Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.104267
Revised: February 28, 2025
Accepted: April 2, 2025
Published online: May 27, 2025
Processing time: 157 Days and 22.2 Hours
As an innovative endoscopic intervention, endoscopic ultrasound-guided pan
Core Tip: Endoscopic ultrasound-guided pancreatic drainage (EUS-PD) is an emerging therapeutic technique used to relieve symptoms caused by pancreatic duct obstruction and high pressure. It provides an effective alternative for cases where endoscopic retrograde pancreatography has failed. This review summarizes the latest research advancements and key procedural considerations of EUS-PD, offering practical guidance for clinicians.
- Citation: Wang SY, Zhao SQ, Wang SP, Zhang Y, Sun SY, Wang S. Endoscopic ultrasound-guided pancreatic duct drainage: Progress and future outlook. World J Gastrointest Surg 2025; 17(5): 104267
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/104267.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.104267
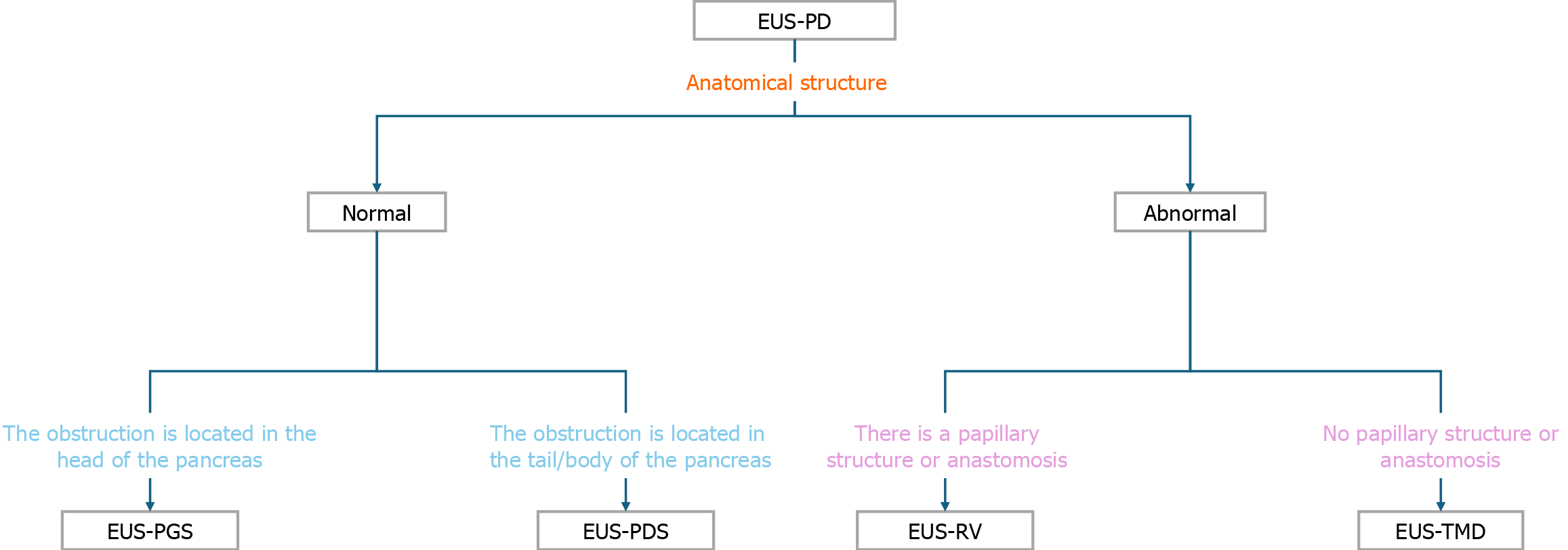
Over the past decade, endoscopic ultrasound (EUS) has revolutionized therapeutic endoscopy[1-7]. Traditional treatment approaches for pancreatic fluid obstruction and elevated pancreatic duct pressure encompass endoscopic retrograde pancreatography (ERP) with stent placement, surgical resection and reconstruction, and percutaneous puncture for pancreatic duct drainage. Among these, surgical interventions entail significant trauma, and percutaneous drainage carries a high risk of complications. Therefore, for symptomatic patients with pancreatic duct hypertension who have experienced ERP treatment failure, a novel alternative has emerged-EUS-guided pancreatic duct drainage (EUS-PD). EUS-PD, apart from minimizing surgical trauma, boasts its primary advantage of precise localization and assessment of pancreatic diseases. It provides clinicians with a more accurate and detailed perspective during the diagnostic and therapeutic processes[8]. Traditional examination methods may pose certain risks and discomfort, while EUS-PD offers a safer and more precise alternative. Through the clarity provided by the EUS probe, physicians can meticulously observe changes in the pancreas, subsequently formulating more precise treatment plans based on these observations. In the last five years, thanks to the widespread application of therapeutic EUS, the operative techniques of EUS-PD have matured. Additionally, the introduction of new complementary instruments and the publication of clinical data from various centers have garnered increasing attention from clinicians[9-11]. EUS-PD, reported for the first time in 2002, encompasses two main techniques: EUS-guided rendezvous (EUS-RV)[12] and EUS-transmural drainage (EUS-TMD)[13]. EUS-RV involves ultrasound-guided puncture of the pancreatic duct, threading a guidewire through the duct into the intestine, followed by the exchange of the duodenoscope to perform classical ERP[14]. EUS-TMD includes two methods: EUS-guided pancreatic-enterostomy, where a stent is placed between the pancreatic duct and the gastrointestinal lumen, and trans enteric antegrade stenting, which involves advancing the stent further into the small intestine through the ampulla or anastomotic site[15,16].
With the continuous advancement of EUS-PD, its potential applications in early diagnosis, therapeutic decision-making, and prognostic evaluation of pancreatic diseases have gained increasing recognition. Although existing studies have demonstrated the significant diagnostic value of EUS-PD in clinical practice, ongoing controversies persist regarding its clinical applicability, technical feasibility, and long-term efficacy. To address these knowledge gaps, this study aims to conduct a systematic evaluation of EUS-PD's diagnostic accuracy, procedural safety profile, and comparative efficacy against conventional imaging modalities [e.g., computed tomography (CT), magnetic resonance imaging] across diverse pancreatic pathologies. Through rigorous analysis of multicenter clinical data, we seek to provide robust theoretical support and empirical evidence to facilitate the clinical adoption and standardized implementation of EUS-PD technology.
The indications for EUS-PD, as summarized in most of the literature, generally encompass patients facing difficulties due to various causes leading to failed ERP or duodenal obstruction (with normal anatomy) and those with surgically altered anatomy following previous surgical interventions. Traditional treatment options, besides ERP, also include surgical resection and reconstruction, as well as percutaneous puncture for pancreatic duct drainage. Surgical interventions involve significant trauma, and percutaneous drainage carries a high risk of complications, making EUS-PD a viable consideration. In addition to the mentioned scenarios, long-standing chronic pancreatitis (CP) leading to main pancreatic duct (MPD) obstruction and pancreatic duct disruption syndrome (DPDS) caused by various factors are other conditions. These etiologies may result in acute recurrent pancreatitis due to potential high-risk factors, making EUS-PD a frontline therapeutic option in such cases[17].
Pancreatic-enteric anastomotic stricture: Pancreatic-enteric anastomotic stricture is a common complication following pancreaticoduodenectomy. Due to the altered anatomy resulting from extensive tissue and organ resection, conventional methods of accessing the duodenum can be challenging. In cases such as post-Whipple procedure with long-standing stents, enteroscopy-assisted ERP (e-ERP) is typically required for treatment. However, the overall success rate of e-ERP is low, especially in cases of severe or complete anastomotic stricture. In such cases, EUS-PD is a feasible and safe alternative option[18-20]. In patients with Pancreatic jejunostomy stricture (PJS) exhibiting anatomical alterations, performing EUS-PD can be challenging. Professor Hiroshi shared a case utilizing forward-viewing echoendoscope (FV-EUS) for the treatment of PJS. Initially, FV-EUS was reported for the drainage of pancreatic pseudocysts. However, studies regarding the drainage of pancreatic pseudocysts primarily focused on demonstrating the feasibility of FV-EUS rather than leveraging its specific characteristics. As literature describing the features of FV-EUS has emerged, its applicability in other procedures has been recognized, such as its use in EUS-guided biliary-duodenal anastomosis, which circumvents the need for dual gastrointestinal punctures[21,22]. Chen et al[23] conducted a comparative study between EUS-guided pancreatic drainage (EUS-PD) and EA-ERP in patients post-pancreaticoduodenectomy. They compared 43 patients who underwent EUS-PD (40 patients with antegrade/transmural approach and 3 patients with RV-ERP) with 35 patients who underwent EA-ERP. EUS-PD showed superior technical success rate (92.5% vs 20%, P < 0.001) and clinical success rate (87.5% vs 23.1%, P < 0.001) compared to EA-ERP[23]. A recent review further confirmed these findings by systematically comparing the outcomes of ERP and EUS-guided PD drainage in patients with pancreatic-enteric anastomotic stricture. The study included a total of 234 patients, with 77 patients undergoing ERP-guided drainage, 145 patients undergoing EUS-guided drainage, and 12 patients undergoing both drainage methods. The results showed that EUS-guided approach was significantly superior to ERP in technical parameters such as pancreatic duct opacification (87% vs 30%, P < 0.001), catheter insertion success rate (79% vs 26%, P < 0.001), and stent placement success rate (72% vs 20%, P < 0.001). Additionally, EUS-PD demonstrated better pain relief[24]. With the further development and maturation of EUS-PD techniques and a decrease in the occurrence of related complications, EUS-PD is expected to become a first-line treatment option for patients with pancreatic-enteric anastomotic stricture and pancreatic ductal hypertension. Due to the altered anatomy, the rendezvous approach can be actively attempted by the operator. Overall, EUS-TMD may be more suitable for patients with anastomotic stricture in terms of success rate.
Inoperable CP: CP is a chronic and refractory disease that requires lifelong treatment and significantly affects patients' quality of life (QoL), imposing a substantial burden on healthcare systems. In recent years, the global incidence of CP has been increasing, and the understanding of its etiology, mechanisms, and treatment options has been evolving[25,26]. The 2018 European Society of Gastrointestinal Endoscopy guidelines recommend extracorporeal shockwave lithotripsy (ESWL) and/or ERP as first-line treatments for CP patients with MPD obstruction in the head/body of the pancreas and no other complications. Surgical treatment may be considered if these methods fail[27,28]. While surgical treatment can provide long-term symptom relief for CP, it is not universally applicable for high-risk individuals such as the elderly, those with poor nutritional status, or those with severe systemic diseases. Additionally, some patients may have distorted and deformed pancreatic ducts due to chronic inflammation, making conventional ERP procedures unsuccessful. EUS-PD offers a minimally invasive and safe decompression treatment for these patients[4,29]. Parhiala et al[30] reviewed multiple databases and analyzed data from 1327 CP patients, of whom 260 (22%) underwent endoscopic treatment. These patients had higher scores on QoL questionnaires (P = 0.047) and better symptom relief. CP patients with pancreatic stents had similar pain patterns compared to the reference population, but further randomized trials are needed to validate the impact of endoscopic procedures on CP patients[30]. Rudler et al[31] conducted a retrospective study comparing the outcomes of EUS-guided pancreaticogastrostomy (EUS-PGS) or pancreatic-duodenostomy for postoperative anastomotic stricture and CP. They included a total of 43 patients, of whom 22 underwent treatment for CP strictures. The technical success rate was 95.3% (41/43), and the technical success rate for CP strictures was 90.9%. The clinical success rate was 72.5% (29/40), and the clinical success rate for CP strictures was 70% (14/20). The overall complication rate was 34.9% (15/43), with postoperative pain being the main complication, occurring in 20.9% (9/43) of patients, and stent migration or obstruction occurring in 27.9% (12/43) of patients. There were no differences in technical success rate, clinical success rate, and complication rate between postoperative and CP stricture patients[31,32]. In the short term, the probability of EUS-PD replacing ESWL as the preferred treatment method is low, and it cannot bridge the gap with surgical treatment. However, it provides a feasible alternative for patients who cannot tolerate surgery. For patients suspected of having resectable malignant lesions, surgical treatment should still be considered as the first choice.
Disconnected pancreatic duct syndrome: DPDS refers to the interruption of the connection between the MPD and the digestive tract, leading to the loss of normal anatomical continuity between the upstream pancreatic tissue and the duodenum. It is predominantly caused by acute necrotizing pancreatitis (occurring in approximately 20%-40% of ANP patients[33]), with a smaller number of cases attributed to CP or abdominal trauma. The fractured pancreatic duct fails to transport continuously secreted pancreatic fluid to the duodenum, resulting in pancreatic fluid collection (PFC), pancreatic external fistula, and pancreatitis, among other complications[34-36]. In 2022, Kale et al[37] utilized EUS-RV technique to treat a patient with DPDS and pancreatic pleural fistula caused by acute necrotizing pancreatitis. The patient had a pancreatic duct diameter of approximately 1.7 mm, so the operator employed a 22G fine-needle aspiration (FNA) needle for puncture. Following contrast injection, a 0.018-inch guidewire was passed through the duodenum while maintaining its position. A duodenoscope was then substituted while the guidewire was pulled out using grasping forceps. Subsequently, a retrograde cholangiopancreatography catheter was threaded through the pancreatic duct, and the guidewire was replaced with a 0.025 inch one, advancing it all the way to the tail of the pancreas. After sphincterotomy, a 5 Fr pancreatic stent was placed at the site of leakage in the tail region, which was removed on postoperative day 10. The patient was discharged on day 14 after stabilization[38]. Most DPDS (approximately 47%) are in the body of the pancreas. A quantitative analysis conducted by Chong et al[39] included 30 studies and collected data from 1355 patients. The weighted success rate of endoscopic EUS-TMD (90.6%, 95%CI: 81.0%-95.6%) was significantly higher than that of trans papillary drainage (58.5%, 95%CI: 36.7%-77.4%). A paired meta-analysis showed comparable success rates between endoscopic and surgical interventions, with 82% (weighted 95%CI: 68.6-90.5) and 87.4% (95%CI: 81.2-91.8), respectively (P = 0.389)[39].
Contraindications for EUS-PD encompass general contraindications for endoscopic procedures, such as severe cardiopulmonary dysfunction. Additionally, factors like the inability to visualize the pancreatic duct with EUS and hemodynamic instability leading to inadequate sedation are considered contraindications[17]. Due to the necessity of puncture and dilation during the procedure, patients are required to have appropriate anticoagulant medications and normal coagulation function. In cases of extensive ascites, EUS-PD poses a risk of pancreatic fistula and peritonitis; hence, the presence of ascites is generally considered a contraindication to the procedure.
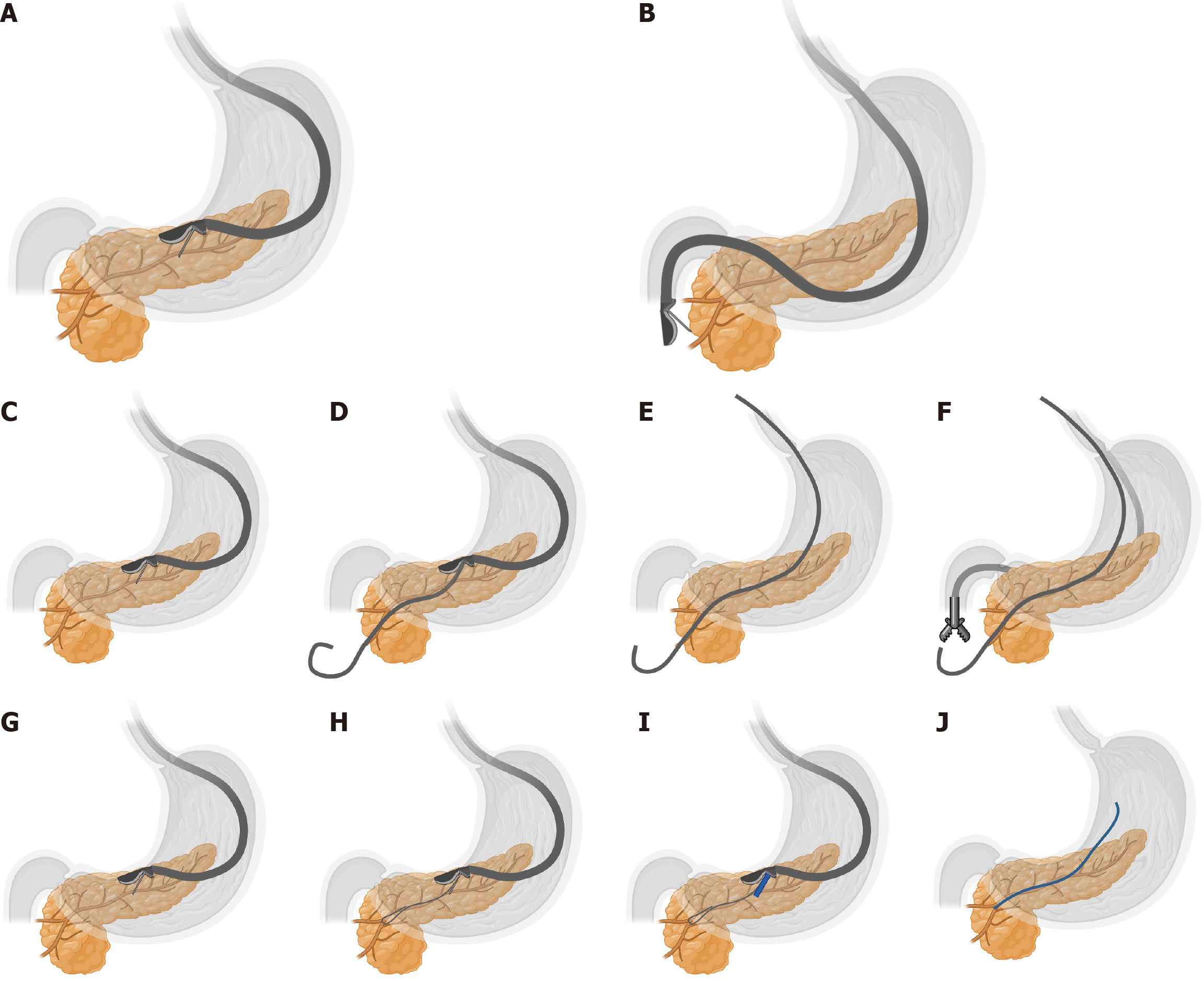
Advance the EUS scope transorally into the gastric or duodenal lumen. Under real-time ultrasonographic guidance, clearly identify the target pancreatic duct branch (diameter ≥ 3 mm) and dynamically avoid adjacent vasculature using color Doppler imaging[10]. Select the puncture route based on anatomical localization: (1) EUS-PGS: Preferred for pancreatic body/tail lesions, with the puncture site typically located on the posterior gastric wall; and (2) EUS-guided pancreaticoduodenostomy: Suitable for pancreatic head lesions, usually performed in the descending duodenum[16] (Figure 1).
During the procedure, retract the 19-gauge (19G) puncture needle into its sheath and advance it through the EUS working channel. Under continuous ultrasound monitoring, gradually extend the needle tip and adjust the puncture trajectory to ensure complete avoidance of vascular structures before penetrating the pancreatic duct (Figure 2A and B).
Stent placement direction criteria: (1) Antegrade placement: Puncture from the pancreatic tail toward the head, ideal for long-segment strictures to maximize stent coverage and drainage efficacy (Figure 2C-F); and (2) Retrograde placement: Puncture from the pancreatic head toward the tail, indicated for proximal obstructions to ensure stent bridging across the stenosis (Figure 2G-J).
Withdraw a small amount of fluid with the puncture needle to confirm clear, colorless pancreatic fluid. Once successful puncture is confirmed, under the supervision of the endoscope and X-ray, inject contrast agent into the pancreatic duct through the needle lumen for duct opacification. Insert a guidewire along the needle lumen, leaving a length of guidewire extending 2-3 coils within the pancreatic duct.
Remove the puncture needle and use the guidewire to guide the insertion of a dilation dilator. The diameter of the dilation tract should match the outer diameter of the drainage tube. Perform pancreatic fluid drainage through the stomach.
The key step in establishing a pathway between the stomach and the pancreatic duct is the successful placement of the stent within the pathway between the stomach and the pancreatic duct.
Insert an internal drainage tube under the guidance of the guidewire, then withdraw the endoscope.
Assuming there is an accessible papillary structure (or an anastomotic site) reachable by endoscopy, one may consider attempting EUS-RV. The steps of RV-ERP differ from guidewire cannulation, requiring the wire to be threaded through the papilla or anastomotic site into the duodenum. Subsequently, a small intestine endoscope is switched, and under the guidance of the wire, a stent is inserted. Compared to transmural methods, RV-ERP preserves anatomical structures, reducing the probability of pancreatic injury and bleeding, and providing better physiological drainage for ductal strictures[14,25,40-42]. Therefore, in the presence of normal anatomical structures, EUS-RV should be attempted first. If passage through the papillary structure is not possible due to severe pancreatic duct stenosis or duodenal obstruction, EUS-TMD can be considered. Additionally, avoiding the need for electrocoagulation, cauterization, or ductal dilation to prevent fistula formation can lower the risks of bleeding, pancreatic leakage, and gastric fluid entering the retroperitoneal space[1]. A large retrospective study indicated that, compared to trans gastric pancreaticogastrostomy (77.8%), RV-ERP (95.6%) exhibited an improved technical success rate with a lower adverse event rate[43]. As a salvage therapy, the technical and clinical success rates of antegrade drainage were 89% and 87%, respectively[44]. EUS-RV presents a more challenging procedure than transmural drainage, as threading the wire through the papilla, switching to the duodenoscope, and maintaining the wire in place throughout the process can be difficult[45]. The technical success rate for antegrade pancreatic duct interventions is 87%, including RV and antegrade stenting. Puncture of unexpanded duct is more challenging due to the difficulty in guiding the wire through and across the papilla[46]. To date, only a limited number of publications have reported successful cases of EUS-RV[12,37,46-48].
The most used in clinical practice is a 19G FNA needle, paired with a hydrophilic guidewire of either 0.035 inches or 0.025 inches. A 22G puncture needle is often used with a 0.018-inch or 0.021-inch guidewire. However, these guidewires may have issues with insufficient stiffness or poor visibility under X-ray guidance. Nevertheless, in cases involving fibrotic pancreas or undilated pancreatic ducts, the use of a 22G puncture needle may be considered to increase the success rate of puncture. The use of a 22G puncture needle for puncture is a good method to reduce complications such as pancreatic fluid leakage. However, due to the lack of a 0.018-inch guidewire suitable for EUS-PD with good X-ray visibility and operability, it is not recommended as the first choice[49].
Prior to stent placement, tract dilation is necessary, and the size of dilation depends on the size of the PD stent. In EUS-PD, tract dilation is a challenging step. Non-cautery or cautery dilation can be performed at the puncture site[46,50]. Non-cautery dilation can be done using progressive dilators such as dilation catheters or balloon catheters. Cautery dilation options include the use of a 6 Fr cystotome or a three-lumen needle knife. Solely using non-cautery dilation for tract dilation may present difficulties for endoscopists when puncturing fibrotic pancreatic tissue, thus some teams recommend using mechanical dilators before cautery dilation to reduce the risk of associated bleeding[51-53]. Examples of this approach include using a tapered injection catheter as the first probe, followed using a 5F to 7F dilating catheter. Additionally, there is literature suggesting the use of an the over-the-wire type diathermy catheter to facilitate channel dilation and anastomosis. If necessary, a 4 mm balloon dilating catheter, which is only compatible with a 5F or similarly sized tapered catheter, can be used for dilation of the intestinal passage and/or anastomosis in order to facilitate stent placement[52]. Compared to a 6Fr cautery dilator, a newly developed ultra-high-efficiency mechanical dilator for EUS-PD demonstrated a lower risk of bleeding (0% vs 18.2%, P = 0.04), with a similar rate of successful dilation (93.3% vs 95.0%, P < 0.05)[54,55]. Recently, a spiral dilator (Tornus ES; Olympus Co., Tokyo, Japan) has been documented for use in EUS-TMD. This dilator features a helical tip design that offers excellent penetration through hard pancreatic tissue and operates without the need for electrical power, effectively reducing the risk of bleeding[22]. The ongoing development of dilation devices is keeping pace with advancements in the field, resulting in more flexible applications in EUS. The continuous innovation of these instruments is poised to further enhance the success rate of EUS-PD procedures in the future.
Statistical analysis of relevant literature reveals that for pancreatic duct drainage, double-pigtail plastic or metal stents designed for endoscopic retrograde cholangiopancreatography are commonly employed, with the latter further classified into semi-covered, fully covered, and self-expanding metal stents (SEMS)[56-58]. Plastic stents are typically favored due to their ease of placement, although they are prone to dislodgement[59]. Recently, a study published by Professor Fukuda et al[60] reported the development of a novel plastic stent with an internal lumen (HarmoRay 5Fr; Hanaco Medical, Tokyo, Japan), which facilitates placement compared to traditional plastic stents that lack an internal lumen. The authors reviewed clinical data from ten patients who underwent EUS-PD using the new plastic stent at the National Cancer Center Hospital from March 2021 to October 2023. Among the ten patients, five exhibited benign post-operative pancreatic anastomotic strictures, while the other five had malignant pancreatic duct obstructions. Both technical and clinical success rates were 100% (10/10). One patient (10.0%) experienced an adverse event (self-limiting abdominal pain). Two patients (20.0%) died from their primary disease during the follow-up period, with a median follow-up of 44 days (range: 25-272 days). The rate of recurrent pancreatic duct obstruction (RPO) was 10.0% (1/10), and the non-RPO rate at three months was 83.3%. These findings indicate the efficacy and safety of the new plastic stent in EUS-PD procedures[60]. In recent years, the application of SEMS has increased. Their advantage lies in a larger drainage diameter compared to plastic stents, facilitating smoother drainage. A literature survey in 2020 investigated the long-term outcomes of fully covered SEMS (FCSEMS) in treating EUS-PD for pancreatic-duodenal anastomotic strictures. All patients achieved technical and clinical success, with 11 cases of complete stricture and 12 cases of partial stricture. In partial pancreatic jejunal anastomosis (PJA) strictures, direct trans anastomotic placement of plastic stents succeeded in only 3 patients (13%), while 20 patients underwent EUS-guided transmural FCSEMS placement on the first attempt. Early adverse events occurred in 4 patients (17.4%), including abdominal pain (n = 3) and peripancreatic fluid collection (n = 1). During follow-up [median, 27.2 months; interquartile range (IQR), 18.7-40.6], late adverse events were observed in 5 patients (21.7%), including asymptomatic stent fracture at the gastric end (n = 3), asymptomatic stent migration (n = 1), and stent obstruction (n = 1). The total duration of stent placement was 27.2 months (IQR, 18.7-40.6), with a median of 2 stent replacements (IQR, 1-2)[61]. In terms of safety and efficacy, FCSEMS demonstrates promising results in EUS-PD, and it is anticipated that the future utilization of FCSEMS in EUS-PD procedures will increase. In palliative treatment, it is recommended to choose easily deployable plastic stents to ensure procedural simplicity and meet the needs for short-term symptom relief. In contrast, for curative treatment, metal stents are preferred to prolong stent durability, reduce the likelihood of re-interventions, and ultimately improve the long-term therapeutic outcomes and QoL for patients.
Adverse events of EUS-PD encompass early complications such as abdominal pain, pancreatitis, fever, minor bleeding, and guide wire dislodgment, with a reported overall incidence of approximately 21.8%. Most patients requiring this procedure are inherently susceptible to pancreatitis due to elevated pancreatic duct pressure. Additionally, the risks of pancreatic leakage are associated with necessary punctures and dilations during the intervention. Therefore, postoperative assessment of adverse events involves dynamic monitoring of patient symptoms and laboratory examinations, coupled with regular imaging studies. Compared to EUS-TMD, the correlation between ERP-RV and AEs was smaller. Early technical challenges and insufficient equipment in the literature resulted in an adverse event incidence rate ranging from 3.4% to 38.6%, with an average rate of 17.0% to 18.9%[51,62]. Ogura et al[63] compiled clinical data from a total of 334 patients, reporting technical and clinical success rates for EUS-PD ranging from 63% to 100% and 76% to 100%, respectively. In contrast, procedure-related adverse event rates were higher at 26.4% (89/334). The most common adverse events included abdominal pain (n = 38), acute pancreatitis (n = 15), bleeding (n = 9), and data related to pancreatic fluid leakage, such as peripancreatic fluid collection or pancreatic leakage (n = 8)[63]. A recent study aimed to reduce the risk of pancreatic leakage and stent migration by using a technique involving balloon dilation of the pancreatoenteric anastomotic orifice to 4Fr and placing a 3Fr stent with pigtail extensions into the pancreatic duct, extending at least 3 cm into the gastric lumen. The authors reported a technical success rate of 88% and a clinical success rate of 62.5%, with no stent-related AEs[64]. Data analysis by Sakai et al[65] described the outcomes of patients with benign pancreatic duct obstruction undergoing endoscopic trans papillary pancreatic drainage (ETPD) and EUS-PD. Among 10 ETPD failures, 8 patients underwent EUS-PD, while 2 patients received EUS-PD as the primary procedure. Following the addition of EUS-PD to ETPD, the technical success rate of endoscopic interventions for CP increased from 82% to 91%, and the technical success rate for patients with pancreatoenteric anastomotic strictures increased from 0% to 80%. The overall clinical success rate of endoscopic interventions was 97%. The postoperative pancreatitis rate in the EUS-PD group was 30% (n = 3). Sakai et al's study[65] suggests that EUS-PD is more likely to achieve technical success in PJA strictures compared to ETPD. When used as a salvage procedure in conjunction with ETPD, it reduces the number of patients requiring surgical drainage due to pancreatic duct obstruction[65].
From 2012 to 2022, a total of 278 publications on EUS-PD were released worldwide, with a steady increase in publication volume. The highest number of publications was observed in 2022, with 34 articles. In terms of country/region analysis, Japan had the highest number of publications, accounting for 29.7% (89 articles), while the application of EUS-PD and the publication volume in China were relatively low[66,67]. The learning process for therapeutic EUS is both challenging and expensive, with many cases concentrated in tertiary hospitals. Physicians need to spend several weeks accumulating experience in teaching hospitals. For international students, the opportunities for hands-on experience are often limited due to variations in regulatory policies among different countries, resulting in primarily observational roles[66,68]. In China, the training for EUS primarily relies on an apprentice system, and there is significant variability in the amount of training required before being allowed to operate independently. Establishing a standardized EUS training curriculum and providing certification to newly trained EUS operators based on effective competence assessment would be beneficial for the standardization of this technique[69].
EUS-PD is still in its early stages, and there is currently limited literature available on ETPD using EUS. Although there are studies that demonstrate its safety and efficacy, the procedure remains challenging to perform and difficult to popularize. The indications for EUS-PD are limited, and the evidence for its feasibility is also limited. Despite improvements in various devices, EUS-PD still poses technical challenges. Therefore, long-term, large-scale, multicenter studies are needed to establish this technique as a viable alternative drainage method[67,70]. In 2023, Mizumachi et al[71] treated a patient with pancreatic stones using EUS-PD. The patient had a history of pancreatic cancer and underwent Whipple surgery, after which benign post-operative scarring led to stenosis at the pancreatojejunostomy site, resulting in recurrent pancreatitis. CT scan results revealed diffuse dilation of the MPD and an 8 mm pancreatic duct stone, which may represent a viable option for managing cases of CP that are not amenable to surgical intervention[71]. The development of EUS has brought significant advancements in the diagnosis and treatment of pancreatic diseases. Various derivative techniques related to EUS have emerged and evolved over the past few decades. Compared to traditional imaging-assisted diagnostic methods, EUS has certain advantages in the management of chronic inflammation and solid lesions, as well as in the accuracy, convenience, and applicability of diagnosis. EUS has significantly improved the early detection and differential diagnosis of pancreatic pathologies. The combination of multiple treatment modalities with EUS is the driving force behind its technological development. This means that the continuous innovation of different treatment methods also brings new vitality to EUS, which is the direction of its application in the treatment of pancreatic diseases. It presents increasingly higher demands on the proficiency of endoscopists. Although some EUS-related procedures are still in the stage of lacking long-term evidence or outcomes, as they continue to develop, their applications in pancreatic diseases will become more widespread. Various adjunctive techniques will become more profound and accessible, ultimately benefiting patients to a new extent. Future research should independently report the results of EUS-RV and transmural stenting as these procedures have different technical considerations and success rates. Additionally, the definition of technical success and clinical success for EUS-PD has not been standardized. The lack of standardization in placing the stents in the pancreatic duct makes it difficult to compare results, as evident in meta-analyses of EUS-PD outcomes[72,73].
The overall utilization rate of EUS-PD in clinical practice is still low, and the development of dedicated accessories, such as specialized and small-caliber accessories, may improve the success rate of the procedure. However, direct comparisons with standard accessories are lacking[64]. The pooled adverse event rate of EUS-PD remains substantial (20.3%, 95%CI: 16.8%-24.2%) based on a meta-analysis of 714 patients[69], and guidelines suggest that experience with other EUS-guided drainage procedures may improve success rates and reduce the risk of adverse events[74]. Given recent literature analysis on the learning curve of EUS-PD, even experienced endoscopists need to perform at least 40 cases of this procedure to achieve basic proficiency[75]. Therefore, it is necessary to study how to improve the success and safety of the procedure, overcome the learning curve of EUS-PD in fewer cases, and enhance standardization through better training and the provision of improved accessories[45]. Standardization of techniques for entering the pancreatic duct, definition of mid-term and long-term postoperative management strategies, and unified criteria for EUS-PD procedures, as well as long-term, large-scale, multicenter studies and training for experienced endoscopists, will contribute to a comprehensive understanding of EUS-PD techniques.
EUS-PD is technically challenging and has limited indications, resulting in its description primarily in small-sample retrospective studies. Future studies must stratify outcomes by technique (antegrade vs rendezvous), as their distinct procedural challenges (e.g., guidewire navigation in undilated ducts) significantly impact success rates[46,72]. The success rate of EUS-PD is strongly correlated with the proficiency of the physician. Standardization of the technique, introduction of dedicated accessories, and systemic training of physicians will improve outcomes for patients undergoing EUS-PD. Furthermore, in cases where stent obstruction is untreatable, long-term follow-up of a larger cohort is required to assess the timeframe for the restoration of fistula patency after the removal of the drainage stent. Meanwhile, we should actively encourage collaboration among large tertiary hospitals to address the numerous unresolved issues related to this effective and minimally invasive procedure.
| 1. | Giovannini M. Endoscopic ultrasonography-guided pancreatic drainage. Gastrointest Endosc Clin N Am. 2012;22:221-230, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Onodera M, Kawakami H, Kuwatani M, Kudo T, Haba S, Abe Y, Kawahata S, Eto K, Nasu Y, Tanaka E, Hirano S, Asaka M. Endoscopic ultrasound-guided transmural drainage for pancreatic fistula or pancreatic duct dilation after pancreatic surgery. Surg Endosc. 2012;26:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, Radaelli F, Knight E, Gralnek IM, Hassan C, Dumonceau JM. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Vanella G, Bronswijk M, Arcidiacono PG, Larghi A, Wanrooij RLJV, de Boer YS, Rimbas M, Khashab M, van der Merwe SW. Current landscape of therapeutic EUS: Changing paradigms in gastroenterology practice. Endosc Ultrasound. 2023;12:16-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Möller K, Jenssen C, Braden B, Hocke M, Hollerbach S, Ignee A, Faiss S, Iglesias-Garcia J, Sun S, Dong Y, Carrara S, Dietrich CF. Pancreatic changes with lifestyle and age: What is normal and what is concerning? Endosc Ultrasound. 2023;12:213-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 6. | Li T, Kang C, Ren G, Lv Y, Luo H, Kang X, Liang S, Wang X, Pan Y. Top 100 cited articles related to EUS: A bibliometric analysis. Endosc Ultrasound. 2024;13:259-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Zhang D, Wu C, Yang Z, Yin H, Liu Y, Li W, Huang H, Jin Z. The application of artificial intelligence in EUS. Endosc Ultrasound. 2024;13:65-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Abdelqader A, Kahaleh M. When ERCP Fails: EUS-Guided Access to Biliary and Pancreatic Ducts. Dig Dis Sci. 2022;67:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Jearth V, Sundaram S, Kale A, Sachan A, Rana SS. Current paradigm of endoscopic ultrasound in biliary and pancreatic duct drainage: an update. Ann Gastroenterol. 2024;37:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Sadek A, Hara K, Okuno N, Haba S, Kuwahara T, Fukui T, Urata M, Kondo T, Yamamoto Y, Tachi K. Safety and efficacy of endoscopic ultrasound-guided pancreatic duct drainage using a drill dilator: a retrospective study in Japan. Clin Endosc. 2024;57:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Trieu JA, Seven G, Baron TH. Endoscopic Ultrasound-Guided Pancreatic Duct Drainage. Gastrointest Endosc Clin N Am. 2024;34:501-510. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Nakai Y, Isayama H, Matsubara S, Kogure H, Mizuno S, Hamada T, Takahara N, Nakamura T, Sato T, Takeda T, Hakuta R, Ishigaki K, Saito K, Tada M, Koike K. A novel "hitch-and-ride" deep biliary cannulation method during rendezvous endoscopic ultrasound-guided ERCP technique. Endoscopy. 2017;49:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Bataille L, Deprez P. A new application for therapeutic EUS: main pancreatic duct drainage with a "pancreatic rendezvous technique". Gastrointest Endosc. 2002;55:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Itoi T, Kasuya K, Sofuni A, Itokawa F, Kurihara T, Yasuda I, Nakai Y, Isayama H, Moriyasu F. Endoscopic ultrasonography-guided pancreatic duct access: techniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Dig Endosc. 2013;25:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Dhir V, Isayama H, Itoi T, Almadi M, Siripun A, Teoh AYB, Ho KY. Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig Endosc. 2017;29:472-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Nakai Y, Kogure H, Isayama H, Koike K. Endoscopic ultrasound-guided pancreatic duct drainage. Saudi J Gastroenterol. 2019;25:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Canakis A, Baron TH. Therapeutic Endoscopic Ultrasound: Current Indications and Future Perspectives. GE Port J Gastroenterol. 2023;30:4-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Li JS, Zheng KL, Lv SL, Su XJ, Wang KX, Li ZS, Chen J, Chen Y. Endoscopic ultrasound-guided versus surgical pancreatic duct drainage after failed endoscopic retrograde pancreatography: a pilot comparative study. Surg Endosc. 2024;38:4422-4430. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Kim JH, Wang J, Hassan KM, Sharaiha RZ, Mahadev S, Sampath K. EUS-guided hepaticojejunostomy for biliary obstruction in near-total gastrectomy with Roux-en-Y: Trials and tribulations (with videos). Endosc Ultrasound. 2024;13:278-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Lin Z, Wang Y, Liu W, Yan X, Chang H, Huang Y. The first case to decompress the pancreatic duct by reopening a surgical cystogastrostomical fistula using EUS-guided pancreatic drainage. Endosc Ultrasound. 2023;12:479-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Hara K, Okuno N, Haba S, Kuwahara T. Forward viewing liner echoendoscopy for therapeutic interventions. Clin Endosc. 2024;57:175-180. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Ogawa T, Kanno Y, Koshita S, Kusunose H, Sakai T, Yonamine K, Miyamoto K, Kozakai F, Okano H, Anan H, Hosokawa K, Ito K. Prospective feasibility study on the efficacy and safety of a novel spiral dilator for endoscopic ultrasound-guided drainage. DEN Open. 2023;3:e170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Chen YI, Miller CS, Hashim A, Valenti D, Metrakos P, Barkun J, Zogopoulos G, Barkun AN, Bessissow A. EUS-IR-Guided Revision of Whipple Anatomy for Concomitant Afferent and Efferent Limb Obstruction. Am J Gastroenterol. 2018;113:1747. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Basiliya K, Veldhuijzen G, Gerges C, Maubach J, Will U, Elmunzer BJ, Stommel MWJ, Akkermans R, Siersema PD, van Geenen EM. Endoscopic retrograde pancreatography-guided versus endoscopic ultrasound-guided technique for pancreatic duct cannulation in patients with pancreaticojejunostomy stenosis: a systematic literature review. Endoscopy. 2021;53:266-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Kunovský L, Dítě P, Bojková M, Dolina J, Vaculová J, Kolovratníková H, Uvírová M, Janeček P, Kala Z, Jabandžiev P. Diagnostics and therapy of chronic pancreatitis according to UEG guidelines. Vnitř Lék. 2021;67:85-91. [DOI] [Full Text] |
| 26. | Bai Y, Qin X, Ao X, Ran T, Zhou C, Zou D. The role of EUS in the diagnosis of early chronic pancreatitis. Endosc Ultrasound. 2024;13:232-238. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 28. | He H, Yu T, Hou S, Li Y, Zhang L. EUS-guided drainage for pancreatic duct stone combined with main pancreatic duct stenosis after pancreaticoduodenectomy: a case report. Med Ultrason. 2023;25:112-113. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Falque A, Gasmi M, Barthet M, Gonzalez JM. Safety and efficacy of EUS-guided pancreatic duct drainage in symptomatic main pancreatic duct obstruction: Is there still a place for surgery? Endosc Int Open. 2021;9:E934-E942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Parhiala M, Nøjgaard C, Bartholdy A, Waage A, Ignatavičius P, Engjom T, Dimcevski G, Nordaas IK, Kalaitzakis E, Drewes AM, Hadi A, Olesen SS, Poulsen JL, Laukkarinen J; Scandinavian Baltic Pancreatic Club. Quality of life after endoscopic procedures for chronic pancreatitis: A multicentre study. United European Gastroenterol J. 2023;11:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Rudler F, Caillol F, Ratone JP, Pesenti C, Valats JC, Soloveyv A, Giovannini M. EUS-guided drainage of the pancreatic duct for the treatment of postoperative stenosis of pancreatico-digestive anastomosis or pancreatic duct stenosis complicating chronic pancreatitis: Experience at a tertiary care center. Endosc Ultrasound. 2022;11:296-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Buchberg J, de Stricker K, Pfeiffer P, Mortensen MB, Detlefsen S. Mutational profiling of 103 unresectable pancreatic ductal adenocarcinomas using EUS-guided fine-needle biopsy. Endosc Ultrasound. 2024;13:154-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Thiruvengadam NR, Forde KA, Miranda J, Kim C, Behr S, Masharani U, Arain MA. Disconnected Pancreatic Duct Syndrome: Pancreatitis of the Disconnected Pancreas and Its Role in the Development of Diabetes Mellitus. Clin Transl Gastroenterol. 2022;13:e00457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Möller K, Jenssen C, Ignee A, Hocke M, Faiss S, Iglesias-Garcia J, Sun S, Dong Y, Dietrich CF. Pancreatic duct imaging during aging. Endosc Ultrasound. 2023;12:200-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 35. | Takenaka M, Saito T, Hamada T, Omoto S, Shiomi H, Iwashita T, Masuda A, Matsubara S, Maruta A, Iwata K, Mukai T, Isayama H, Yasuda I, Nakai Y. Disconnected pancreatic duct syndrome: diagnostic and therapeutic challenges and future directions. Expert Rev Gastroenterol Hepatol. 2024;18:631-645. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Hou Y, Tian Y, Dong J, Zhang L. Pancreatic fistula after pancreaticoduodenectomy - Alternative therapy of endoscopic ultrasound guided pancreatic duct puncture drainage. Rev Esp Enferm Dig. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Kale A, Sundaram S, Satai M, Harindranath S, Garg L, Aggarwal M. EUS-guided pancreatic rendezvous for management of pancreaticopleural fistula with an undilated duct and pancreas divisum. Endosc Ultrasound. 2022;11:515-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 38. | Wang ZJ, Song YH, Li SY, He ZX, Li ZS, Wang SL, Bai Y. Endoscopic management of pancreatic fluid collections with disconnected pancreatic duct syndrome. Endosc Ultrasound. 2023;12:29-37. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Chong E, Ratnayake CB, Saikia S, Nayar M, Oppong K, French JJ, Windsor JA, Pandanaboyana S. Endoscopic transmural drainage is associated with improved outcomes in disconnected pancreatic duct syndrome: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Kovacevic B, Toxværd A, Klausen P, Larsen MH, Grützmeier S, Detlefsen S, Karstensen JG, Brink L, Hassan H, Høgdall E, Vilmann P. Tissue amount and diagnostic yield of a novel franseen EUS-FNB and a standard EUS-FNA needle-A randomized controlled study in solid pancreatic lesions. Endosc Ultrasound. 2023;12:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 41. | Giovannini M. EUS-guided transenteric pancreatic duct drainage. Best Pract Res Clin Gastroenterol. 2022;60-61:101815. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Conti Bellocchi MC, Amodio A, Bernardoni L, Gabbrielli A, Crinò SF. A penetrating foreign body mimicking pancreatic cancer (with videos). Endosc Ultrasound. 2023;12:286-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Dalal A, Patil G, Maydeo A. Six-year retrospective analysis of endoscopic ultrasonography-guided pancreatic ductal interventions at a tertiary referral center. Dig Endosc. 2020;32:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | van Wanrooij RLJ, Bronswijk M, Kunda R, Everett SM, Lakhtakia S, Rimbas M, Hucl T, Badaoui A, Law R, Arcidiacono PG, Larghi A, Giovannini M, Khashab MA, Binmoeller KF, Barthet M, Pérez-Miranda M, van Hooft JE, van der Merwe SW. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2022;54:310-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 45. | Teh JL, Teoh AYB. Techniques and Outcomes of Endoscopic Ultrasound Guided-Pancreatic Duct Drainage (EUS- PDD). J Clin Med. 2023;12:1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Bronswijk M, Persyn D, van Malenstein H, Laleman W, van der Merwe S. Outcomes of minor versus major papilla rendez-vous for EUS-guided pancreatic duct drainage. Dig Liver Dis. 2024;56:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Prakash A, Koshy AK, Rao HB, Venu RP. Endoscopic Ultrasound-Guided Rendezvous Procedure for a Nondilated, Leaking Pancreatic Duct. ACG Case Rep J. 2018;5:e105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Okamoto T, Nakamura K, Takasu A, Sunagawa H, Fukuda K. EUS-guided pancreatic duct drainage with rendezvous technique for post-Whipple pancreatic duct stone. VideoGIE. 2021;6:512-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 49. | Singh AD, Madhu D, Pathiyil MM, Ramai D, Mohan BP, Shah B, Adler DG. Device malfunctions with use of EUS-guided fine-needle biopsy devices: Analysis of the MAUDE database. Endosc Ultrasound. 2023;12:424-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 50. | Takenaka M, Omoto S, Kudo M. EUS-guided drainage of the gallbladder using a novel 0.018-inch guidewire for preventing bile leakage (with video). Endosc Ultrasound. 2022;11:520-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Uchida D, Kato H, Saragai Y, Takada S, Mizukawa S, Muro S, Akimoto Y, Tomoda T, Matsumoto K, Horiguchi S, Okada H. Indications for Endoscopic Ultrasound-Guided Pancreatic Drainage: For Benign or Malignant Cases? Can J Gastroenterol Hepatol. 2018;2018:8216109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Itoi T, Yasuda I, Kurihara T, Itokawa F, Kasuya K. Technique of endoscopic ultrasonography-guided pancreatic duct intervention (with videos). J Hepatobiliary Pancreat Sci. 2014;21:E4-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Park DH, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 54. | Honjo M, Itoi T, Tsuchiya T, Tanaka R, Tonozuka R, Mukai S, Sofuni A, Nagakawa Y, Iwasaki H, Kanai T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc Ultrasound. 2018;7:376-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 55. | Kongkam P, Tiankanon K, Seo DW, Luangsukrerk T, Sriuranpong V, Nantavithya C, Jantarattana T, Cañones A, Kerr SJ, Tantitanawat K, Angsuwatcharakon P, Ridtitid W, Kullavanijaya P, Rerknimitr R. EUS-guided radiofrequency ablation plus chemotherapy versus chemotherapy alone for pancreatic cancer (ERAP): An observational open-label pilot study. Endosc Ultrasound. 2023;12:402-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 56. | Xu N, Li L, Su S, Zhao D, Xiang J, Wang P, Cheng Y, Linghu E, Chai N. A novel lumen-apposing metal stent for endoscopic drainage of symptomatic pancreatic fluid collections: a retrospective study. Endosc Ultrasound. 2024;13:40-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 57. | Vanek P, Falt P, Vitek P, Zoundjiekpon V, Horinkova M, Zapletalova J, Lovecek M, Urban O. EUS-guided transluminal drainage using lumen-apposing metal stents with or without coaxial plastic stents for treatment of walled-off necrotizing pancreatitis: a prospective bicentric randomized controlled trial. Gastrointest Endosc. 2023;97:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 58. | Braden B, Hocke M, Selvaraj E, Kaushal K, Möller K, Ignee A, Vanella G, Arcidiacono PG, Teoh A, Larghi A, Rimbas M, Hollerbach S, Napoleon B, Dong Y, Dietrich CF. Mishaps with EUS-guided lumen-apposing metal stents in therapeutic pancreatic EUS: Management and prevention. Endosc Ultrasound. 2023;12:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 59. | Ishii S, Isayama H, Sasahira N, Matsubara S, Nakai Y, Fujisawa T, Tomishima K, Sasaki T, Ishigaki K, Kogure H, Okamoto T, Otsuka T, Takasaki Y, Suzuki A. A pilot study of Spring Stopper Stents: Novel partially covered self-expandable metallic stents with anti-migration properties for EUS-guided hepaticogastrostomy. Endosc Ultrasound. 2023;12:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Reference Citation Analysis (0)] |
| 60. | Fukuda S, Hijioka S, Nagashio Y, Maruki Y, Ohba A, Agarie D, Hagiwara Y, Hara H, Okamoto K, Yamashige D, Yagi S, Kuwada M, Chatto M, Kondo S, Morizane C, Ueno H, Saito Y, Okusaka T. Feasibility and safety of a novel plastic stent designed specifically for endoscopic ultrasound-guided pancreatic duct drainage. Endosc Int Open. 2024;12:E715-E722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 61. | Oh D, Park DH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. Long-term outcome of endoscopic ultrasound-guided pancreatic duct drainage using a fully covered self-expandable metal stent for pancreaticojejunal anastomosis stricture. J Gastroenterol Hepatol. 2020;35:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Tyberg A, Sharaiha RZ, Kedia P, Kumta N, Gaidhane M, Artifon E, Giovannini M, Kahaleh M. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc. 2017;85:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Ogura T, Ohama H, Higuchi K. Endoscopic Ultrasound-Guided Pancreatic Transmural Stenting and Transmural Intervention. Clin Endosc. 2020;53:429-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Hayat U, Freeman ML, Trikudanathan G, Azeem N, Amateau SK, Mallery J. Endoscopic ultrasound-guided pancreatic duct intervention and pancreaticogastrostomy using a novel cross-platform technique with small-caliber devices. Endosc Int Open. 2020;8:E196-E202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Sakai T, Koshita S, Kanno Y, Ogawa T, Kusunose H, Yonamine K, Miyamoto K, Kozakai F, Okano H, Ohira T, Horaguchi J, Oikawa M, Tsuchiya T, Noda Y, Ito K. Early and long-term clinical outcomes of endoscopic interventions for benign pancreatic duct stricture/obstruction-the possibility of additional clinical effects of endoscopic ultrasonography-guided pancreatic drainage. Pancreatology. 2022;22:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Xin L, Gao Y, Wang TJ, Meng QQ, Jin ZD, Fu ZJ, Wang YL, Lin H, Li ZS, Wang LW. EUS development in China: Results from national surveys in 2013 and 2020. Endosc Ultrasound. 2023;12:90-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Ishii S, Isayama H, Suzuki M, Koga H, Tomishima K, Fujisawa T, Shimizu T, Yamataka A. Recent progress and current status of pancreatobiliary interventional endoscopic ultrasound in children. Dig Endosc. 2025;37:53-67. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Chandan S, Mohan BP, Khan SR, Kassab LL, Ponnada S, Ofosu A, Bhat I, Singh S, Adler DG. Efficacy and safety of endoscopic ultrasound-guided pancreatic duct drainage (EUS-PDD): A systematic review and meta-analysis of 714 patients. Endosc Int Open. 2020;8:E1664-E1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Bhurwal A, Tawadros A, Mutneja H, Gjeorgjievski M, Shah I, Bansal V, Patel A, Sarkar A, Bartel M, Brahmbhatt B. EUS guided pancreatic duct decompression in surgically altered anatomy or failed ERCP - A systematic review, meta-analysis and meta-regression. Pancreatology. 2021;21:990-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Fujii-Lau LL, Levy MJ. Endoscopic ultrasound-guided pancreatic duct drainage. J Hepatobiliary Pancreat Sci. 2015;22:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Mizumachi M, Mukai S, Itoi T. Transmural pancreatoscopy-assisted lithotripsy through endoscopic ultrasound-guided pancreatic duct drainage tract created by a novel drill dilator. Dig Endosc. 2023;35:e93-e94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 72. | Chantarojanasiri T, Siripun A, Kongkam P, Pausawasdi N, Ratanachu-Ek T. Three-year evaluation of a novel, nonfluoroscopic, all-artificial model for EUS-guided biliary drainage training for the impact to practice: A prospective observational study (with videos). Endosc Ultrasound. 2023;12:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 73. | Gutta A, Fogel E, Sherman S. Identification and management of pancreas divisum. Expert Rev Gastroenterol Hepatol. 2019;13:1089-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Teoh AYB, Dhir V, Kida M, Yasuda I, Jin ZD, Seo DW, Almadi M, Ang TL, Hara K, Hilmi I, Itoi T, Lakhtakia S, Matsuda K, Pausawasdi N, Puri R, Tang RS, Wang HP, Yang AM, Hawes R, Varadarajulu S, Yasuda K, Ho LKY. Consensus guidelines on the optimal management in interventional EUS procedures: results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018;67:1209-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 75. | Ramai D, Ahmed Z, Chandan S, Facciorusso A, Deliwala SS, Alastal Y, Nawras A, Maida M, Barakat MT, Anderloni A, Adler DG. Safety and efficacy of the EndoRotor device for the treatment of walled-off pancreatic necrosis after EUS-guided cystenterostomy: A systematic review and meta-analysis. Endosc Ultrasound. 2024;13:165-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |