INTRODUCTION
Gastric cancer is the second most common malignant tumour in terms of incidence and the third leading cause of cancer-related deaths in China. With advancements in endoscopic techniques and increased accessibility to health screenings, the detection rate of early gastric cancer has steadily increased. Early gastric cancer is characterized by a low risk of metastasis and a high cure rate, with patients achieving a 5-year survival rate exceeding 90% following standardized treatment[1]. Compared with patients with locally advanced gastric cancer, those with early-stage disease place greater emphasis on postoperative quality of life. Consequently, surgical approaches for early gastric cancer are evolving towards minimally invasive, precise, and function-preserving techniques. These approaches aim to maximize the preservation of gastric structure and function while ensuring oncological safety, thereby enhancing patients’ postoperative quality of life. As a result, function-preserving gastrectomy procedures, including pylorus-preserving gastrectomy (PPG), proximal gastrectomy, segmental gastrectomy, and local gastric resection, have emerged[2,3].
Function-preserving gastrectomy is distinguished by its limited gastric resection, preservation of the pylorus, and retention of the vagus nerve, all while ensuring adequate lymph node dissection. Laparoscopic surgery, with its benefits of minimal invasiveness and rapid recovery, combined with function-preserving techniques, has become the standard of care for early gastric cancer[4,5]. Laparoscopic PPG, in particular, has gained recognition for its safety and efficacy, and its implementation is becoming increasingly widespread. This article, which draws from the current literature, explores the controversies and consensus surrounding the use of laparoscopic PPG for early gastric cancer.
DEFINITION AND INDICATIONS OF LAPAROSCOPIC PPG
PPG requires preservation of the infrapyloric and right gastric vessels; conservation of the hepatic, pyloric, and celiac branches of the vagus nerve (to the extent possible); and retention of the upper 1/3 of the stomach along with 3–4 cm of the pylorus. The concept of PPG was first introduced by Japanese researchers in the 1960s, primarily to treat benign ulcers on the lesser curvature of the stomach or those distant from the pylorus[6]. Maki et al[6] preserved 1.5 cm of the gastric antrum from the pylorus during gastrectomy, thereby maintaining normal pyloric function. Following the recognition of early gastric cancer as a distinct entity, Japanese researchers in 1989 incorporated lymph node dissection into traditional PPG, adapting it for the treatment of early gastric cancer[7].
According to the Japanese Gastric Cancer Treatment Guidelines, a gross resection margin of 2 cm should be achieved for T1 tumours[8]. Therefore, the indications for PPG include tumours in the middle 1/3 of the stomach, with the distal end of the tumour ≥ 4 cm from the pylorus (2 cm from the lower tumour margin to the distal resection margin, and ≥ 2 cm from the distal resection margin to the pylorus), and clinical stage T1N0M0 early gastric cancer. Patients with benign gastric ulcers meeting these criteria are also candidates for PPG. Compared with endoscopic treatment, laparoscopic PPG offers a safer resection margin and allows for the intraoperative management of potential lymph node metastases.
Given that most PPG surgeries currently employ laparoscopic dissection followed by small-incision resection and anastomosis, preoperative gastroscopy is recommended to accurately locate the proximal and distal margins of the tumour. Furthermore, prior to surgery, it is essential to determine both the location and horizontal infiltration range of the tumour with precision. For certain superficially diffuse early gastric cancers, if conventional gastroscopy fails to clearly identify the tumour margin, it is advisable to use magnifying gastroscopy in conjunction with narrow-band imaging technology. This approach allows for assessing the distance between the lesion margin and pylorus while precisely marking both the proximal and distal ends of the lesion using metal clips (recommended). Such measures are beneficial for intraoperative margin determination.
Recently, various strategies have been explored within laparoscopic surgery for effective margin localization. These include indocyanine green fluorescence tracking, India ink staining, and nanocarbon marking techniques[9]. Owing to the absence of direct tactile feedback, laparoscopic surgeons must rely predominantly on visual assessment when identifying margins. This reliance becomes particularly critical in cases involving early gastric cancer as well as advanced gastric cancer that has not penetrated the serosa; in these instances, tumour boundaries can be ambiguous, often leading to positive margins, which may result in either residual tumour or excessive resection of healthy tissue. In accordance with the Chinese Quality Control Indices for Standardized Diagnosis and Treatment of Gastric Cancer, intraoperative endoscopic-assisted localization is recommended for T1-stage tumours with unclear boundaries[10]. Some experts posit that for certain early gastric cancers in the upper 1/3 of the stomach, where the proximal tumour margin is ≥ 3 cm from the oesophagogastric junction, PPG can be performed with oncological safety and postoperative complication rates comparable to those of total gastrectomy while offering superior postoperative quality of life[11].
SAFETY OF LAPAROSCOPIC PPG
There is a significant degree of controversy regarding the surgical safety of PPG. This debate stems primarily from several critical factors, including the extent of lymph node dissection, postoperative complications, and overall prognosis. Lymph node metastasis is a critical determinant of gastric cancer prognosis. Standardized and thorough lymph node dissection during surgery significantly increases the long-term survival of patients. Current guidelines recommend D1 lymph node dissection for PPG, encompassing lymph node No. 1, No. 3, No. 4sb, No. 4d, No. 6, and No. 7. For D1+ dissection, No. 8a and No. 9 are additionally included. However, the PPG requirement to preserve the blood supply and innervation to the gastric antrum, particularly where the right gastric artery runs alongside the pyloric branch of the vagus nerve, precludes complete dissection of lymph nodes No. 5 and No. 6. This balance between functional preservation and radical resection remains a primary point of contention in PPG.
To address oncological safety concerns, numerous studies have been conducted. Yanzhang et al[12] conducted a retrospective analysis of the clinical and pathological data from 354 patients diagnosed with early gastric cancer. Their findings indicated that the rate of lymph node metastasis above the pylorus in patients with middle-T1 stage gastric cancer was merely 3.05%. Thus, for early gastric cancer in the middle third of the stomach, the rate of metastasis to No. 5 and No. 6 lymph nodes is negligible, obviating the need for dissection[13]. A recent retrospective study conducted by the National Cancer Center of South Korea revealed that for upper and middle gastric cancers with a diameter of less than 4 cm, classified as stage T1 and well to moderately differentiated stage T2, the metastasis rate for No. 5 lymph nodes was 0%, whereas that for No. 6 lymph nodes was only 0.4%[14]. These findings suggest that although PPG may not permit complete pyloric lymph node dissection, it does not compromise oncological safety. Laparoscopic PPG provides safe and effective lymph node dissection within the appropriate range. Furthermore, PPG based on sentinel lymph node biopsy can further mitigate the risk of incomplete lymph node dissection[15].
With respect to surgical safety, PPG has outcomes comparable to those of traditional distal gastrectomy and even has a lower overall postoperative complication rate[16-18]. Hiki et al[19] reported no significant differences in operation time, intraoperative blood loss, or number of dissected lymph nodes between laparoscopic PPG and open PPG. Japanese researchers Akiyama et al[17] demonstrated that, compared with laparoscopy-assisted PPG, total laparoscopic PPG results in less trauma, reduced intraoperative blood loss, and earlier postoperative oral intake, underscoring the advantages of fully laparoscopic procedures.
The safety and feasibility of laparoscopic radical gastrectomy for both early and advanced gastric cancer patients have been substantiated by evidence-based medicine. However, laparoscopic techniques still present certain disadvantages, including the "chopstick effect" associated with operating instruments, muscle fatigue experienced by surgeons, and microtremors during surgery. With advancements in surgical techniques, the Da Vinci robotic surgical system has gradually been integrated into the field of surgery, ushering minimally invasive procedures into a new era. Compared with traditional open surgery and laparoscopic approaches, a robotic surgical system offers several technical advantages, such as three-dimensional magnified vision, multijoint movement capabilities, tremor filtration from hand movements, and support for remote operation. These features effectively address some of the technical limitations inherent in conventional laparoscopic gastric cancer surgeries.
A meta-analysis conducted in 2022 included data from 12401 gastric cancer patients to evaluate the efficacy and safety of both robotic and laparoscopic gastrectomies. This analysis revealed that, while the robotic group had extended operation durations compared with those who underwent laparoscopy alongside reduced intraoperative blood loss, they also performed more lymph node dissections than the laparoscopic group did[20].
The findings from two randomized controlled trials indicate that the postoperative hospital stay following robotic gastric surgery is shorter than that following laparoscopic gastric surgery, with durations of 7.9 days vs 8.2 days and 12 days vs 13 days, respectively[21,22]. Additionally, several large-sample clinical studies and meta-analyses have consistently demonstrated no statistically significant difference in the incidence of complications, including gastric emptying disorder, intestinal obstruction, abdominal infection, incision infection, anastomotic leakage, or pancreatic-related complications, between robotic surgery and laparoscopic surgery[23]. Notably, studies involving obese patients yielded similar results[24]. These findings collectively suggest that robotic surgery is not inferior to laparoscopic surgery and may offer enhanced advantages in terms of postoperative recovery. However, owing to its high cost and other factors, robotic surgery has yet to achieve widespread adoption; thus, laparoscopic surgery remains the predominant surgical approach globally at present.
In recent years, reports on PPG outcomes have increased[25]. A meta-analysis conducted by Mao et al[26] involving a total of 4871 patients (1955 in the PPG group and 2916 in the distal gastrectomy group) indicated that the long-term survival rates and recurrence rates for both groups were comparable. Zhu et al[11] reported on 288 early gastric cancer patients and demonstrated that PPG outcomes were not inferior to those of distal or total gastrectomy. According to research conducted by Kasahara et al[27] in 2021, the 5-year overall survival rate of patients with pathological stage I cancer was higher in the laparoscopic-assisted PPG group than in the laparoscopic-assisted distal gastrectomy group (100% vs 82.9%, P = 0.027). We contend that the collective findings of these studies substantiate the assertion that PPG is a safe and effective treatment for early gastric cancer, comparable in oncological safety to conventional surgical methods.
ADVANTAGES OF LAPAROSCOPIC PPG
Patients undergoing traditional distal gastrectomy often experience complications, such as alkaline reflux gastritis and dumping syndrome, due to the loss of the pyloric sphincter, peripyloric blood vessels, and vagus nerve, which decreases the postoperative quality of life. In contrast, PPG preserves the pylorus and vagus nerve, theoretically reducing the incidence of these postoperative complications. Many researchers have performed relevant studies on the clinical effects of PPG, presenting a variety of perspectives. Sun and Wu[28] demonstrated that, compared with distal gastrectomy, PPG substantially decreases the occurrence of dumping syndrome, body weight loss and bile reflux gastritis. A meta-analysis revealed that PPG is associated with lower rates of anastomosis-related complications, remnant gastritis, and bile reflux[25]. Park et al[29] compared the clinical efficacy of PPG with that of distal gastrectomy plus Billroth I reconstruction and reported that PPG resulted in lower incidences of bile reflux gastritis and gallstone formation. Fujita et al[30] reported that PPG outperformed distal gastrectomy with Billroth I reconstruction in terms of supplementary food intake, diarrhoea scores, and dumping syndrome management. Gastric acid and gastrin play crucial roles in the digestion and absorption of food, particularly iron. Following distal gastrectomy with Billroth II reconstruction, food directly enters the jejunum, reducing gastric acid and gastrin secretion and consequently limiting iron absorption. PPG, however, maintains normal food passage, facilitating more efficient digestion and absorption. A Japanese questionnaire-based study revealed that the incidence of dumping syndrome following PPG was significantly lower than that after distal gastrectomy with Roux-en-Y anastomosis[31].
Moreover, long-term follow-up studies have consistently shown that PPG leads to superior nutritional outcomes[32]. Terayama et al[32] reported that PPG has a great advantage in maintaining postoperative skeletal muscle mass as well as the nutritional parameters of older patients. In a comparative study, Eom et al[33] reported that laparoscopic PPG was noninferior to laparoscopic distal gastrectomy in terms of postoperative total serum protein, albumin, and body mass index (BMI), with the added benefit of superior haemoglobin recovery. Therefore, on the basis of the aforementioned research findings, we propose that PPG may effectively reduce the incidence of postoperative reflux and dumping syndrome while ensuring satisfactory nutritional status following surgery.
KEY TECHNICAL ASPECTS OF LAPAROSCOPIC PPG
PPG involves preserving the complex anatomy of infrapyloric vessels and associated branches of the vagus nerve. The most critical aspect of this surgical procedure lies in performing lymph node dissection while safeguarding the associated vessels and nerves, making it a prominent topic for surgeons to study and discuss. Dissection of the infrapyloric lymph node at Station 6 presents both the primary focus and the greatest challenge in laparoscopic PPG. The current classification of Station 6 adheres to the 15th edition of the Japanese Gastric Cancer Treatment Guidelines (2017), which delineates them into Station 6a (along the right gastroepiploic artery), Station 6i (along the infrapyloric artery), and Station 6v (along the right gastroepiploic and infrapyloric veins). Mizuno et al[34] demonstrated the absence of metastasis to Station 6i in patients with T1 middle gastric cancer, providing evidence-based support for reducing the extent of surgery and preserving function in PPG.
Following the Japanese classification, the dissection of Station 6 can be strategically divided into central, lateral, and medial areas. (1) Central area: After separating the gastric and colonic mesentery, the pancreatic head and duodenum are exposed. The right gastroepiploic vein is traced along Henle’s trunk, skeletonizing it from inferior to superior until it reaches the confluence of the infrapyloric vessels, while dissecting Station 6v; (2) Lateral area: This region carries a risk of right gastroepiploic vein injury. The infrapyloric vessels along the lower edge of the greater curvature of the gastric antrum are carefully dissected from left to right, progressively dissecting Station 6i. Caution should be taken when an ultrasonic scalpel is used to avoid direct contact with the infrapyloric vascular plexus; and (3) Medial area: With the assistant elevating the gastric antrum to fully expose the gastropancreatic fold, the right gastroepiploic artery is traced along the gastroduodenal artery. Station 6a is dissected proximally to distally until the first branch of the right gastroepiploic vein is identified. The triangular space formed by the greater curvature, infrapyloric vessels, and main trunk of the right gastroepiploic vessels is observed. Within this space, the main trunk of the right gastroepiploic artery and vein distal to the first branch of the right gastroepiploic vein are transected. We suggest the use of retrograde dissection of the gastroepiploic vascular arch if isolation of the origin of the infrapyloric vessels is challenging intraoperatively.
Anatomical variations in the infrapyloric vasculature necessitate careful intraoperative identification to safeguard the blood supply to the infrapyloric area. Nishizawa et al[35] proposed a four-type classification for infrapyloric vein distribution: (1) Type I: ≥ 2 infrapyloric veins drain into the right gastroepiploic vein; (2) Type IIa: A single infrapyloric vein drains into the right gastroepiploic vein; (3) Type IIb: One infrapyloric vein drains into the right gastroepiploic vein and the other into the superior anterior pancreaticoduodenal vein; and (4) Type III: A single infrapyloric vein drains exclusively into the superior anterior pancreaticoduodenal vein. A 2018 multicentre clinical study involving 419 patients revealed that 95.0% had a single infrapyloric artery, with 36.8% originating from the gastroduodenal artery, 31.0% from the superior anterior pancreaticoduodenal artery, and 27.2% from the right gastroepiploic artery. Multiple infrapyloric arteries were observed in 3.6% of patients, whereas 1.4% lacked an infrapyloric artery[36]. Japanese scholars, through a retrospective analysis of 156 gastric cancer surgery patients, proposed three types of classification for infrapyloric artery origin: (1) Type I (distal type), originating from the superior anterior pancreaticoduodenal artery; (2) Type II (caudal type), originating from the right gastroepiploic artery; and (3) Type III (proximal type), originating from the gastroduodenal artery[37].
Preserving the hepatic branch of the vagus nerve maintains gallbladder contractility while reducing Oddi sphincter tension, thereby lowering the incidence of postoperative gallstones and cholecystitis[38]. We noted that the hepatic branch of the vagus nerve runs between the hepatogastric ligaments and can be clearly exposed laparoscopically by incising the omentum more than 1 cm below the liver. Caution should be exercised to avoid injury when the liver is suspended during surgery. Intraoperatively, dissection along the hepatic branch to the left side of the lower oesophagus reveals that the hepatic and anterior gastric branches originate from the anterior vagal trunk. The anterior gastric branch is transected distal to the bifurcation of the anterior vagal trunk, preserving the hepatic branch. The main trunk of the right gastric artery is dissected approximately 3 cm proximal to the pylorus, preserving the pyloric branch of the vagus nerve. Because PPG does not require dissection of lymph nodes 5 and 12 and the hepatoduodenal ligament does not need to be dissected, preserving these vagal branches is relatively straightforward.
Preserving the celiac branch of the vagus nerve can improve postoperative gastrointestinal motility recovery and reduce the incidence of diarrhoea[39]. According to research conducted by Chinese scholars, the celiac branch of the vagus nerve plays a role in regulating insulin secretion[40]. Consequently, preserving the celiac branch of the vagus nerve may also contribute to improved blood glucose levels. On the basis of surgical experience at our centre, the key points for preserving the celiac branch of the vagus nerve include the following: (1) Dissection along the epineurium at the root of the left gastric artery; (2) Retrograde dissection of the celiac branch to the posterior trunk using nonenergy instruments such as dissecting forceps; (3) Locating the vagus nerve between the right crus of the diaphragm and the abdominal part of the oesophagus is easier, identifying the nerve before transecting vessels is crucial; and (4) Using nerve fibres surrounding arteries as boundaries, lymph nodes outside the nerve fibre membrane are dissected. Because the left side of the gastropancreatic fold has a sparse vascular distribution, separating the celiac branch from the left gastric artery in this area is safer. However, owing to the close relationship between the celiac branch of the vagus nerve and the left gastric artery, simultaneously preserving nerve function and thoroughly dissecting lymph node 7, which is crucial for tumour eradication, is challenging. The Japanese Gastric Cancer Treatment Guidelines stipulate that Station 7 must be dissected during D1 radical gastrectomy for gastric cancer[8]. Therefore, selective preservation of the celiac branch of the vagus nerve should be considered only after ensuring thorough dissection of Station 7. If suspicious lymph node infiltration is found intraoperatively, the lymph nodes should be removed en bloc with the nerve. Some scholars argue that preserving the celiac branch of the vagus nerve does not significantly affect short-term postoperative gastric function recovery[41]. Consequently, controversy remains regarding whether the celiac branch of the vagus nerve should be preserved in PPG.
DIGESTIVE TRACT RECONSTRUCTION METHODS IN LAPAROSCOPIC PPG
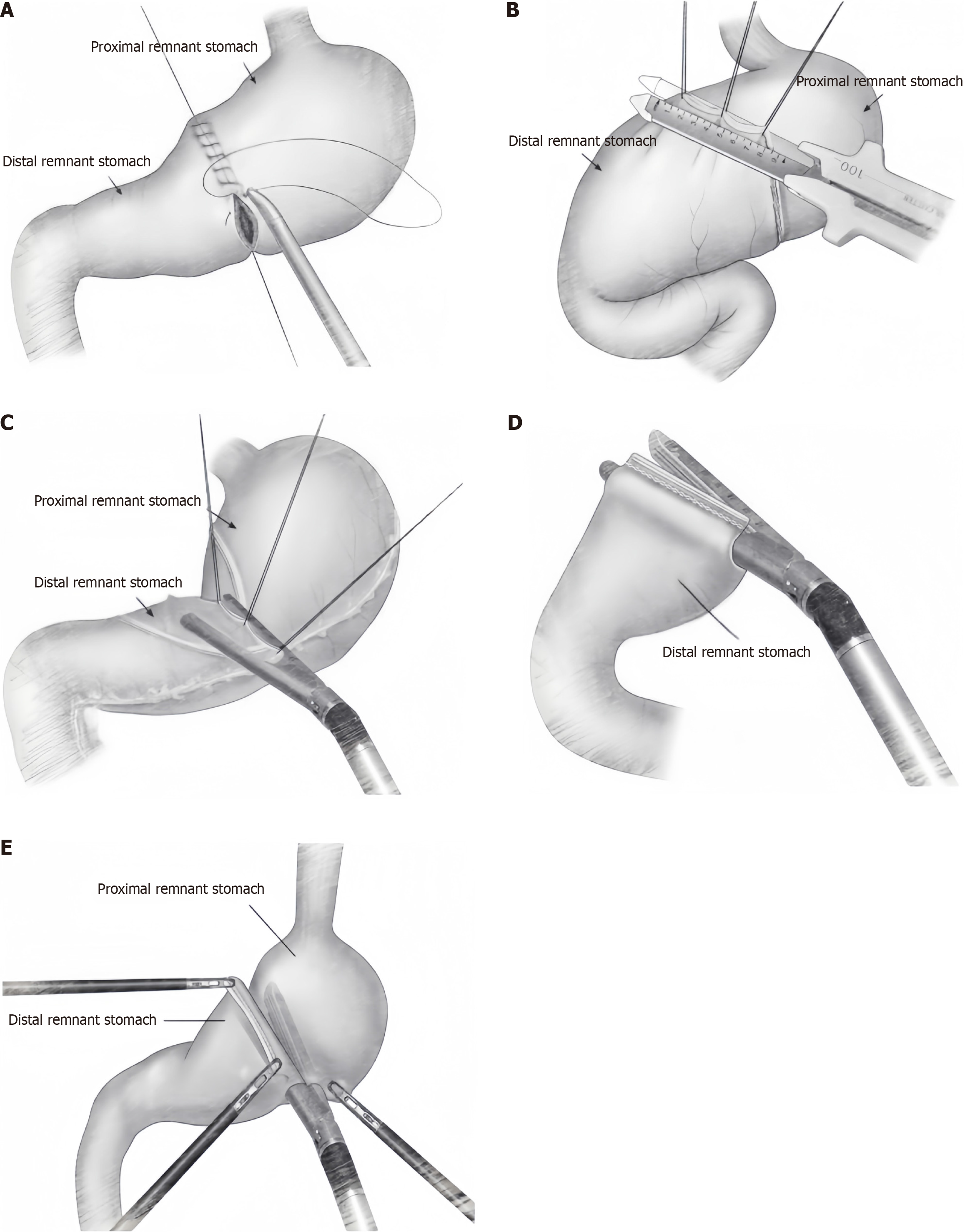
The reconstruction of the digestive tract via PPG involves anastomosis between the remaining parts of the stomach. Numerous scholars have proposed various methods for this type of anastomosis; however, a standardized technique has yet to be established. The traditional digestive tract reconstruction method for PPG involves end-to-end manual anastomosis of the remnant stomach (Figure 1A). This process begins with suturing the seromuscular layer of the posterior gastric wall, followed by full-thickness suturing, and concludes with full-thickness suturing of the anterior gastric wall. As laparoscopic techniques have advanced, the Billroth I triangular anastomosis method has been increasingly adopted for laparoscopic PPG (Figure 1B). However, laparoscopic triangular anastomosis, which is a side-to-side anastomosis of the posterior gastric wall, shortens the effective length of the pyloric sleeve and may increase the risk of postoperative delayed gastric emptying and retention. Some Japanese scholars prefer completely laparoscopic delta anastomosis (Figure 1C), with manual suturing of the common opening, a method that has been proven safe and reliable[42]. Ohashi et al[43] introduced a modified fully laparoscopic “Pierce” anastomosis method using a linear stapler (Figure 1D). This technique involves creating a small opening on the lesser curvature side of the pyloric sleeve stump near the closure line, allowing the anvil of the linear stapler to pass through the lesser curvature of the stomach. This approach addresses the issue of incomplete one-time closure, and subsequently, the common opening is closed and transected together with the opening of the pyloric sleeve. Theoretically, this method more closely mimics physiological end-to-end anastomosis, providing a more satisfactory pyloric sleeve length and larger anastomotic diameter, thus reducing the likelihood of anastomotic stenosis.
Figure 1 Anastomosis surgery.
A: End-to-end manual anastomosis; B: Billroth I triangular anastomosis; C: Laparoscopic delta anastomosis; D: Laparoscopic “Pierce” anastomosis; E: Laparoscopic overlap anastomosis.
For tumours in the upper posterior wall of the gastric body, Park et al[44] developed a novel fully laparoscopic overlap anastomosis method (Figure 1E). This technique involves rotating the proximal stomach to anastomose the anterior wall of the proximal remnant stomach with the posterior wall of the distal remnant stomach, ensuring a safe resection margin and appropriate anastomotic tension. Our centre favours manual anastomosis for digestive tract reconstruction. Through a small abdominal wall incision, direct observation of the blood supply to the resection margin and its distance from the pylorus is possible. If the margin is unsatisfactory, immediate corrective action can be taken. Direct manual suturing offers greater precision and reduces the likelihood of anastomotic stenosis. This approach is particularly beneficial for patients with thickened gastric walls, avoiding potential complications or failures associated with linear staplers. Additionally, intraoperative dilation of the pylorus using oval forceps before anastomosis can prevent postoperative pyloric stenosis. Our centre routinely employs 3–0 absorbable sutures for anastomosis. When anterior gastric wall anastomosis is suboptimal, additional suturing of the seromuscular layer can be performed to reinforce the anastomosis.
Some researchers have conducted comparisons of the various digestive tract reconstruction methods mentioned above in patients with PPG. Their findings indicate that there are no significant differences among these methods regarding oncological safety and postoperative complications[45]. More reports on various laparoscopic PPG digestive tract reconstruction methods are being published in recent years. Surgeons can select an appropriate reconstruction method on the basis of specific intraoperative conditions, personal experience, and preferences. However, determining the superior digestive tract reconstruction method requires more robust evidence-based medical support in future studies.
PREVENTION AND TREATMENT OF POSTOPERATIVE COMPLICATIONS IN LAPAROSCOPIC PPG
The common complications associated with PPG are comparable to those observed following distal subtotal gastrectomy. The Korean KLASS-04 multicentre randomized controlled trial evaluated the postoperative outcomes of 124 patients who underwent PPG and 129 patients who underwent distal subtotal gastrectomy[46]. The overall complication rates were 19.3% for PPG and 15.5% for distal gastrectomy (P = 0.419). No statistically significant differences were noted in the rates of wound infection (4.8% vs 2.3%), intraluminal bleeding (1.6% vs 0.8%), intra-abdominal bleeding (0% vs 1.6%), anastomotic leakage (1.6% vs 1.5%), pancreatitis (0% vs 0.8%), or ileus (0% vs 2.3%) (P > 0.005). Additionally, there was no statistically significant difference in the distribution of complication grades (P > 0.005). Delayed gastric emptying is a notable complication specific to PPG procedures; the results from the KLASS-04 trial indicated that the incidence rate of delayed gastric emptying was significantly higher in the PPG group (6.5%) than in the distal gastrectomy group (no cases) (P = 0.003)[46]. Other related studies have reported that the incidence of delayed gastric emptying ranges from approximately 5.2% to 8.3%[47,48]. Although PPG preserves the physiological structure and function of the pylorus, thereby maintaining food storage and gastric emptying, some patients still experience postprandial fullness, indicating gastric emptying disorders post-PPG. These disorders lead to food retention, resulting in decreased subjective quality of life and impacting endoscopic follow-up examinations. Preventing and managing this condition during and after the surgical procedure is a critical aspect that merits thorough discussion. Eom et al[49] identified delayed gastric emptying as the primary complication of laparoscopic PPG, with a significantly greater incidence than that of laparoscopic distal gastrectomy. Interestingly, two years after surgery, the subjective perception of gastric retention symptoms did not differ between PPG patients and distal gastrectomy patients. However, gastric scintigraphy revealed a significantly greater incidence of food retention in the remnant stomach in the PPG group than in the distal gastrectomy group[50]. Park et al[29] utilized nuclear imaging to demonstrate that although liquid food emptying times were similar between PPG and distal gastrectomy, solid food emptying was significantly prolonged after PPG. Conversely, some studies have shown improvements in postprandial discomfort and food intake two years after PPG, suggesting the possibility of gradual recovery of pyloric function over time[51]. Takahashi et al[52] identified sex, age, BMI, comorbid diabetes, and abdominal infections as factors associated with post-PPG gastric retention, with sex, age, and BMI emerging as independent predictors. Specifically, the likelihood of postoperative gastric retention is greater among elderly, male, and obese patients.
Unlike traditional postoperative gastroparesis syndrome, gastrointestinal motility nerve dysregulation may not be the primary factor in gastric emptying disorders following PPG. Current findings suggest that postoperative pyloric oedema and vagus nerve imbalance are key contributors to these disorders. Consequently, we emphasize the importance of preserving the blood supply to the pyloric area and maintaining an appropriate pyloric canal length during surgery while also preserving relevant vagus nerve branches. Intraoperative preservation of the infrapyloric vein can improve blood return from the pyloric area, with studies demonstrating that this technique significantly reduces the incidence of postoperative gastric retention[53].
The Japanese Gastric Cancer Treatment Guidelines currently recommend PPG for tumours > 4 cm from the pylorus. However, some patients still experience postoperative gastric emptying disorders. Ongoing research into gastric emptying dynamics has led some scholars to explore increasing the distance between the pylorus and the distal gastric resection line to mitigate these disorders. From 1989 to 2000, surgeons typically preserved a pyloric canal length of 1.5 cm; after 2000, increasing this length to > 3.0 cm reduced the incidence of delayed gastric emptying[54]. Namikawa et al[55] suggested that preserving a pyloric canal length of 3.0–5.0 cm via PPG yields optimal postoperative gastric function recovery. On the basis of these findings, we recommend a preserved pyloric canal length of ≥ 3.0 cm in PPG. Crucially, preoperative endoscopic tumour localization is essential to ensure margin safety, with intraoperative gastroscopy relocalization and rapid frozen section pathological examination of margins performed when necessary.
When digestive tract reconstruction is performed through an extracorporeal abdominal incision during surgery, surgeons can employ intestinal clamps to dilate the pyloric canal, thereby mitigating the risk of postoperative complications, such as pyloric stenosis and delayed gastric emptying[56]. Our centre routinely performs intraoperative dilation of the pyloric canal to mitigate the aforementioned complications.
In cases where gastric emptying disorders occur, conservative treatments—including fasting, gastrointestinal decompression, and parenteral nutrition—are typically the initial course of action. For patients who do not respond to conservative treatment, endoscopic balloon dilation often proves beneficial. When endoscopic balloon dilation is ineffective, endoscopic pyloric stent implantation may be considered, but this procedure carries a risk of stent displacement, leading to failure[57]. Notably, some researchers have explored the use of botulinum toxin injections to address postoperative pyloric stenosis, with encouraging results[58].
CONCLUSION
Function-preserving gastrectomy has emerged as the predominant approach for early gastric cancer surgery. PPG ensures oncological safety through radical tumour resection and lymph node dissection while simultaneously reducing both short-term and long-term complications associated with gastrectomy, thus increasing patients’ quality of life. Combining PPG with laparoscopic techniques further highlights its minimally invasive advantages and promotes rapid recovery. In recent years, the maturation of laparoscopic PPG techniques has been driven by increased early detection rates of gastric cancer, advancements in surgical instruments, and the evolution of laparoscopic technology. Continuous validation through numerous clinical studies has established a robust evidence-based foundation for implementing PPG. As a result, laparoscopic PPG represents an ideal surgical approach for eligible early gastric cancer patients. As more compelling evidence-based medical support accumulates and the concept of function preservation gains wider acceptance, we anticipate that laparoscopic PPG will be increasingly widely implemented in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade C
P-Reviewer: Liang H; Wang L S-Editor: Luo ML L-Editor: A P-Editor: Zhao YQ