Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.106784
Revised: March 21, 2025
Accepted: April 15, 2025
Published online: May 27, 2025
Processing time: 77 Days and 20.1 Hours
The treatment strategy for pancreatic pseudocysts (PPC) is comprehensive and warrants multidisciplinary participation. However, at present, the treatment concepts for PPC are inconsistent. Moreover, the timing of interventional therapy is unclear, and complication management is insufficient. Therefore, the deve
In this study, we present a rare case of PPC identified by endoscopy and imaging examination, and successfully managed by endoscopic and percutaneous dra
Ultrasound-guided endoscopic drainage for the management of PPC may provide additional insights to current clinical guidelines.
Core Tip: In this case, the patient underwent endoscopic window drainage for a pancreatic pseudocyst, utilizing ultrasound gastroscopy for the first time. The ultrasound gastroscopy facilitated accurate positioning, while the endoscopic submucosal dissection related technique was employed to incise the gastric wall and access the cyst, allowing for the drainage of cystic fluid. Subsequent dynamic re-examinations revealed a significant reduction in cyst size without any associated complications. During follow-up, the patient’s overall condition remained stable, and our institution has successfully performed four similar procedures without complications.
- Citation: Liu YL, Liu J, Jiang WJ, Zhang KG, Wang YT. Ultrasound-guided endoscopic drainage for the management of pancreatic pseudocysts: A case report. World J Gastrointest Surg 2025; 17(5): 106784
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/106784.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.106784
Pancreatic fluid collection, a common complication of acute and chronic pancreatitis, manifests as pancreatic pseudocysts (PPCs) and walled-off pancreatic necrosis, a type of pancreatic cystic disease[1]. PPC represent a complication associated with both acute and chronic pancreatitis that usually develop four to six weeks from the onset of pancreatitis. Perforation of a pseudocyst is a rare lethal complication of PPCs, occurring in less than 3% of PPC cases[2]. Historically, surgical exploration has been the predominant method employed in the management of perforated pseudocysts. Nevertheless, recent literature indicates that both endoscopic ultrasonography-guided interventions and percutaneous drainage techniques represent feasible alternatives for the treatment of this condition[3].
Currently, the treatment concepts for PPC are inconsistent. The optimal timing of intervention is not yet clear, and strategies for managing complications are not well developed. Therefore, the development of a multidisciplinary expert consensus on PPC is warranted. At present, endoscopic treatment is recommended for managing PPC in American Society for Gastrointestinal Endoscopy guideline[4] and Chinese Consensus guidelines[5]. According to the latest guidelines, case series reported that PPCs were effectively managed through endoscopic cystogastrostomy decom
This case report details the clinical management of a 36-year-old female patient who was admitted on September 6, 2024, presenting with a six-month history of upper abdominal discomfort.
The patient described intermittent sensations of fullness and discomfort in the upper abdomen, without significant abdominal pain, vomiting, or weight loss, and maintained a good appetite.
The patient’s medical history included multiple interventions for recurrent pancreatitis from 2019 to 2023. The patient presented with recurrent episodes of pancreatitis from 2019 to 2023, occurring at an approximate annual frequency. However, there were no indications of pancreatic necrosis. Therapeutic interventions, including fluid resuscitation, dietary fasting, and suppression of pancreatic juice secretion, were implemented at a local medical facility, resulting in clinical improvement. Notably, the patient experienced acute pancreatitis again with pancreatic necrosis in December 2023 and the computed tomography (CT) results from an external hospital in January 2024 indicated the presence of cysts.
The patient reported no prior use of tobacco or alcohol, nor any long-term medication consumption, and denied any personal or familial history of specific genetic disorders.
Upon physical examination, the patient had a body mass index of 38.5, weighing 105 kg, and appeared in good spirits, with no signs of anemia or jaundice. The abdomen was soft, and no palpable masses were detected.
Tumor markers and rheumatological markers, including immunoglobulin G4, were within normal limits.
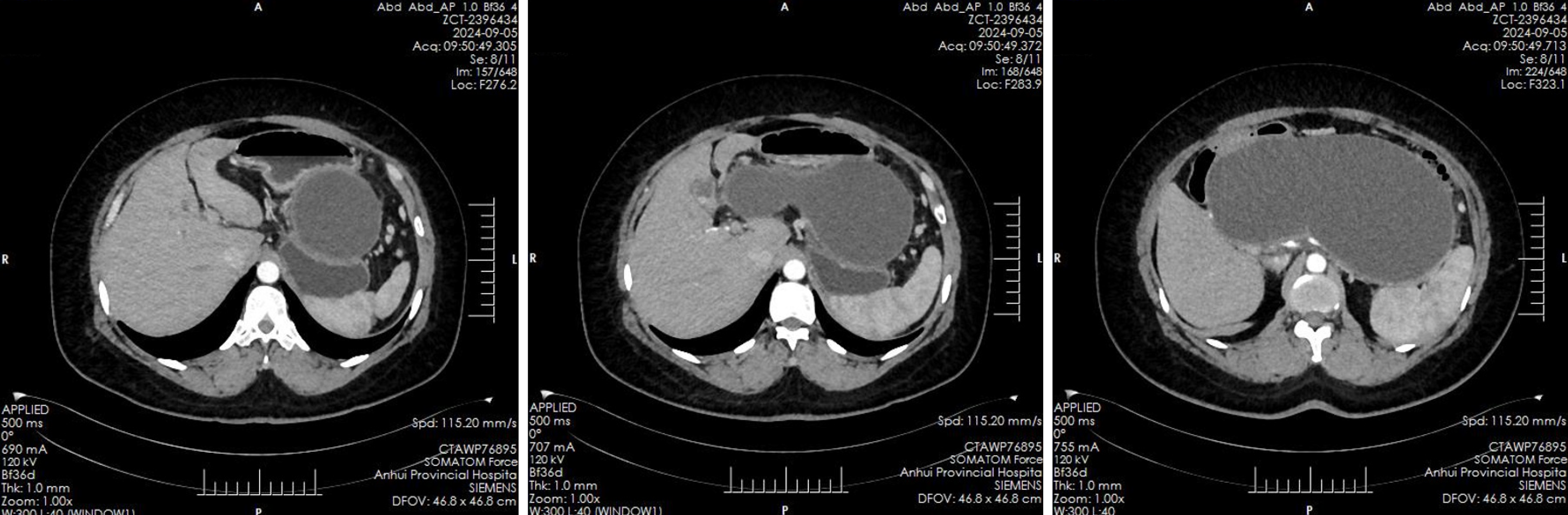
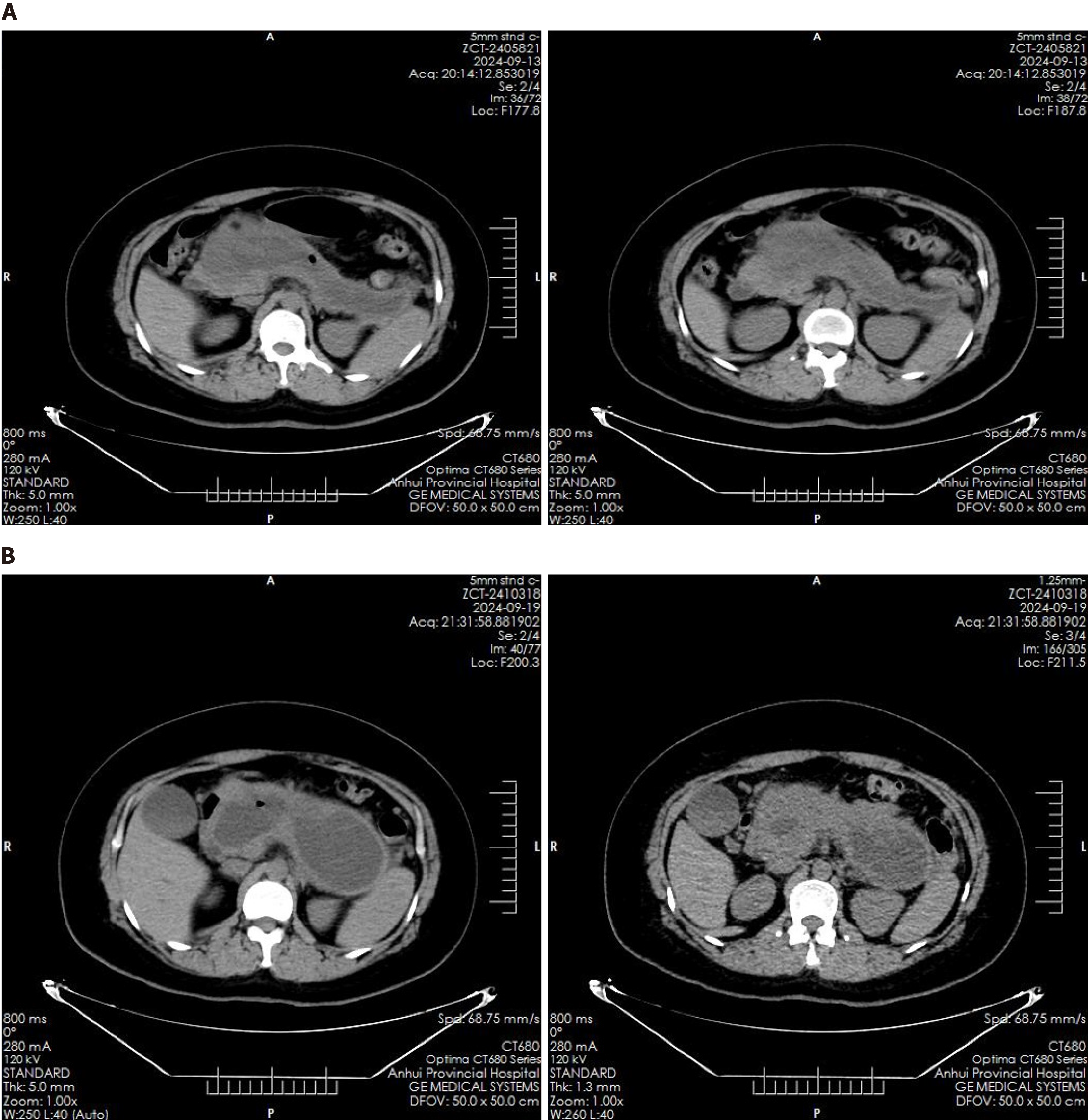
Abdominal ultrasound revealed multiple echogenic clusters with acoustic shadows in the gallbladder, the largest measuring approximately 12 mm × 8 mm. The pancreas exhibited clear, non-echogenic changes, measuring approximately 231 mm × 135 mm × 109 mm. Auxiliary examinations included an enhanced CT scan of the upper abdomen, which revealed an irregular, elliptical cystic low-density shadow approximately 232 mm × 114 mm in size, located in the pancreatic region, with no significant dilation of the intrahepatic or extrahepatic bile ducts (Figure 1). Additionally, multiple gallstones were identified in the gallbladder. Magnetic resonance imaging findings confirmed the presence of a pancreatic cyst and gallstones.
The patient’s history of recurrent pancreatitis and gallstones led to a diagnosis of biliary pancreatitis, with surgical intervention planned for gallstone removal. However, due to the presence of a large pancreatic cyst and associated surgical risks, it was recommended that the cyst be addressed first through pseudocyst drainage. Following multidisciplinary discussions and informed consent from the patient, endoscopic treatment was performed under ultrasound guidance to facilitate the drainage of the PPC.
Biliary pancreatitis and PPCs.
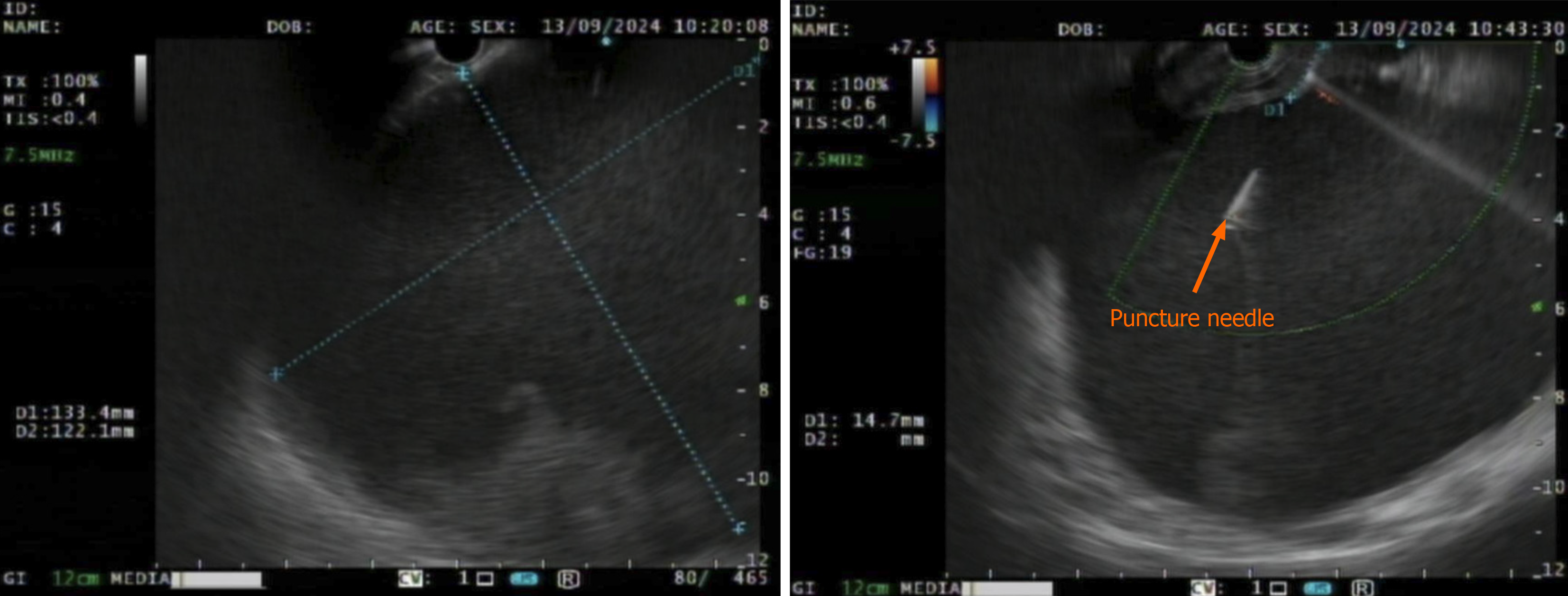
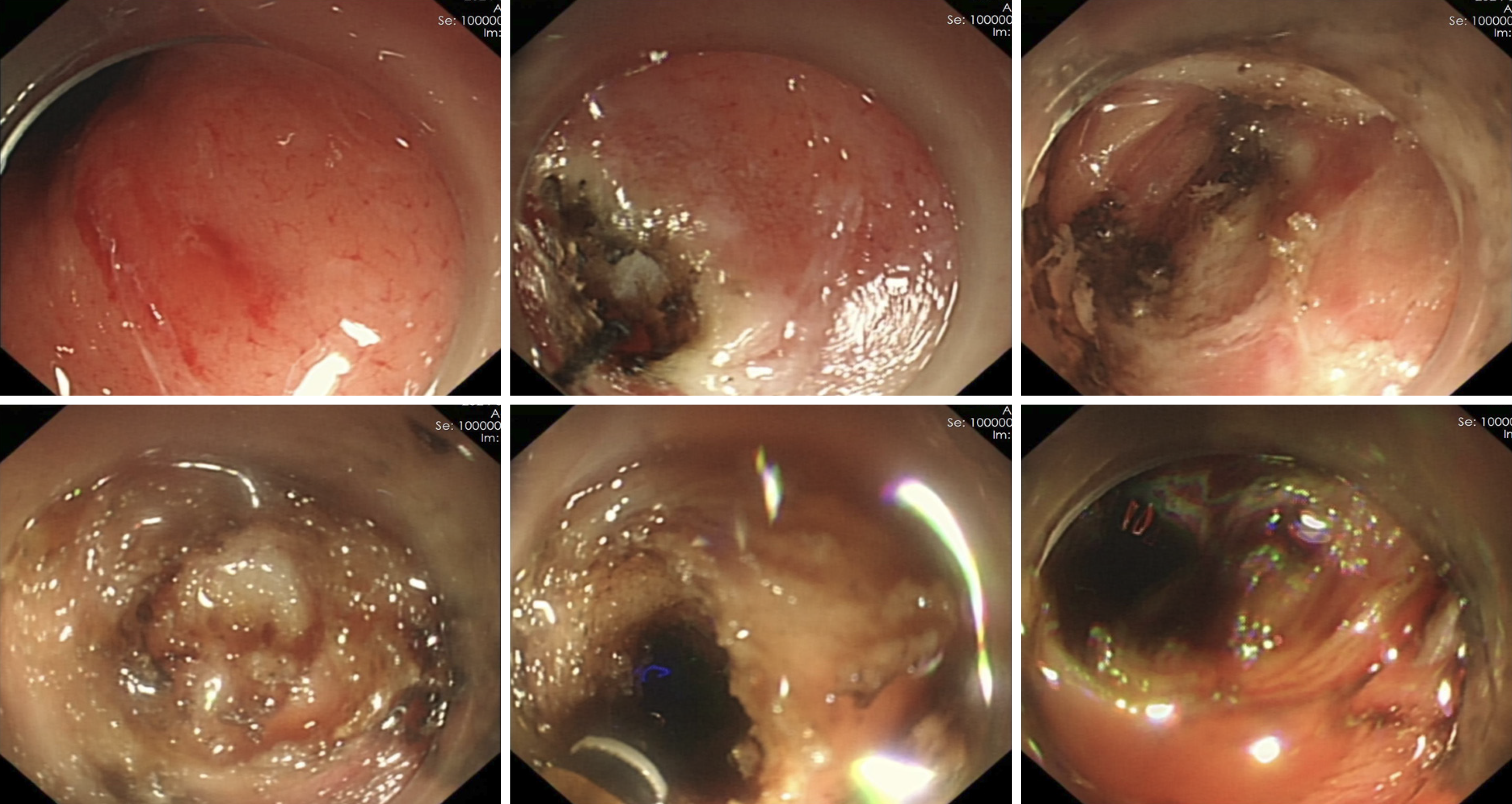
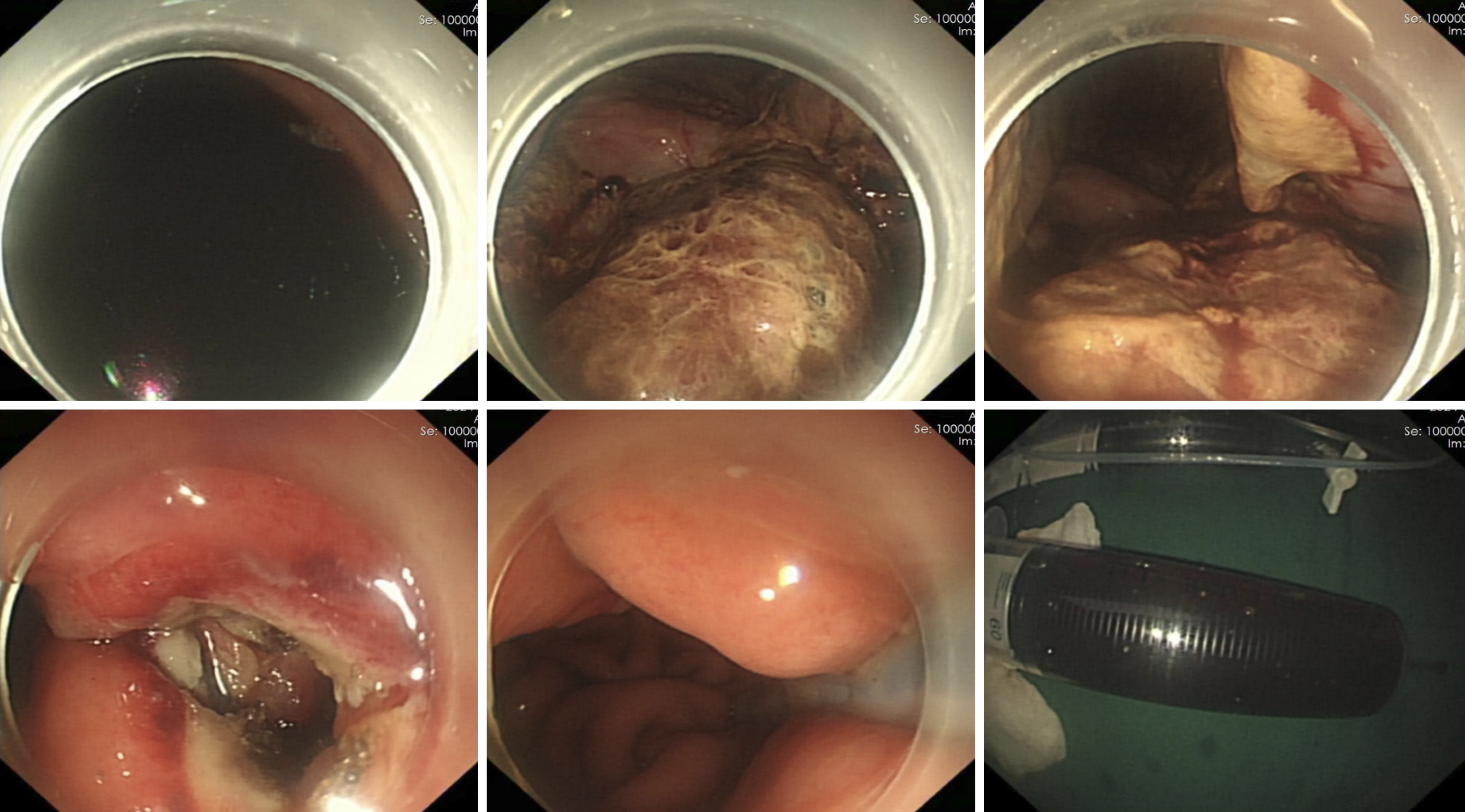
Under general anesthesia with endotracheal intubation, endoscopic ultrasound revealed a circular hypoechoic lesion in the body of the pancreas, with scattered areas of increased echogenicity (Figure 2). A 23G needle was utilized for puncture, yielding a coffee-like fluid. The puncture site was marked, and a Dual knife was employed to incise the gastric wall, exposing white, wall-like tissue. Upon aspiration with the puncture needle, a significant amount of coffee-like fluid was observed, prompting a deeper incision at the site, resulting in a substantial outflow of fluid (Figure 3). The endoscope was subsequently inserted into the cystic cavity to facilitate further aspiration, revealing a flocculent substance at the base. Hemostasis was achieved, and a gastric tube was placed (Figure 4).
Post-procedure imaging via CT scan, conducted eight hours after the endoscopic intervention, demonstrated complete resolution of the pancreatic cyst (Figure 5A). A follow-up CT scan on the sixth postoperative day indicated the re
At present, the optimal timing for interventional therapy for PPC remains ambiguous, and the management of associated complications is inadequate. Consequently, there is a pressing need for the establishment of a multidisciplinary expert consensus regarding PPC[7,8]. Endoscopic intervention is recommended for PPCs that persist for over four weeks and exhibit a diameter of 6 cm or greater, as well as those associated with secondary compression symptoms, progressive swelling, infection, or concurrent pancreatic portal hypertension[5]. Treatment modalities for pseudocysts encompass percutaneous catheter drainage, laparoscopic surgical intervention, and Roux-en-Y anastomosis of the cyst to the jejunum, in addition to endoscopic retrograde cholangiopancreatography drainage[9,10]. Currently, the guidelines indicate a high quality of evidence and strong recommendation for endoscopic ultrasound-guided puncture drainage, advocating for the use of a LAMS. However, the placement of such stents is not without risks, which may include bleeding, displacement, detachment, and embedding[11].
In this case, the patient underwent endoscopic window drainage for a PPC, utilizing ultrasound gastroscopy for the first time. The ultrasound gastroscopy facilitated accurate positioning, while the endoscopic submucosal dissection related technique was employed to incise the gastric wall and access the cyst, allowing for the drainage of cystic fluid. Postoperative CT showed complete disappearance of the cyst, but it recurred 6 days later. We speculated that the causes for recurrence may be related to infection or incomplete drainage[12]. Subsequent dynamic re-examinations revealed a significant reduction in cyst size without any associated complications. During follow-up, the patient’s overall condition remained stable, and our institution has successfully performed four similar procedures without complications, which is similar to previous study[13]. The patient’s cyst and associated symptoms have markedly improved, thereby presenting a novel therapeutic approach for patients with similar conditions.
Recurrence is characterized as the emergence of a new pseudocyst, as detected through imaging techniques during follow-up assessments following a previously documented resolution. Previous studies have indicated instances of recurrence associated with the therapeutic interventions employed. However, no statistically significant differences in recurrence rates were observed between endoscopic drainage and laparoscopic surgery[14,15]. In addition, adverse events have been reported in the literature. A recent meta-analysis revealed an overall adverse event rate of 11.35% in the endoscopy cohort and 14.66% in the laparoscopy cohort[16]. Furthermore, a multicenter trial conducted by Teoh et al[17] indicates that the application of a novel self-approximating LAMS for the drainage of PPCs is both safe and effective, with a low incidence (6.8%) of stent-related adverse events associated with its use[17], which is also recommended by the 2024 American Society for Gastrointestinal Endoscopy guide[4]. However, some limitations should be noted. First, although tumor markers and rheumatological markers, including immunoglobulin G4, were within normal limits, we cannot confirm whether tumor or autoimmune pancreatitis was completely excluded due to the lack of pancreatic puncture pathology examination. Second, the follow-up was only up to 50 days after surgery, and the long-term efficacy was not evaluated.
In summary, we report a rare case with PPC which was successfully treated by ultrasound-guided endoscopic drainage.
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4315] [Article Influence: 359.6] [Reference Citation Analysis (45)] |
| 2. | Koseki M, Hashimoto Y. Perforation of pancreatic pseudocyst diagnosed with endoscopy and treated with percutaneous drainage. DEN Open. 2024;4:e295. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Son TQ, Hoc TH, Huong TT, Dinh NQ, Van Tuyen P. A ruptured pancreatic pseudocyst causes acute peritonitis with clinical characteristics of a gastrointestinal tract perforation. J Surg Case Rep. 2022;2022:rjac164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | ASGE Standards of Practice Committee; Sheth SG, Machicado JD, Chalhoub JM, Forsmark C, Zyromski N, Thosani NC, Thiruvengadam NR, Ruan W, Pawa S, Ngamruengphong S, Marya NB, Kohli DR, Fujii-Lau LL, Forbes N, Elhanafi SE, Desai M, Cosgrove N, Coelho-Prabhu N, Amateau SK, Alipour O, Abidi W, Qumseya BJ; ASGE Standards of Practice Committee Chair. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the management of chronic pancreatitis: summary and recommendations. Gastrointest Endosc. 2024;100:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 5. | Zhu H, Du Y, Wang K, Li Z, Jin Z. Consensus guidelines on the diagnosis and treatment of pancreatic pseudocyst and walled-off necrosis from a Chinese multiple disciplinary team expert panel. Endosc Ultrasound. 2024;13:205-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Duggal S, Nagineni L, Trivedi BS, Zuckerman M, Badillo R. Evolving Endoscopic Approaches to Pancreatic Pseudocysts and Walled-Off Necrosis: Case Series and Review of Evidence. J Investig Med High Impact Case Rep. 2024;12:23247096241304521. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Law R, Baron TH. Endoscopic management of pancreatic pseudocysts and necrosis. Expert Rev Gastroenterol Hepatol. 2015;9:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Bang JY, Varadarajulu S. Endoscopic ultrasound-guided management of pancreatic pseudocysts and walled-off necrosis. Clin Endosc. 2014;47:429-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Bofill-Garcia A, Lupianez-Merly C. Endoscopic Retrograde Cholangiopancreatography for Management of Chronic Pancreatitis. Gastrointest Endosc Clin N Am. 2024;34:449-473. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Rasch S, Nötzel B, Phillip V, Lahmer T, Schmid RM, Algül H. Management of pancreatic pseudocysts-A retrospective analysis. PLoS One. 2017;12:e0184374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Bang JY, Hasan MK, Navaneethan U, Sutton B, Frandah W, Siddique S, Hawes RH, Varadarajulu S. Lumen-apposing metal stents for drainage of pancreatic fluid collections: When and for whom? Dig Endosc. 2017;29:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Guerra JG, Takenaka C, Fernandes MO, Ardengh JC. Necrotizing pancreatitis with a recurring pancreatic pseudocyst treated by endoscopic duodenum-gastropancreatic anastomosis. Arq Gastroenterol. 2023;60:158-160. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Rückert F, Lietzmann A, Wilhelm TJ, Sold M, Kähler G, Schneider A. Long-term results after endoscopic drainage of pancreatic pseudocysts: A single-center experience. Pancreatology. 2017;17:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Redwan AA, Hamad MA, Omar MA. Pancreatic Pseudocyst Dilemma: Cumulative Multicenter Experience in Management Using Endoscopy, Laparoscopy, and Open Surgery. J Laparoendosc Adv Surg Tech A. 2017;27:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Zhao X, Feng T, Ji W. Endoscopic versus surgical treatment for pancreatic pseudocyst. Dig Endosc. 2016;28:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Melman L, Azar R, Beddow K, Brunt LM, Halpin VJ, Eagon JC, Frisella MM, Edmundowicz S, Jonnalagadda S, Matthews BD. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc. 2009;23:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Teoh AYB, Bapaye A, Lakhtakia S, Ratanachu T, Reknimitr R, Chan SM, Choi HJ, Gadhikar HP, Kongkam P, Korrapati SK, Lee YN, Medarapalem J, Ridtitid W, Moon JH. Prospective multicenter international study on the outcomes of a newly developed self-approximating lumen-apposing metallic stent for drainage of pancreatic fluid collections and endoscopic necrosectomy. Dig Endosc. 2020;32:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |