishi T, Yamamoto N, Ueno M, Fukunaga Y. Systemic Inflammatory Markers Combined with Tumor-Infiltrating Lymphocyte Density for the Improved Prediction of Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Ann Surg Oncol. 2021;28:6189-6198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Duque-Santana V, López-Campos F, Martin-Martin M, Valero M, Zafra-Martín J, Couñago F, Sancho S. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Factors in Locally Advanced Rectal Cancer. Oncology. 2023;101:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Cho MC, Yoo S, Choo MS, Son H, Jeong H. Lymphocyte-to-monocyte ratio is a predictor of clinically significant prostate cancer at prostate biopsy. Prostate. 2021;81:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 406] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 8. | Manoochehry S, Rasouli HR, Ahmadpour F, Keramati A. Evaluation of the role of inflammatory blood markers in predicting the pathological response after neoadjuvant chemoradiation in patients with locally advanced rectal cancer. Radiat Oncol J. 2023;41:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Ergen ŞA, Barlas C, Yıldırım C, Öksüz DÇ. Prognostic Role of Peripheral Neutrophil-Lymphocyte Ratio (NLR) and Platelet-Lymphocyte Ratio (PLR) in Patients with Rectal Cancer Undergoing Neoadjuvant Chemoradiotherapy. J Gastrointest Cancer. 2022;53:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Mo CJ, Hu ZJ, Qin SZ, Chen HP, Huang L, Li S, Cao Z. Diagnostic value of platelet-lymphocyte ratio and hemoglobin-platelet ratio in patients with rectal cancer. J Clin Lab Anal. 2020;34:e23153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Gawiński C, Hołdakowska A, Wyrwicz L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in Locally Advanced Rectal Cancer. Curr Oncol. 2022;30:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Bulut G, Ozdemir ZN. Prognostic Significance of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Metastatic Colorectal Cancer. J Gastrointest Cancer. 2022;53:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Fülöp ZZ, Gurzu S, Fülöp RL, Bara T Jr, Tímár J, Drágus E, Jung I. Prognostic Impact of the Neutrophil-to-Lymphocyte and Lymphocyte-to-Monocyte Ratio, in Patients with Rectal Cancer: A Retrospective Study of 1052 Patients. J Pers Med. 2020;10:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Portale G, Bartolotta P, Azzolina D, Gregori D, Fiscon V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: systematic review and meta-analysis. Langenbecks Arch Surg. 2023;408:85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Colloca G, Venturino A, Guarneri D. Neutrophil-to-lymphocyte ratio predicts survival of patients with rectal cancer receiving neo-adjuvant chemoradiation followed by radical resection: a meta-analysis. Expert Rev Anticancer Ther. 2023;23:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Lisanti C, Basile D, Garattini SK, Parnofiello A, Corvaja C, Cortiula F, Bertoli E, Ongaro E, Foltran L, Casagrande M, Di Nardo P, Cardellino GG, Fasola G, Buonadonna A, Pella N, Aprile G, Puglisi F. The SAFFO Study: Sex-Related Prognostic Role and Cut-Off Definition of Monocyte-to-Lymphocyte Ratio (MLR) in Metastatic Colorectal Cancer. Cancers (Basel). 2022;15:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Gawiński C, Mróz A, Roszkowska-Purska K, Sosnowska I, Derezińska-Wołek E, Michalski W, Wyrwicz L. A Prospective Study on the Roles of the Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) in Patients with Locally Advanced Rectal Cancer. Biomedicines. 2023;11:3048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 18. | Sanger CB. Research Perspective on Prognostic Significance of Lymphocyte-to-Monocyte and Platelet-to-Lymphocyte Ratio in Rectal Cancer: A Systematic Review, Meta-analysis, and Meta-regression. Dis Colon Rectum. 2022;65:188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Hamid HKS, Emile SH, Davis GN. Prognostic Significance of Lymphocyte-to-Monocyte and Platelet-to-Lymphocyte Ratio in Rectal Cancer: A Systematic Review, Meta-analysis, and Meta-regression. Dis Colon Rectum. 2022;65:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |