Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.103867
Revised: March 6, 2025
Accepted: April 28, 2025
Published online: June 27, 2025
Processing time: 140 Days and 2.8 Hours
Primary sclerosing cholangitis (PSC) is a long-term liver condition defined by the inflammation and scarring of the bile ducts, resulting in complications such as liver cirrhosis, portal hypertension, and cholangiocarcinoma. Although PSC predominantly affects adults, the incidence in pediatric patients is rising. For individuals in the advanced stages of liver disease, liver transplantation (LT) is the sole curative treatment option. However, the recurrence of PSC in the transplanted liver, known as recurrent PSC (rPSC), remains a significant concern.
To identify the potential risk factors for the recurrence of PSC in pediatric patients after undergoing LT.
A literature search was carried out across databases, including PubMed, Embase, Cochrane Library, and Scopus, covering studies published from 1990 through 2024. The Newcastle-Ottawa scale was utilized to assess the quality of the selected studies. Statistical analyses were conducted using RevMan 5.3 software, where the risk of recurrence was quantified using hazard ratios (HR) with 95%CI.
A total of nine reports with 2524 pediatric patients with PSC were included in this analysis. The findings revealed several important risk factors connected to the rPSC in pediatric patients who had received a liver transplant, including concurrent inflammatory bowel disease (IBD), elevated liver enzyme levels, and the presence of PSC-autoimmune hepatitis (AIH) overlap syndrome (all P < 0.05). No statistically significant association was found between acute allograft rejection, Epstein-Barr virus infection, and the risk of rPSC recurrence in the pediatric liver transplant recipients.
The present systematic review and meta-analysis have identified various risk factors associated with the recurrence of PSC in pediatric patients who underwent LT, including IBD, elevated liver enzyme levels, and PSC-AIH overlap syndrome.
Core Tip: This meta-analysis identifies risk factors for the recurrence of primary sclerosing cholangitis (PSC) in pediatric patients after liver transplantation. Key findings include the association of concurrent inflammatory bowel disease, elevated liver enzyme levels at the time of liver transplant procedure, and PSC-autoimmune hepatitis overlap syndrome with a higher risk of recurrent PSC. These insights can inform clinical decision-making and patient management strategies for pediatric patients with PSC after transplantation.
- Citation: Sun B, Guan D, Gao YG, Chen JY, Rong YH, Guo ZM. Risk factors for recurrence of primary sclerosing cholangitis in pediatric liver transplant recipients: A meta-analysis. World J Gastrointest Surg 2025; 17(6): 103867
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/103867.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.103867
Primary sclerosing cholangitis (PSC) is a persistent and gradually worsening liver disease that affects the bile ducts[1]. It is characterized by inflammation and fibrosis of these ducts, which can result in serious complications, including liver cirrhosis, portal hypertension, and cholangiocarcinoma[2]. Although PSC commonly affects adults, its incidence rate in the pediatric population is increasing[3]. Hepatic transplantation is currently the definitive treatment for children with PSC who have reached end-stage liver disease[4]. However, the possibility of PSC recurring in the transplanted liver, referred to as recurrent PSC (rPSC), poses a substantial concern. rPSC affects the quality of life for pediatric patients, leading to significant physical and psychosocial challenges. Children with rPSC often experience fatigue that can hinder their ability to participate in daily activities, such as attending school[5]. Additionally, recurrent cholangitis, a common complication of rPSC, can result in frequent hospitalizations. These physical burdens are accompanied by the psychological and social impacts of long-term immunosuppression and repeated medical interventions, which can disrupt social development and lead to feelings of isolation, particularly among adolescents[6].
The etiology of PSC is intricate, involving a combination of genetic, immunological, and environmental elements. Notably, PSC is frequently linked to inflammatory bowel disease (IBD), especially ulcerative colitis[7]. About 80% of individuals with PSC will develop IBD at some stage during their illness[8]. The occurrence of IBD in patients with PSC is correlated with a more severe disease progression and an elevated risk of rPSC[9,10]. Despite progress in our understanding of the pathophysiology and treatment of PSC, the incidence of rPSC after liver transplantation (LT) remains significant. A cohort study revealed that pediatric patients with rPSC face a significantly higher risk of graft failure in contrast to individuals without recurrence, with an earlier need for re-transplantation, presenting approximately 5 years sooner than in non-recurrent cases[11]. Identifying the risk factors for rPSC in pediatric patients who have undergone LT for PSC is essential to enhance patient outcomes and tailor therapeutic interventions. Thus, this meta-analysis was performed to identify the risk factors for the recurrence of PSC in pediatric liver transplant recipients by aggregating data from observational studies to guide clinical management decisions.
The research adhered to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[12]. Literature search was carried out using the databases PubMed, Embase, Cochrane Library, and Scopus from January 1990 to December 2024. The search approach incorporated a blend of keywords such as “Primary sclerosing cholangitis”, “PSC”, “liver transplantation”, “pediatric”, “children”, “recurrence”, and “risk factors”. The search was not limited by language or publication date. The reference lists of the chosen articles were meticulously examined.
The inclusion criteria were as follows: (1) Children diagnosed with PSC who were under the age of 18 years; (2) LT performed to treat PSC; (3) Study design included cohort studies, case-control studies, or cross-sectional studies that evaluated PSC recurrence in the pediatric population; and (4) The study presented data on potential risk factors associated with the recurrence of PSC. The exclusion criteria included case reports, studies with small sample sizes, and those with substantial methodological weaknesses.
Two investigators independently collected data following a standardized extraction template. The following details were collected from each study: Author names, publication year, sample size, demographic characteristics of the patients (age at PSC diagnosis, gender, etc.), presence of IBD, follow-up duration, recurrence rates, time to recurrence, and any reported risk factors associated with PSC recurrence. To ensure the integrity and accuracy of the extracted data, a systematic approach was adopted to address missing or incomplete information. For studies with missing critical data points, such as recurrence rates or follow-up durations, efforts were made to contact the original authors to obtain the necessary information. When the contact was unsuccessful or the data remained inaccessible, the respective studies were excluded from the analysis, and the reasons for their exclusion were meticulously documented. In cases where studies contained incomplete data, we thoroughly reviewed the full text of the articles and, when possible, reached out to the authors to gather missing details. If the information could not be supplemented, these studies were included in the analysis with the available data, and the limitations associated with incomplete data were clearly acknowledged in the results section. This methodological approach aimed to mitigate the influence of missing or incomplete data on the study outcomes and maintain the rigor of our findings.
The quality of the included studies was assessed by two reviewers using the Newcastle-Ottawa scale designed for cohort studies. This scale assesses the quality of non-randomized studies based on three primary criteria: Selection, comparability, and outcome assessment. Studies that achieved a score of 6 or above were considered to be of high quality.
The primary outcome was the recurrence of PSC after hepatic transplantation. The risk of recurrence was evaluated using hazard ratios (HR) with corresponding 95%CI. Considering the expected variability among studies, a random-effects model was employed to combine the data. Statistical analyses were conducted using RevMan 5.3 software. The I2 statistic was used to measure the heterogeneity among the studies. Statistical significance was defined as a P value < 0.05. Funnel plots were used to assess the possibility of publication bias. Subgroup analyses were carried out to explore potential sources of heterogeneity. Subgroups were formed based on duration of follow-up (< 5 years vs ≥ 5 years), recurrence rate (cohort vs case-control), and patient age at the time of transplantation (low vs high). The statistical significance of differences in pooled HRs across subgroups was evaluated using the Q statistic and the I2 statistic for heterogeneity.
Sensitivity analyses were performed to evaluate the stability of the primary results. This involved the sequential removal of one study at a time to determine whether the overall effect estimate remained stable. Additionally, a leave-one-out analysis was performed to estimate the influence of individual studies on the pooled HRs. The stability of the results was further evaluated by excluding studies with a high risk of bias or those that did not meet predefined quality criteria.
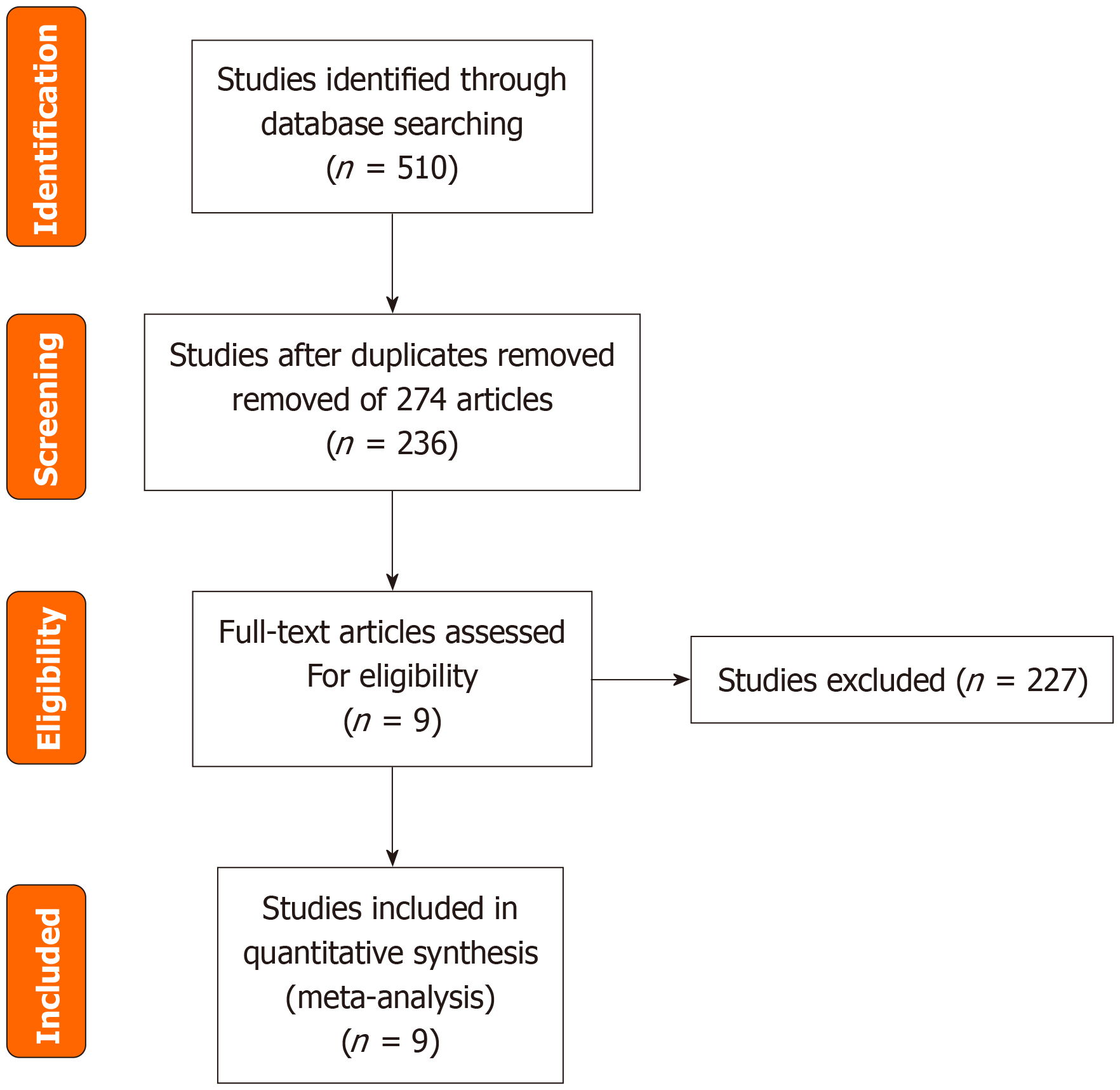
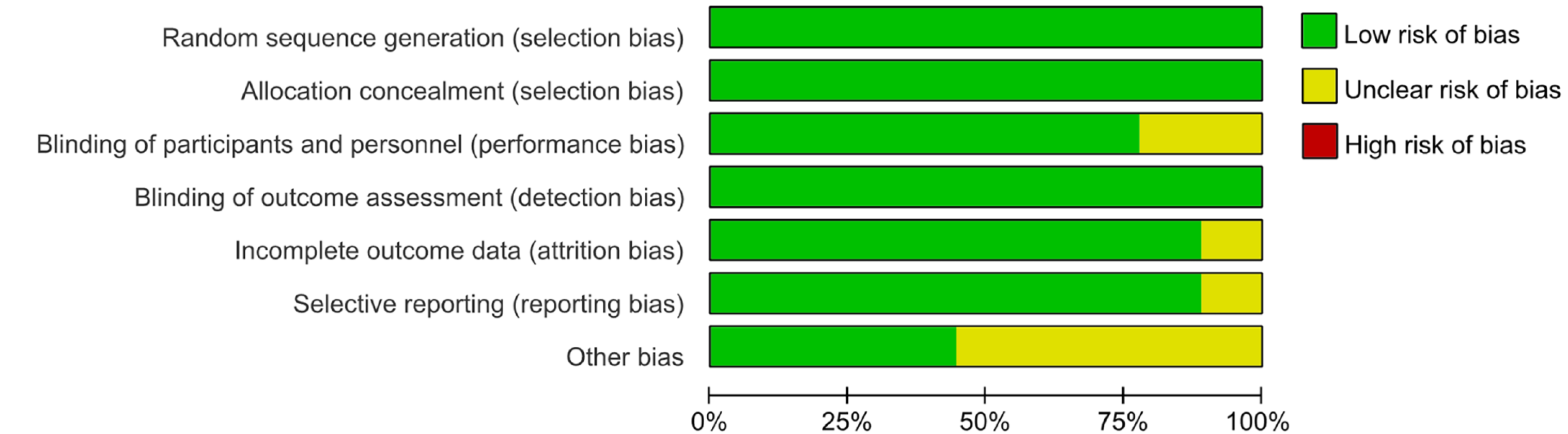
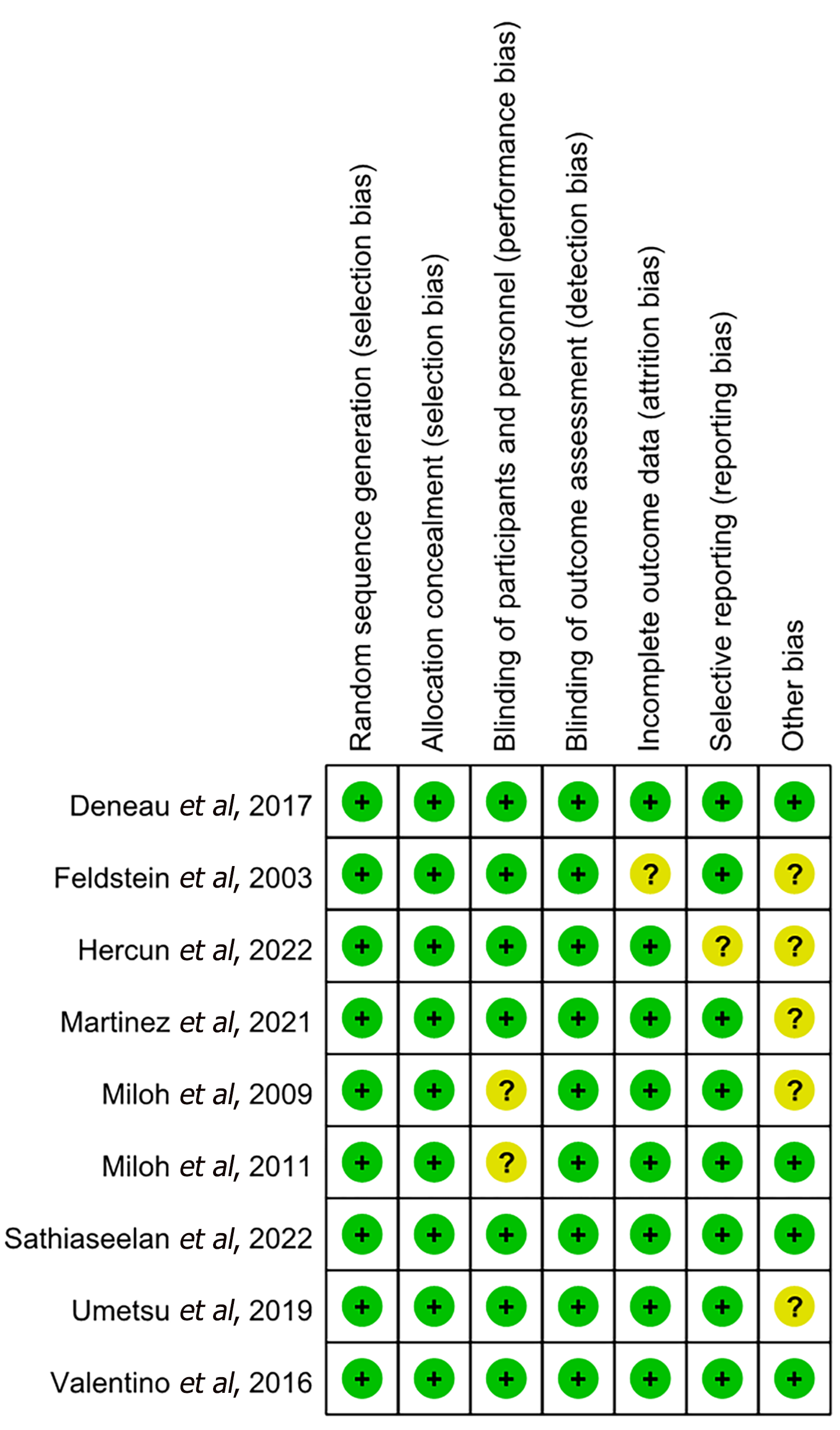
The process of literature selection is illustrated in Figure 1. By utilizing the search strategies, 510 reports were initially identified. After the initial retrieval, a review of titles and abstracts led to the exclusion of 274 duplicate articles. This process left a subset of 236 articles, which were then selected for a more detailed assessment through full-text examination. Finally, nine reports with 2524 pediatric patients with PSC met the inclusion criteria and passed the subsequent quality evaluation; thus, they were included in the current study. Figures 2 and 3 provide an assessment of the risk of bias associated with the included articles.
Across the articles included in this study, the potential risk factors for recurrence were identified from five investigations conducted in the United States[13-17], three studies involving participants from Canada[18-20], one conducted in Australia, and another in Japan[21]. The age at diagnosis for patients with PSC varied considerably across these studies, with median ages ranging from 4.7 to 15 years (Table 1). Furthermore, the duration of post-transplantation follow-up exhibited remarkable variation, with follow-up periods ranging from 1.1 to 6.1 years. The observed recurrence rates were also variable, spanning from 8.8% (low) to 55.6% (high).
| Ref. | Age at PSC diagnosis (median, range, years) | Gender (male, %) | PSC with LT/total PSC | IBD presence (%) | Follow-up, years | Recurrence n/N (%) | Median time to recurrence, months | Risk factors |
| Martinez et al[18] | 9.5 (7.8-12.3) | 61 | 140/1325 | 86 | 3 (1.1-6.1) | 36/140 (25.7) | 44.4 (19.2-70.8) | 1-5 |
| Miloh et al[19] | 12.6 ± 3.9 (mean ± SD) | 58.2 | 79/113 | 51 | 3.1 ± 2.7 | 7/79 (8.8) | 18.7 ± 13.8 | 2,3 |
| Feldstein et al[11] | 4.7 (1.5-19.6) | 65.4 | 11/52 | 81 | 6.6 ± 4.4 | 3/11 (27.3) | 48.4 | 2,3 |
| Miloh et al[20] | 12 (2-20) | 62 | 9/47 | 59 | 6.5 (0.5-19) | 5/9 (55.6) | 84 (48-228) | 2,5 |
| Deneau et al[9] | 15 (11.9-17.7) | 45 | 113/781 | 76 | 4.4 (2-7.9) | 51/113 (45.1) | 46.1 | 2-4 |
| Hercun et al[13] | 10.85 ± 3.78 (mean ± SD) | 59.3 | 5/27 | 77.8 | 8.9 ± 7.3 | 1/5 (20) | 48.6 | 2-4 |
| Valentino et al[29] | 13.4 (10.1, 16.0) | 60 | 6/89 | 85 | 3.7 (1.5, 6.9) | 1/6 (16.7) | 48 | 2-4 |
| Sathiaseelan et al[24] | 11.3 | 39.2 | 8/51 | 73 | 4.5 | 4/8 (50) | 22 | 2,4 |
| Umetsu et al[28] | 9.0 (6.0-13.5) | 56.4 | 9/39 | 94.8 | 5.5 (3.4-8.7) | 5/9 (55.56) | 84.9 ± 23.3 | 4 |
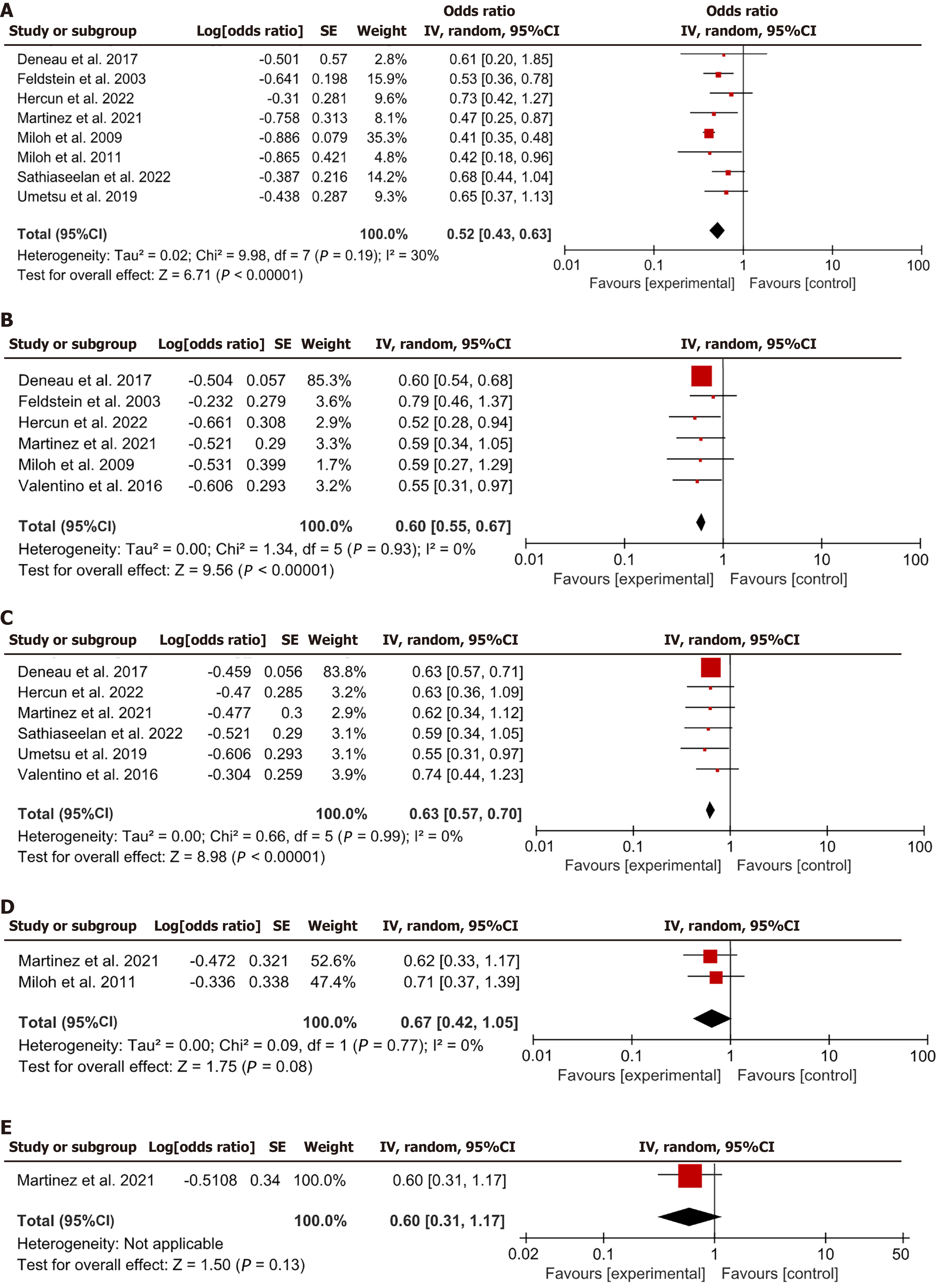
This meta-analysis integrated data from eight articles regarding the incidence of IBD before LT. Pooled analysis of these studies revealed a combined HR of 0.52, with a 95%CI of 0.43-0.63, P < 0.05 (Figure 4A). These results indicated that having IBD could be a substantial risk factor for rPSC after transplantation.
Six studies described the assessment of elevated liver enzyme levels at the time of LT. The results from these studies indicated a pooled HR of 0.60, with a 95%CI ranging from 0.55 to 0.67, P < 0.05 (Figure 4B). The results indicated that elevated liver enzyme levels might serve as a critical risk factor for rPSC in children.
Six studies supplied data regarding the incidence of PSC-autoimmune hepatitis (AIH) overlap in pediatric patients with PSC. The aggregated data from these six studies revealed a trend toward an amplified risk of rPSC in patients with overlap syndrome (HR = 0.63, 95%CI: 0.57-0.70, P < 0.05; Figure 4C). The findings implied that the incidence of PSC-AIH overlap might serve as an important risk factor for rPSC in children.
Two studies described the incidence of acute allograft rejection and its relationship with PSC recurrence. The results from these studies indicated that the incidence of acute allograft rejection was not markedly related to the risk of developing rPSC (HR = 0.67, 95%CI: 0.42-1.05, P = 0.08; Figure 4D).
Only one study described the incidence and impact of Epstein-Barr virus (EBV) viremia on rPSC. The findings of this study indicated that EBV viremia was not markedly associated with the risk of developing rPSC (HR = 0.60, 95%CI: 0.31-1.17, P = 0.13; Figure 4E).
The subgroup analyses were conducted to explore heterogeneity and to provide a more nuanced understanding of the risk factors for rPSC in pediatric patients. The findings are displayed in Table 2. The subgroup analysis implied that the risk of rPSC was lower among patients under the age of 10 years, as compared to those aged 10 years and above. Similarly, patients with a follow-up time of less than 5 years had a lower risk compared to those with a follow-up duration of 5 years or more. The recurrence rate itself also influenced the risk, with a lower risk in patients with a low recurrence rate compared to those with a high recurrence rate.
| Ref. | Subgroup characteristics | Hazard ratio (95%CI) | P value |
| [13,15,21] | Age group: < 10 years | 0.58 (0.45-0.75) | < 0.05 |
| [14,16-20] | Age group: ≥ 10 years | 0.54 (0.42-0.70) | < 0.05 |
| [13,14,19,20] | Follow-up time: < 5 years | 0.56 (0.44-0.72) | < 0.05 |
| [15,16,18,21] | Follow-up time: ≥ 5 years | 0.50 (0.38-0.67) | < 0.05 |
| [15,19] | Recurrence rate: Low | 0.62 (0.48-0.81) | < 0.05 |
| [13,14,16-18,20,21] | Recurrence rate: High | 0.53 (0.41-0.68) | < 0.05 |
Considering that each analysis for individual risk factors involved fewer than 10 studies, the sample sizes were insufficient to achieve adequate statistical power to differentiate between random variation and actual asymmetry in funnel plots. Consequently, funnel plots were not evaluated to estimate the risk of bias.
Sensitivity analyses were conducted to assess the robustness of the primary findings. The sensitivity analyses validated that the main findings of the meta-analysis were consistent and robust, suggesting that the identified risk factors for recurrence of PSC in pediatric patients after LT are not influenced by the exclusion of any individual study or by the presence of studies with a high risk of bias.
PSC is a rare but progressive liver condition that predominantly affects the bile ducts[22]. Although PSC can occur at any age, it is most commonly diagnosed in adults. However, the incidence of PSC in children is increasing, although it remains relatively rare[23]. The symptoms of PSC in children can include jaundice, fatigue, abdominal pain, and itching. However, PSC has no cure, and it can lead to complications such as cirrhosis, portal hypertension, and cholangiocarcinoma[24].
LT is the definitive treatment option for end-stage liver disease in children with PSC. Although LT can improve survival and quality of life in these patients, the disease can recur in the transplanted liver, a phenomenon known as rPSC[25]. At present, the risk factors for rPSC in children remain unclear, and it is crucial to identify these factors to improve patient outcomes and develop targeted therapies. Therefore, this study primarily aimed to identify and evaluate the potential risk factors for the recurrence of PSC in pediatric patients after LT. By analyzing data from nine observational studies, several factors that may be connected to an augmented risk of PSC recurrence in the pediatric population were found, including the presence of IBD, elevated liver enzyme levels at the time of transplantation, and PSC-AIH overlap syndrome.
Understanding the theoretical basis and mechanism of PSC recurrence is essential for developing strategies to prevent and manage this condition. The pathophysiology of PSC recurrence after LT is believed to involve a combination of immune-mediated processes, alterations in the gut-liver axis, and genetic predisposition. Autoimmune reactions may target the bile ducts of the transplanted liver, leading to chronic inflammation and fibrosis. The gut microbiome’s role in modulating the immune response and promoting liver inflammation is also attracting attention. Additionally, genetic factors may contribute to an individual’s susceptibility to PSC recurrence. These mechanisms are complex and interrelated, and further research is needed to fully elucidate them.
IBD was the most consistent risk factor identified across studies[26]. Eight of the nine included studies provided data on IBD, and the meta-analysis revealed a pooled HR of 0.52 (95%CI: 0.43-0.63, P < 0.05), indicating that the presence of IBD might increase the risk of PSC recurrence in pediatric liver transplant recipients. This finding is supported by previous research[27], which has shown that IBD is a strong risk factor for PSC in pediatric and adult populations. However, the pathophysiological mechanism underlying the relationship between IBD and PSC recurrence after LT remains unclear. However, the shared immune-mediated pathogenesis of IBD and PSC may contribute to the increased risk of recurrence in this patient population.
Increased levels of liver enzymes at the time of LT were identified as a significant risk factor for PSC recurrence in pediatric patients[28,29]. Six studies reported on liver enzyme levels, with a pooled HR of 0.60 (95%CI: 0.55-0.67). Elevated liver enzymes, particularly alanine aminotransferase and aspartate aminotransferase, have been associated with liver inflammation and injury, which may accelerate the progression of PSC and enhance the risk of recurrence after transplantation[30]. Given these findings, clinicians must supervise liver enzyme levels in pediatric patients with PSC who are undergoing LT and consider proactive management strategies to reduce liver inflammation and injury in these high-risk patients.
PSC-AIH overlap syndrome was another risk factor identified in this meta-analysis. Data from six studies were included in the analysis, and the pooled HR for PSC-AIH overlap syndrome was 0.63 (95%CI: 0.42-0.99). The presence of AIH features in patients with PSC has been connected to more aggressive disease progression and an elevated risk of LT[31]. In addition, the overlap syndrome may be more resistant to immunosuppressive therapy, which is a critical component of post-transplant management[32]. These factors may contribute to the increased risk of PSC recurrence in pediatric patients with PSC-AIH overlap syndrome.
Other factors that were evaluated in this systematic review include acute allograft rejection and EBV viremia. However, evidence to support a marked connection between these factors and the risk of PSC recurrence in pediatric liver transplant recipients is lacking. Only two studies reported on acute allograft rejection, and the results did not show a marked connection with PSC recurrence. Similarly, only one study described the incidence and impact of EBV viremia on PSC recurrence, and the findings did not indicate a remarkable association.
Despite the strengths of this analysis, several limitations need to be considered. Firstly, most of the included articles were retrospective in design, which may introduce bias and confounding factors. Secondly, the sample sizes of individual articles were generally small, which might limit the precision of the risk estimates and the generalizability of the findings. Finally, a significant heterogeneity was observed among studies in terms of study design, patient characteristics, and definitions of recurrence, which may have influenced the results of the analysis.
This meta-analysis identified several risk factors for the recurrence of PSC in pediatric patients after LT, including the presence of IBD, elevated liver enzyme levels at the time of transplantation, and PSC-AIH overlap syndrome. These findings may guide clinical decision-making and help identify high-risk patients who may benefit from proactive monitoring or tailored therapeutic interventions. However, these results should be interpreted with caution because of the inherent limitations in the included studies and the observational nature of the data.
| 1. | Akamatsu N. Liver transplantation for primary sclerosing cholangitis-morbidities including disease recurrence. Hepatobiliary Surg Nutr. 2024;13:143-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ali AH, Damman J, Shah SB, Davies Y, Hurwitz M, Stephen M, Lemos LM, Carey EJ, Lindor KD, Buness CW, Alrabadi L, Berquist WE, Cox KL. Open-label prospective therapeutic clinical trials: oral vancomycin in children and adults with primary sclerosing cholangitis. Scand J Gastroenterol. 2020;55:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Assis DN, Bowlus CL. Recent Advances in the Management of Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2023;21:2065-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Aziz B, Kok B, Cheah M, Lytvyak E, Moctezuma-Velazquez C, Wasilenko S, Tsochatzis E, Ravikumar R, Jose S, Allison M, Gunson B, Manas D, Monaco A, Mirza D, Fusai G, Owen N, Thorburn D, Roberts K, Srinivasan P, Wigmore S, Athale A, Creamer F, Fernando B, Iyer V, Madanur M, Sen G, Montano-Loza AJ, Hansen B, Mason AL. Severe Cholestasis Predicts Recurrent Primary Sclerosing Cholangitis Following Liver Transplantation. Am J Gastroenterol. 2025;120:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Bedke T, Stumme F, Tomczak M, Steglich B, Jia R, Bohmann S, Wittek A, Kempski J, Göke E, Böttcher M, Reher D, Franke A, Lennartz M, Clauditz T, Sauter G, Fründt T, Weidemann S, Tiegs G, Schramm C, Gagliani N, Pelczar P, Huber S. Protective function of sclerosing cholangitis on IBD. Gut. 2024;73:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Catassi G, D'Arcangelo G, Norsa L, Bramuzzo M, Hojsak I, Kolho KL, Romano C, Gasparetto M, Di Giorgio A, Hussey S, Yerushalmy-Feler A, Turner D, Matar M, Weiss B, Karoliny A, Alvisi P, Tzivinikos C, Aloi M. Outcome of Very Early Onset Inflammatory Bowel Disease Associated With Primary Sclerosing Cholangitis: A Multicenter Study From the Pediatric IBD Porto Group of ESPGHAN. Inflamm Bowel Dis. 2024;30:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Couchonnal E, Jacquemin E, Lachaux A, Ackermann O, Gonzales E, Lacaille F, Debray D, Boillot O, Guillaud O, Wildhaber BE, Chouik Y, McLin V, Dumortier J. Long-term results of pediatric liver transplantation for autoimmune liver disease. Clin Res Hepatol Gastroenterol. 2021;45:101537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Del Chierico F, Cardile S, Baldelli V, Alterio T, Reddel S, Bramuzzo M, Knafelz D, Lega S, Bracci F, Torre G, Maggiore G, Putignani L. Characterization of the Gut Microbiota and Mycobiota in Italian Pediatric Patients With Primary Sclerosing Cholangitis and Ulcerative Colitis. Inflamm Bowel Dis. 2024;30:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Deneau MR, El-Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, Chan A, Cotter J, Doan S, El-Youssef M, Ferrari F, Furuya KN, Gottrand M, Gottrand F, Gupta N, Homan M, Kamath BM, Kim KM, Kolho KL, Konidari A, Koot B, Iorio R, Ledder O, Mack C, Martinez M, Miloh T, Mohan P, O'Cathain N, Papadopoulou A, Ricciuto A, Saubermann L, Sathya P, Shteyer E, Smolka V, Tanaka A, Varier R, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Jensen MK. The natural history of primary sclerosing cholangitis in 781 children: A multicenter, international collaboration. Hepatology. 2017;66:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Di Giorgio A, Vergani D, Mieli-Vergani G. Cutting edge issues in juvenile sclerosing cholangitis. Dig Liver Dis. 2022;54:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Feldstein AE, Perrault J, El-Youssif M, Lindor KD, Freese DK, Angulo P. Primary sclerosing cholangitis in children: a long-term follow-up study. Hepatology. 2003;38:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Helgadottir H, Folseraas T, Kemmerich G, Aabakken L, Jørgensen KK, Vesterhus M. [Primary sclerosing cholangitis]. Tidsskr Nor Laegeforen. 2023;143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Hercun J, Willems P, Bilodeau M, Vincent C, Alvarez F. Long-Term Follow-Up into Adulthood of Pediatric-Onset Primary Sclerosing Cholangitis and Autoimmune Sclerosing Cholangitis. JPGN Rep. 2022;3:e220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Jerregård Skarby A, Casswall T, Bergquist A, Lindström L. Good long-term outcomes of primary sclerosing cholangitis in childhood. JHEP Rep. 2024;6:101123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Lapin B, Boehnke JR. Introduction to "PRISMA-COSMIN for outcome measurement instruments 2024". Qual Life Res. 2024;33:2025-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Lehan E, Wang T, Soboleski D, Acker A, Kehar M. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis in a Pediatric Patient With Neurofibromatosis Type 1. ACG Case Rep J. 2021;8:e00605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Leung KK, Deeb M, Fischer SE, Gulamhusein A. Recurrent Primary Sclerosing Cholangitis: Current Understanding, Management, and Future Directions. Semin Liver Dis. 2021;41:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Martinez M, Perito ER, Valentino P, Mack CL, Aumar M, Broderick A, Draijer LG, Fagundes EDT, Furuya KN, Gupta N, Horslen S, Jonas MM, Kamath BM, Kerkar N, Kim KM, Kolho KL, Koot BGP, Laborda TJ, Lee CK, Loomes KM, Miloh T, Mogul D, Mohammed S, Ovchinsky N, Rao G, Ricciuto A, Rodrigues Ferreira A, Schwarz KB, Smolka V, Tanaka A, Tessier MEM, Venkat VL, Vitola BE, Woynarowski M, Zerofsky M, Deneau MR. Recurrence of Primary Sclerosing Cholangitis After Liver Transplant in Children: An International Observational Study. Hepatology. 2021;74:2047-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Miloh T, Anand R, Yin W, Vos M, Kerkar N, Alonso E; Studies of Pediatric Liver Transplantation Research Group. Pediatric liver transplantation for primary sclerosing cholangitis. Liver Transpl. 2011;17:925-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Miloh T, Arnon R, Shneider B, Suchy F, Kerkar N. A retrospective single-center review of primary sclerosing cholangitis in children. Clin Gastroenterol Hepatol. 2009;7:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Pinnuck B, Lynch KD. Navigating the pharmacotherapeutic management of comorbid inflammatory bowel disease and primary sclerosing cholangitis. Expert Opin Pharmacother. 2024;25:1835-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Räisänen L, Nikkonen A, Kolho KL. Liver enzyme profiles after initiating biological treatment in children with inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. 2024;79:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Ricciuto A, Kamath BM, Hirschfield GM, Trivedi PJ. Primary sclerosing cholangitis and overlap features of autoimmune hepatitis: A coming of age or an age-ist problem? J Hepatol. 2023;79:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 24. | Sathiaseelan M, Bolia R, Barallon R, Alex G, Hardikar W, Rajanayagam J. Impact of ulcerative colitis on liver-related outcomes of children with primary sclerosing cholangitis. J Paediatr Child Health. 2022;58:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Sohal A, Kayani S, Kowdley KV. Primary Sclerosing Cholangitis: Epidemiology, Diagnosis, and Presentation. Clin Liver Dis. 2024;28:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Tenca A, Kolho KL, Consonni D, Jokelainen K, Färkkilä M. Dominant stricture in paediatric-onset primary sclerosing cholangitis is associated with impaired prognosis in a long-term follow-up. United European Gastroenterol J. 2024;12:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Tica S, Alghamdi S, Tait C, Nemera B, Turmelle Y, Fleckenstein J, Stoll J, Kulkarni S. Diagnosis of Primary Sclerosing Cholangitis Beyond Childhood is Associated with Worse Outcomes. J Clin Exp Hepatol. 2022;12:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Umetsu S, Notohara K, Nakazawa T, Tsunoda T, Sogo T, Komatsu H, Tanaka A, Tazuma S, Takikawa H, Inui A, Fujisawa T. Long-term outcomes of pediatric-onset primary sclerosing cholangitis: A single-center experience in Japan. Hepatol Res. 2019;49:1386-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Valentino PL, Wiggins S, Harney S, Raza R, Lee CK, Jonas MM. The Natural History of Primary Sclerosing Cholangitis in Children: A Large Single-Center Longitudinal Cohort Study. J Pediatr Gastroenterol Nutr. 2016;63:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | van Munster KN, Bergquist A, Ponsioen CY. Inflammatory bowel disease and primary sclerosing cholangitis: One disease or two? J Hepatol. 2024;80:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 31. | Wheless WH, Russo MW. Treatment of Primary Sclerosing Cholangitis Including Transplantation. Clin Liver Dis. 2024;28:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Zhang X, Lin X, Li X, Guan L, Li Y, Wang N. Ulcerative colitis complicated by primary sclerosing cholangitis and autoimmune hepatitis overlap syndrome: a case report and literature review. Front Immunol. 2023;14:1132072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |