Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.105220
Revised: February 27, 2025
Accepted: March 21, 2025
Published online: May 27, 2025
Processing time: 128 Days and 21.1 Hours
Hepatic perivascular epithelioid cell tumor (PEComa) is an extremely rare neo
A 36-year-old woman presented with a liver mass discovered during a routine physical examination. Initially diagnosed as focal nodular hyperplasia based on contrast-enhanced computed tomography, she declined surgical intervention. Two years later, re-examination revealed an approximately 60% increase in the size of the mass and suggested the possibility of hepatocellular carcinoma. She subsequently underwent surgical resection. Postoperative histopathological and immunohistochemical analysis revealed positivity for HMB-45 and Melan-A, confirming the diagnosis of hepatic PEComa. A 13-month follow-up revealed no recurrence or metastasis.
Hepatic PEComa requires a combination of radiological and immunohistoche
Core Tip: Hepatic perivascular epithelioid cell tumor is a rare liver tumor with nonspecific clinical and imaging features, often leading to misdiagnosis. This case highlights the importance of combining radiological and immunohistochemical evaluations for accurate diagnosis. The significant growth of the lesion observed in this patient emphasizes the need for timely surgical intervention. Long-term follow-up is essential to monitor for recurrence or metastasis.
- Citation: Zhou JA, Fan ZC, Zheng RJ, Guo QX, Su S. Diagnostic challenges and radiological insights of rare hepatic perivascular epithelioid cell tumor: A case report and review of the literature. World J Gastrointest Surg 2025; 17(5): 105220
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/105220.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.105220
Perivascular epithelioid cell tumors (PEComas) are a rare group of neoplasms characterized by epithelioid cells with perivascular disposition, expressing myogenic and melanocytic markers[1]. In most cases, PEComas primarily affect the uterus. However, PEComas can also occur in other organs such as the intestines, lungs, and stomach[2]. Only a few cases of PEComa growing from the liver have been reported, with the majority being benign[3]. Malignant hepatic PEComa is extremely rare. Due to the current lack of globally standardized imaging and laboratory diagnostic criteria, primary hepatic PEComa can easily be misidentified as other liver tumors[4]. Accurate identification of this rare liver tumor is crucial, as misdiagnosis can have a significant adverse impact on the patient's prognosis and treatment[5]. We report a case of hepatic PEComa discovered in a young woman that was misdiagnosed as hepatic focal nodular hyperplasia (FNH) and hepatocellular carcinoma (HCC).
A 36-year-old woman was admitted due to a liver lesion discovered during physical examination.
At the time of presentation, the patient was asymptomatic, with no complaints related to the liver lesion. She denied experiencing fever, weight loss, or any other systemic symptoms.
The patient had no relevant past medical history that could suggest the origin of the liver lesion.
The patient had no history of alcohol consumption or tobacco use. There were no reports of viral hepatitis or previous abdominal surgeries. She had no known genetic disorders, and her family medical history was not notable.
Physical examination was unremarkable.
Liver function tests, viral markers, and digestive system tumor markers (such as alpha-fetoprotein and carbohydrate antigen 19-9) were within normal limits.
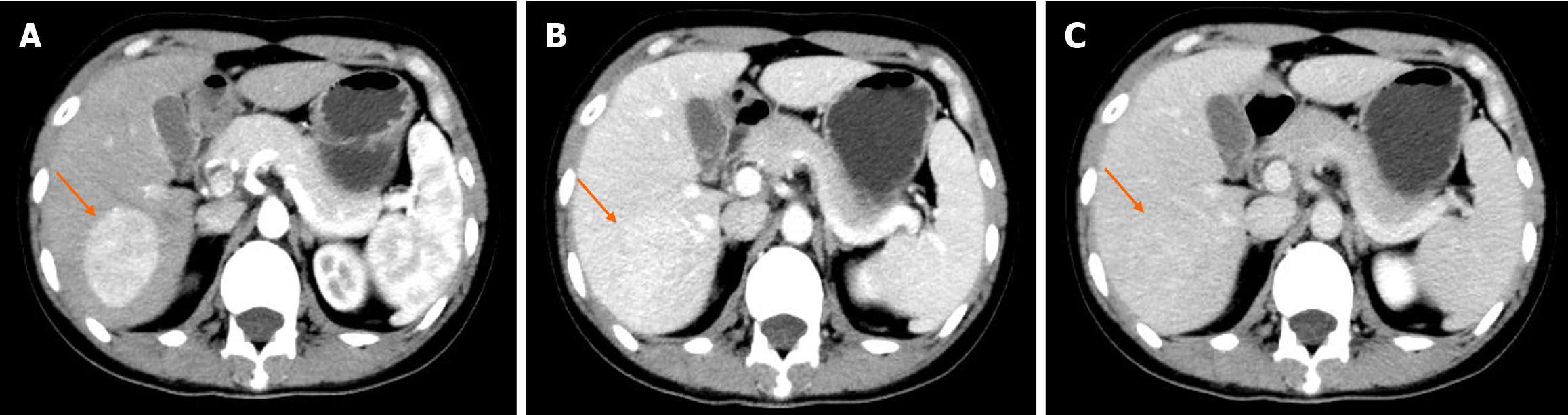
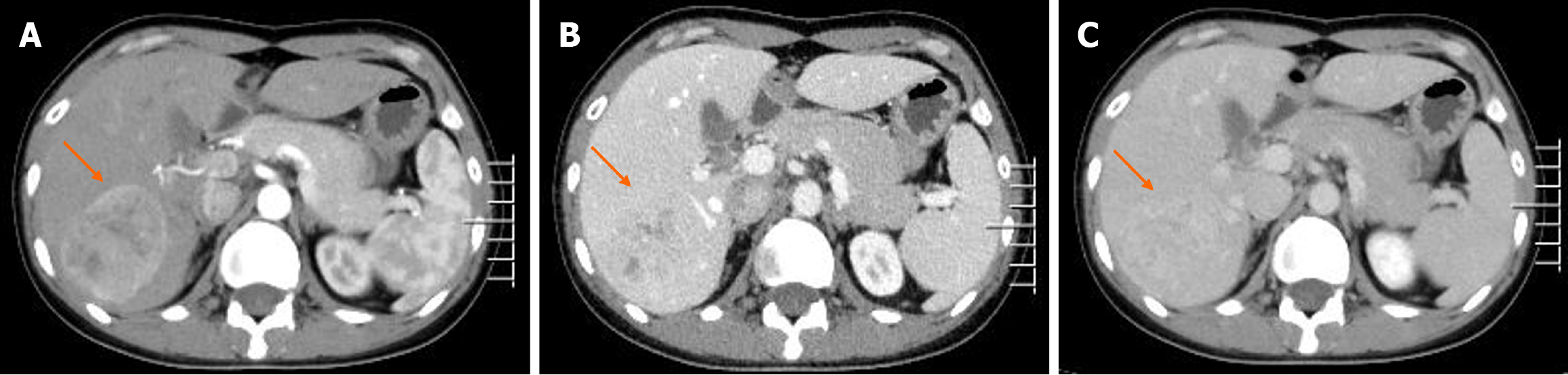
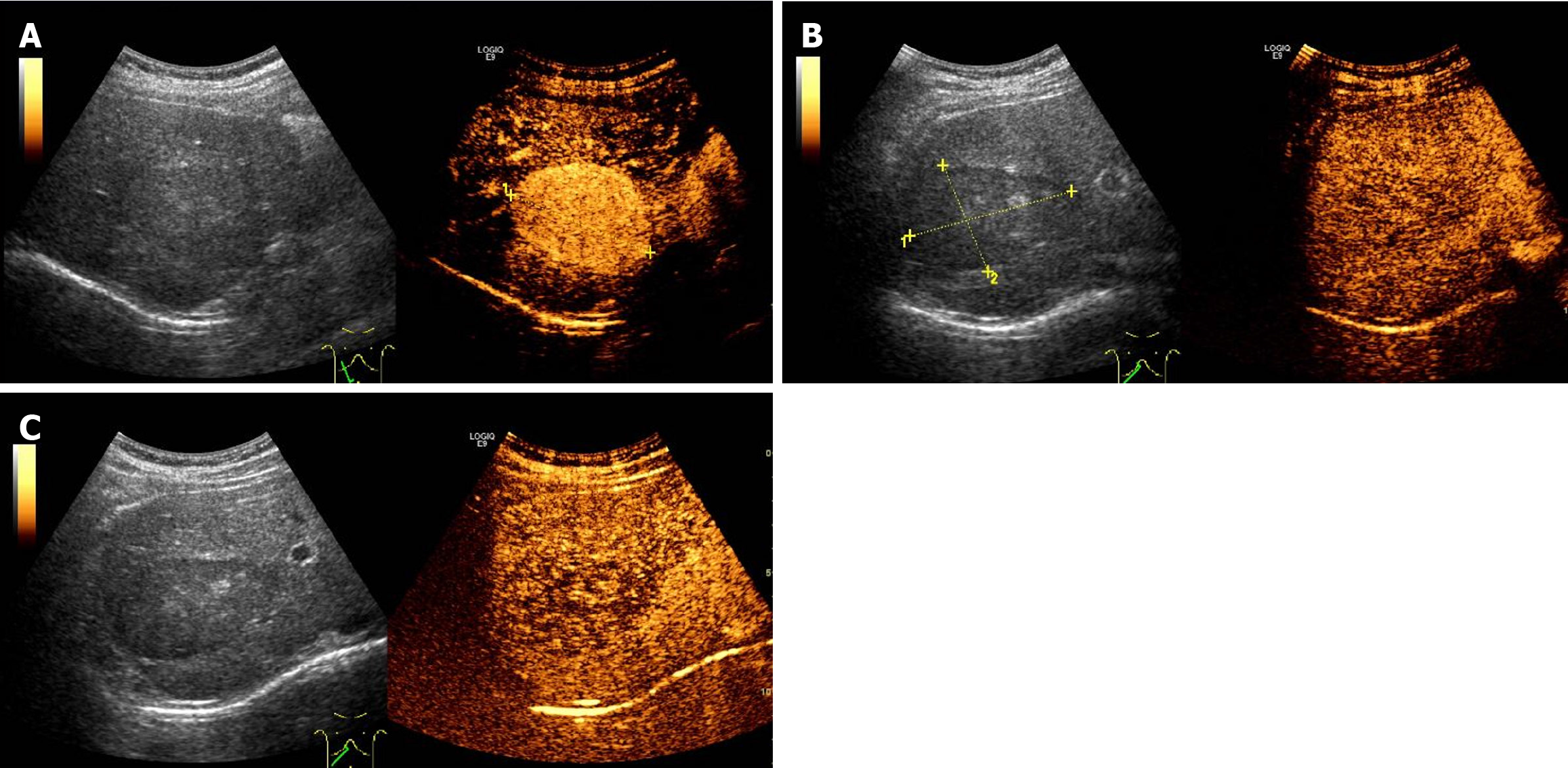
Contrast-enhanced computed tomography (CT) showed a well-demarcated lesion measuring 5.0 cm × 3.9 cm in hepatic segment VII. The lesion exhibited significant and heterogeneous enhancement in the arterial phase, and less enhancement in the portal vein and delayed phases. However, the density of the lesion remained higher than that of the liver parenchyma (Figure 1). Initially, a diagnosis of hepatic FNH was suspected, but other diseases were not ruled out. We suggested surgical treatment, but the patient declined. Consequently, we recommended follow-up every 3 months, but the patient was lost to follow-up. Two years later, the patient was admitted to the hospital again. Contrast-enhanced CT revealed that the size of the lesion was approximately 6.2 cm × 5.2 cm, which represents an increase of approximately 60% compared to 2 years ago (Figure 2). Additionally, contrast-enhanced ultrasound (CEUS) revealed a well-defined, regularly shaped lesion. In the arterial phase, the lesion demonstrated homogeneous centripetal enhancement. In the portal vein phase, the lesion began to wash out slowly, and this washout persisted in the delayed phase, presenting as slightly hypoechoic (Figure 3). Imaging examinations suggested the possibility of HCC.
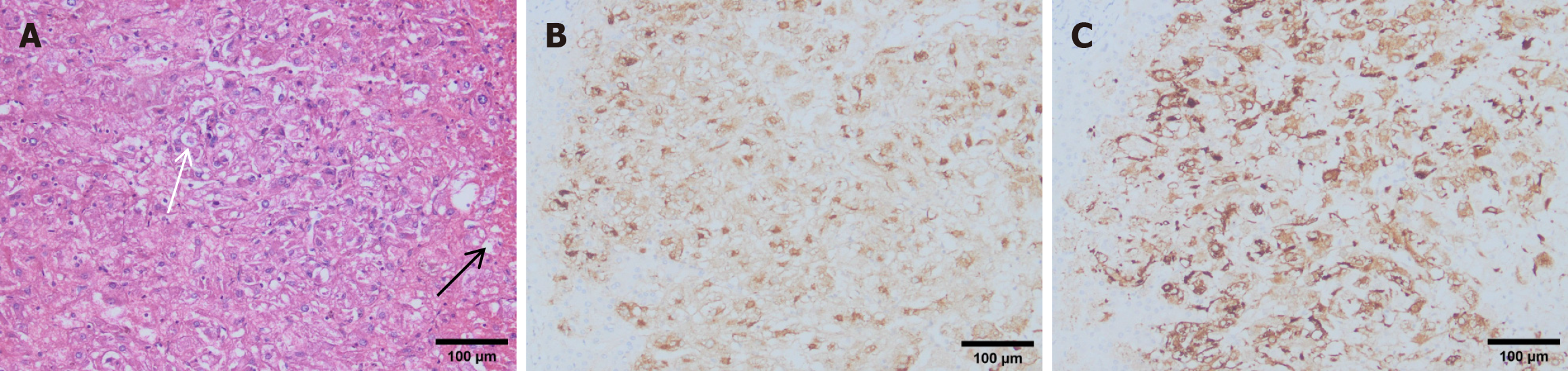
Histologically, the tumor was primarily composed of proliferating epithelioid cells, with a small number of adipocytes (Figure 4A). Immunohistochemical staining was negative for CD10 and S-100 but positive for CD34. The melanocyte markers HMB-45 and Melan-A were strongly expressed (Figure 4B and C). The pathological examination results supported the diagnosis of hepatic PEComa.
Because of the concern for an enlarging hepatic mass with unknown malignant potential, the clinician recommended surgical resection. The patient ultimately underwent laparoscopic hepatic segmentectomy using a right posterior approach. Intraoperative ultrasound was used to precisely locate the tumor, allowing for an anatomical liver resection that ensured adequate surgical margins while preserving as much normal liver tissue as possible. Special care was taken to protect major vascular and bile duct structures during the procedure, and no significant complications were observed. Postoperative management included close monitoring of vital signs and liver function, prevention of complications, rational use of antibiotics and analgesics, gradual initiation of rehabilitation activities, and nutritional support to promote liver function recovery.
After 13 months of close follow-up, the patient remained asymptomatic with good quality of life.
In this report, we describe a young woman with a liver lesion that was initially misdiagnosed as FNH and subsequently as HCC. However, the postoperative pathological results revealed hepatic PEComa.
PEComa was first reported in 1992 by Bonetti et al[6]. The World Health Organization firstly defined it as mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells in 2002[7]. PEComa most commonly occurs in the uterus, followed by the urinary system, gastrointestinal tract, and retroperitoneum, while its occurrence in the liver is extremely rare[8,9].
Currently, most reported cases of hepatic PEComa in the literature exhibit benign characteristics[10-14]. However, cases of locally invasive and metastatic hepatic PEComa have also been documented[15-18]. Among these, malignant hepatic PEComas > 5 cm in diameter are more prone to recurrence or metastasis, involving multiple abdominal organs, including the omentum, and in severe cases, causing massive abdominal hemorrhage. In the present case, the patient initially refused surgical intervention, and the tumor volume increased by approximately 60% over 2 years. This observation suggests that even benign hepatic PEComas have a high potential for proliferation. This finding underscores the importance of early intervention to mitigate potential risks.
Currently, diagnostic experience with hepatic PEComa remains limited, and the preoperative misdiagnosis rate is high. This phenomenon may result from multiple contributing factors. Most patients with hepatic PEComa are asymptomatic or present only with nonspecific symptoms, such as abdominal pain or nausea[16,19]. In rare cases, an abdominal mass may be the only clinical sign[20]. Furthermore, there are no specific biochemical markers for hepatic PEComa. Common gastrointestinal tumor markers, such as carcinoembryonic antigen, alpha-fetoprotein, and carbohydrate antigen 19-9, are usually not elevated and are only useful for differential diagnosis of hepatic masses.
In terms of imaging features, enhanced CT and CEUS findings in this case demonstrated significant arterial-phase enhancement with washout in the portal and delayed phases. This pattern is consistent with the reports by Matrood et al[2] and Liu et al[21]. On magnetic resonance imaging (MRI), hepatic PEComa exhibits a similar enhancement pattern[17,22]. However, these imaging characteristics are not specific, as HCC, hepatic adenoma (HCA), and FNH can also display similar imaging features[23,24]. Some studies have suggested that vascular proliferation and arteriovenous features observed on enhanced CT or MRI may serve as important diagnostic clues for PEComa[25]. Gao et al[26] highlighted specific CEUS characteristics of hepatic PEComa, including lateral shadows, a "fast-in and slow-out" enhancement pattern, peripheral hyperechoic areas, large surrounding vessels, and rich tumor vascularity. Another distinctive imaging feature of hepatic PEComa is the coexistence of fatty and solid components, manifested as fat-density regions. In contrast, FNH typically lacks fatty components, and HCA only contains a small amount of fat. Therefore, the presence of pronounced fatty components in PEComa offers a diagnostic advantage. However, variations in the proportions of fat, smooth muscle, and vascular tissue in PEComa result in diverse imaging presentations. Moreover, fat-containing HCC may mimic PEComa on imaging, increasing the likelihood of diagnostic errors by radiologists[27]. The overlap of imaging features, the heterogeneity of tumor composition, and the rarity of hepatic PEComa collectively contribute to the high rate of preoperative misdiagnosis based on imaging studies.
Preoperative fine-needle aspiration pathology assessment plays a crucial role in the accurate diagnosis of PEComa. However, studies have reported a misdiagnosis rate of approximately 15% even after pathological biopsy, which may be attributed to the histological heterogeneity of the tumor and insufficient biopsy sample size[28]. Immunohistochemical markers are therefore critical in diagnosing PEComa. HMB-45 and Melan-A, along with smooth muscle actin, exhibit strong positive reactions in nearly all reported cases of hepatic PEComa. These markers are essential for differentiating PEComa from other hepatic tumors such as FNH, HCA, and HCC. The expression of other markers, such as S100 and vimentin, shows variability across studies[29,30], potentially due to tumor heterogeneity, liver-specific microenvironments, and differentiation characteristics. As a result, preoperative biopsy testing for specific immunohistochemical markers such as HMB-45 and Melan-A is recommended and may significantly enhance the accuracy of preoperative diagnosis.
The development of PEComa is closely associated with mutations in the TSC1 and TSC2 genes. Tuberous sclerosis complex (TSC) is a hereditary disorder that can cause benign tumors in multiple organs. Mutations in TSC1 and TSC2 genes lead to abnormal activation of the mammalian target of rapamycin (mTOR) signaling pathway, promoting tumor progression. This mechanism suggests that PEComa represents an extrarenal manifestation of TSC, particularly in patients with a family history of TSC[31,32]. This finding has opened new avenues for targeted therapy of PEComa. Wagner et al[33] demonstrated the efficacy of the mTOR inhibitor sirolimus in treating three cases of PEComa, with CT scans confirming the effectiveness of monotherapy. Similarly, another study reported significant tumor shrinkage in a hepatic PEComa patient after 8 months of neoadjuvant sirolimus treatment, enabling successful surgical resection with favorable outcomes[34]. These findings underscore the potential of mTOR inhibitors in PEComa treatment. However, larger-scale clinical trials and studies exploring combination therapies, such as mTOR inhibitors with immunotherapy, are needed to further optimize patient outcomes.
Currently, complete surgical resection with negative margins remains the gold standard for treating hepatic PEComa, and it is curative in most cases. Nevertheless, given the risks of recurrence or metastasis, long-term postoperative follow-up is essential. In 2000, Dalle et al[35] reported the first case of malignant hepatic PEComa, where the patient died of recurrent disease 7 months after tumor resection. Subsequently, Liu et al[17] reported a recurrence and death 2 years after surgery, and Parfitt et al[19] described a case of recurrence 9 years after surgery. These reports highlight the potential for late recurrence, even in the absence of early postoperative signs. In the current case, the patient remained recurrence-free 13 months after surgery, indicating a favorable short-term prognosis. However, ongoing monitoring is crucial to evaluate long-term outcomes.
The prevalence of PEComa varies across populations and geographical regions. Overall, the incidence is low, with most cases being sporadic. In patients with TSC, the risk of developing PEComa is significantly increased, and the age of onset tends to be younger. Asia, particularly China and Japan, has reported a higher number of cases. Currently, the association with environmental factors, such as pollution or radiation, remains unclear. Future studies are needed to elucidate the roles of genetic and environmental factors in PEComa development and to clarify its epidemiological and pathophysiological characteristics through multicenter investigations.
We have reported a case of liver mass initially misdiagnosed as FNH and HCC, which was later confirmed as benign hepatic PEComa following surgical resection. Accurate diagnosis relies on comprehensive imaging examinations and pathological biopsy, particularly immunohistochemical staining (e.g., positive for Melan-A and HMB-45). Surgical resection remains the primary treatment modality, with mTOR inhibitors potentially offering effective adjunctive therapy. In the present case, the tumor grew approximately 60% over 2 years, indicating significant proliferative potential and underscoring the importance of early surgical intervention. Postoperative follow-up is essential, and a long-term management plan should be implemented to optimize patient outcomes.
| 1. | Perán Fernández C, de Paco Navaro Á, Castañer Ramón-Llin J, Bertelli Puche J, Sánchez Espinosa A. Perivascular epithelioid cell tumor (PEComa) of the liver. An extremely rare diagnosis. Rev Esp Enferm Dig. 2023;115:348-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Matrood S, Görg C, Safai Zadeh E, Alhyari A. Hepatic perivascular epithelioid cell tumor (PEComa): contrast-enhanced ultrasound (CEUS) characteristics-a case report and literature review. Clin J Gastroenterol. 2023;16:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Revilla López J, Enciso Chancahuana R, Meza Cruzado S, Meléndez Ríos F, Negrón Abril YL, Sumarriva D, Samec T, Sullcahuaman Allende Y, Chávez Passiuri I, Casanova Marquez L, Carracedo Gonzáles C. Next-Generation Sequencing: Key for Diagnosing Angiomyolipoma - A Case Report. Case Rep Oncol. 2025;18:247-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Kou YQ, Yang YP, Ye WX, Yuan WN, Du SS, Nie B. Perivascular epithelioid cell tumors of the liver misdiagnosed as hepatocellular carcinoma: Three case reports. World J Clin Cases. 2023;11:426-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Skaret MM, Vicente DA, Deising AC. An Enlarging Hepatic Mass of Unknown Etiology. Gastroenterology. 2021;160:e14-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 308] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Jo VY, Doyle LA. Refinements in Sarcoma Classification in the Current 2013 World Health Organization Classification of Tumours of Soft Tissue and Bone. Surg Oncol Clin N Am. 2016;25:621-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 9. | Calame P, Tyrode G, Weil Verhoeven D, Félix S, Klompenhouwer AJ, Di Martino V, Delabrousse E, Thévenot T. Clinical characteristics and outcomes of patients with hepatic angiomyolipoma: A literature review. World J Gastroenterol. 2021;27:2299-2311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Baek JH, Chung MG, Jung DH, Oh JH. Perivascular epithelioid cell tumor (PEComa) in the transverse colon of an adolescent: a case report. Tumori. 2007;93:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Abhirup B, Kaushal K, Sanket M, Ganesh N. Malignant hepatic perivascular epithelioid cell tumor (PEComa) - Case report and a brief review. J Egypt Natl Canc Inst. 2015;27:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Son HJ, Kang DW, Kim JH, Han HY, Lee MK. Hepatic perivascular epithelioid cell tumor (PEComa): a case report with a review of literatures. Clin Mol Hepatol. 2017;23:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ma Y, Huang P, Gao H, Zhai W. Hepatic perivascular epithelioid cell tumor (PEComa): analyses of 13 cases and review of the literature. Int J Clin Exp Pathol. 2018;11:2759-2767. [PubMed] |
| 14. | Krawczyk M, Ziarkiewicz-Wróblewska B, Wróblewski T, Podgórska J, Grzybowski J, Gierej B, Krawczyk P, Nyckowski P, Kornasiewicz O, Patkowski W, Remiszewski P, Zając K, Grąt M. PEComa-A Rare Liver Tumor. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Rouquie D, Eggenspieler P, Algayres JP, Béchade D, Camparo P, Baranger B. [Malignant-like angiomyolipoma of the liver: report of one case and review of the literature]. Ann Chir. 2006;131:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Selvaggi F, Risio D, Claudi R, Cianci R, Angelucci D, Pulcini D, D'Aulerio A, Legnini M, Cotellese R, Innocenti P. Malignant PEComa: a case report with emphasis on clinical and morphological criteria. BMC Surg. 2011;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Liu D, Shi D, Xu Y, Cao L. Management of perivascular epithelioid cell tumor of the liver: A case report and review of the literature. Oncol Lett. 2014;7:148-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 637] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 19. | Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Strzelczyk JM, Durczynski A, Szymanski D, Jablkowski M, Dworniak D, Sporny S. Primary perivascular epithelioid cell tumor (PEComa) of the liver: report of a case. Surg Today. 2009;39:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Liu J, Zhang CW, Hong DF, Tao R, Chen Y, Shang MJ, Zhang YH. Primary hepatic epithelioid angiomyolipoma: A malignant potential tumor which should be recognized. World J Gastroenterol. 2016;22:4908-4917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Tan Y, Xiao EH. Hepatic perivascular epithelioid cell tumor (PEComa): dynamic CT, MRI, ultrasonography, and pathologic features--analysis of 7 cases and review of the literature. Abdom Imaging. 2012;37:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Harwal R, Joseph Rosemary LJ, Raju P, Chidambaranathan S, Bharathi Vidya Jayanthi J, Obla Lakshmanamoorthy NB. Hepatic Perivascular Epithelioid Cell Tumor Mimicking Hepatocellular Carcinoma. ACG Case Rep J. 2023;10:e00962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D'Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 25. | Fang SH, Zhou LN, Jin M, Hu JB. Perivascular epithelioid cell tumor of the liver: a report of two cases and review of the literature. World J Gastroenterol. 2007;13:5537-5539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Gao X, Tang H, Wang J, Yao Q, Wang H, Wang Y, Ma M, Yang W, Yan K, Wu W. Specific imaging features indicate the clinical features of patients with hepatic perivascular epithelioid cell tumor by comparative analysis of CT and ultrasound imaging. Front Oncol. 2022;12:908189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Klompenhouwer AJ, Verver D, Janki S, Bramer WM, Doukas M, Dwarkasing RS, de Man RA, IJzermans JNM. Management of hepatic angiomyolipoma: A systematic review. Liver Int. 2017;37:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Yan S, Lu JJ, Chen L, Cai WH, Wu JZ. Hepatic perivascular epithelioid cell tumors: The importance of preoperative diagnosis. World J Gastroenterol. 2024;30:1926-1933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Amante MF. Hepatic perivascular epithelioid cell tumors: Benign, malignant, and uncertain malignant potential. World J Gastroenterol. 2024;30:2374-2378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Zhang S, Yang PP, Huang YC, Chen HC, Chen DL, Yan WT, Yang NN, Li Y, Li N, Feng ZZ. Hepatic perivascular epithelioid cell tumor: Clinicopathological analysis of 26 cases with emphasis on disease management and prognosis. World J Gastroenterol. 2021;27:5967-5977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Plas DR, Thomas G. Tubers and tumors: rapamycin therapy for benign and malignant tumors. Curr Opin Cell Biol. 2009;21:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Scheppach W, Reissmann N, Sprinz T, Schippers E, Schoettker B, Mueller JG. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J Gastroenterol. 2013;19:1657-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH, Demetri GD, Kwiatkowski DJ, Maki RG. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 34. | Bergamo F, Maruzzo M, Basso U, Montesco MC, Zagonel V, Gringeri E, Cillo U. Neoadjuvant sirolimus for a large hepatic perivascular epithelioid cell tumor (PEComa). World J Surg Oncol. 2014;12:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |