Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1436
Revised: April 10, 2024
Accepted: April 22, 2024
Published online: May 27, 2024
Processing time: 140 Days and 2.4 Hours
Pulmonary lymphoepithelioma-like carcinoma (PLELC) is a rare type of non-small-cell lung cancer. Stomach lymphoepithelioma-like carcinoma (LELC) me
A 64-year-old female was admitted to our hospital for a regular gastroscopy examination with a 6-year history of surgical resection for left PLELC. Positron emission tomography/computed tomography suggested high accumulation of 18F-fludeoxyglucose in the gastric cardia region. Upper gastrointestinal endo
For gastric LELC metastasis, PD-1 inhibitor therapy could become a new therapeutic approach, though there is still no evidence from large data sets to support this.
Core Tip: Pulmonary lymphoepithelioma-like carcinoma (PLELC) is a rare type of non-small-cell lung cancer. Stomach lymphoepithelioma-like carcinoma (LELC) metastasis secondary to PLELC has not been reported recently. We present a 64-year-old female patient who was admitted to our hospital for a regular gastroscopy examination with a 6-year history of surgical resection for left PLELC. After proximal gastrectomy, histopathological examination showed a poorly differentiated carcinoma with prominent lymphoplasmacytic infiltration, suggesting stomach LELC metastasis. Tumor programmed cell death ligand 1 (PD-L1) expression showed a tumor proportion score of 98% and a combined positive score of 100. After discharge, this patient underwent PD-1 inhibitor treatment for ten cycles and has not experienced tumor recurrence. These findings suggest that for gastric LELC metastasis, PD-1 inhibitor therapy could become a potential therapeutic approach.
- Citation: Chen GF, Wang J, Yan Y, Xu S, Chen J. Metastatic stomach lymphoepithelioma-like carcinoma and immune checkpoint inhibitor therapy: A case report. World J Gastrointest Surg 2024; 16(5): 1436-1442
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1436.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1436
Pulmonary lymphoepithelioma-like carcinoma (PLELC) is a rare type of non-small cell lung cancer that tends to occur in young, nonsmoking, and Asian populations[1]. It has unique clinical and pathological features that are similar to those of undifferentiated nasopharyngeal carcinoma[2,3]. PLELC is characterized by Epstein-Barr virus (EBV) infection. Ac
A 64-year-old female patient was admitted to our hospital for a regular gastroscopy examination.
There is no history of present illness.
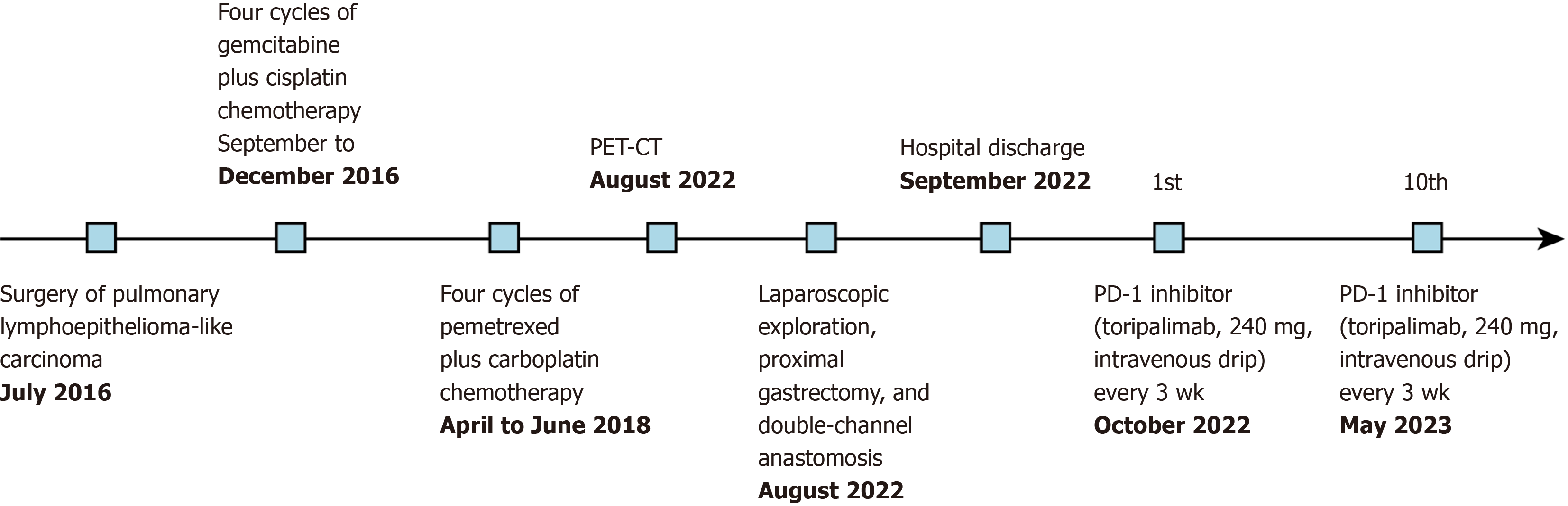
The patient had a 6-year history of surgical resection for left PLELC. She underwent four cycles of gemcitabine plus cisplatin chemotherapy, four cycles of pemetrexed plus carboplatin chemotherapy, and 30 cycles of radiotherapy (Figure 1).
The patient denied any chronic medical history, such as hypertension, diabetes, heart disease, or tobacco or alcohol (illicit drug) use.
On physical examination, the patient reported no obvious discomfort.
Laboratory findings included increased levels of tumor markers, such as carbohydrate antigen 125 (CA125) (36.9 U/mL; reference range, < 35 U/mL) and CA211 (5.9 ng/mL; reference range, < 5 ng/mL). A stool occult blood test was positive.
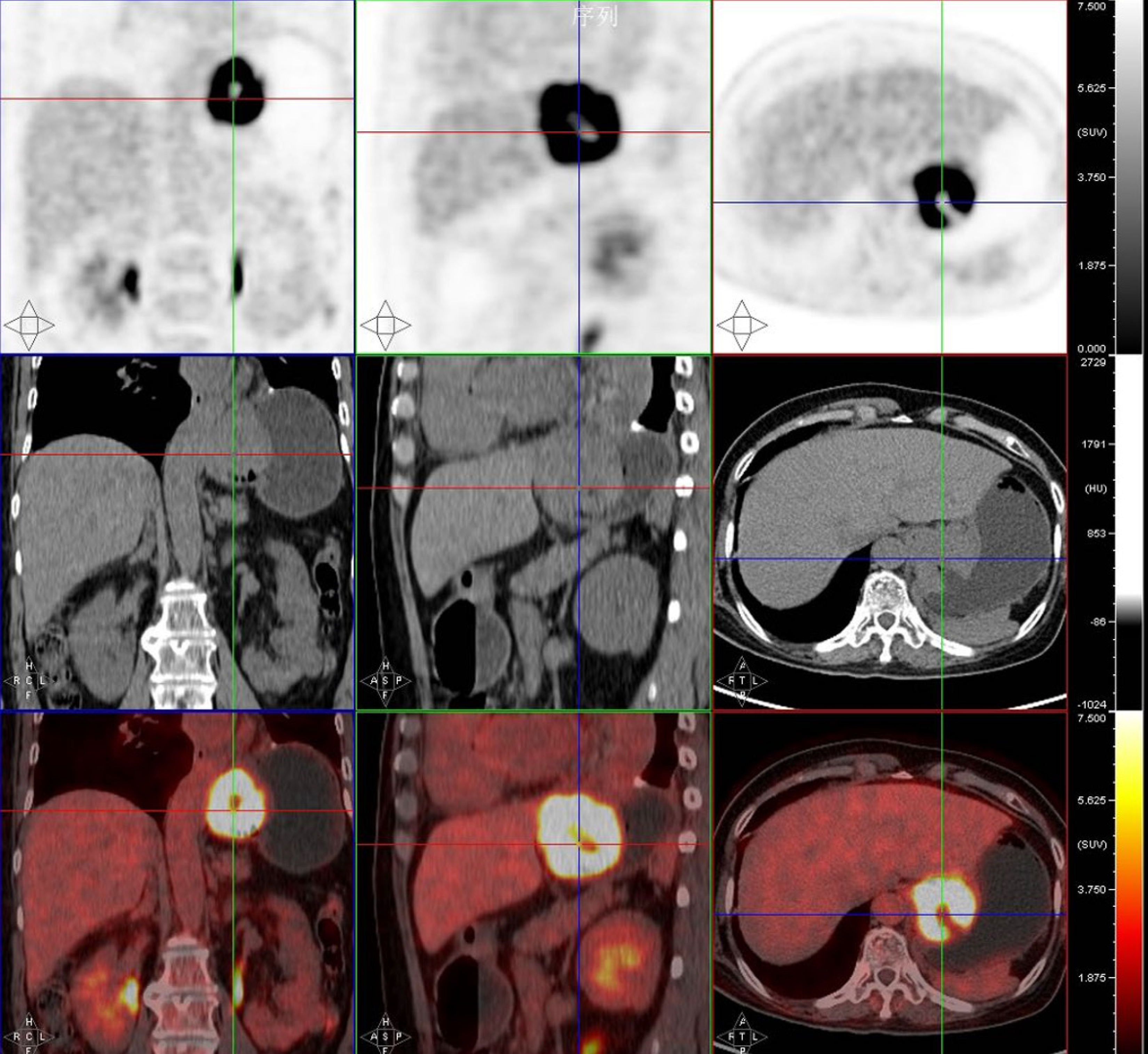
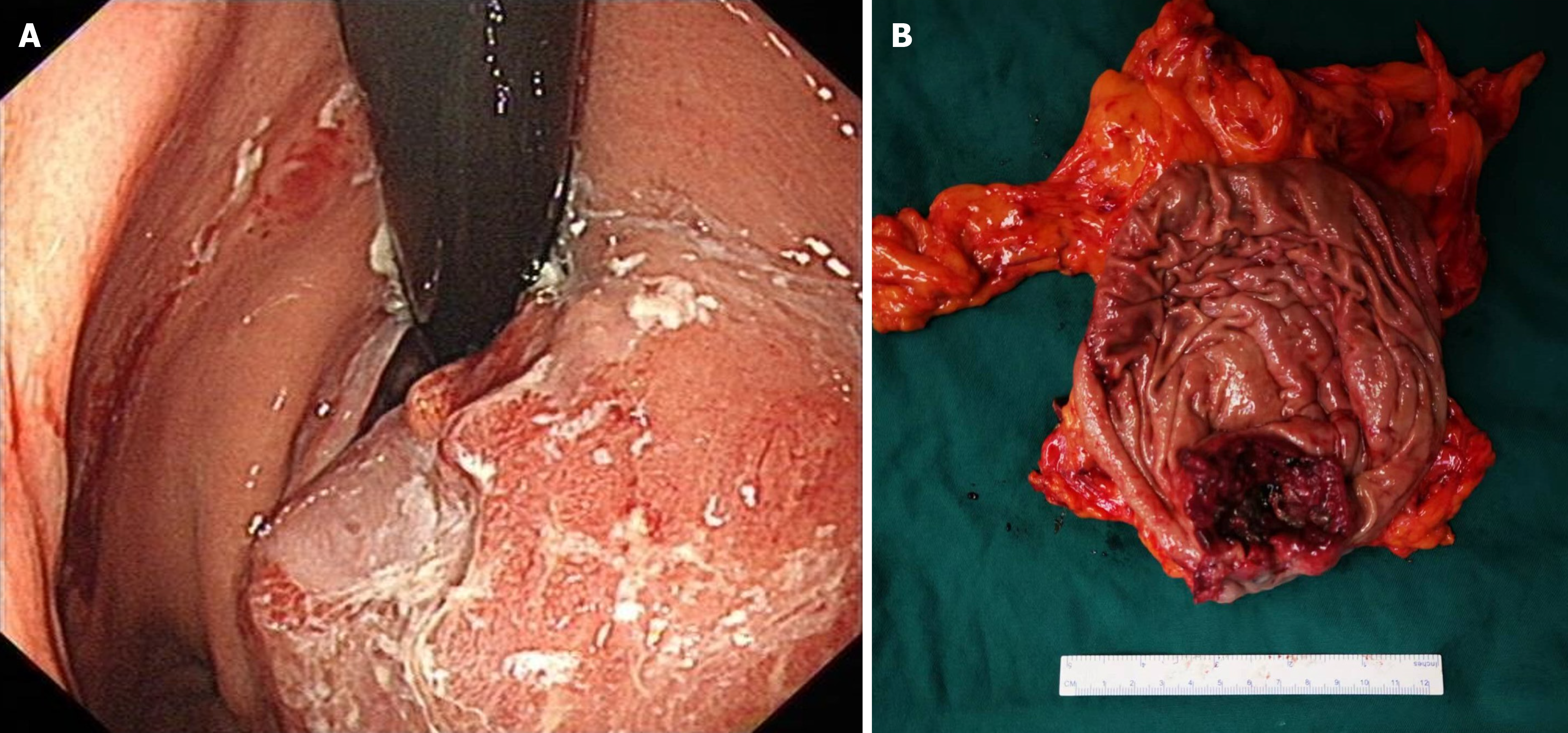
Gastroscopy revealed a space-occupying lesion in the cardia/fundus region of the stomach. A whole-abdominal contrast-enhanced computed tomography (CT) scan showed an irregular mass in the gastric cardia. Positron emission tomo
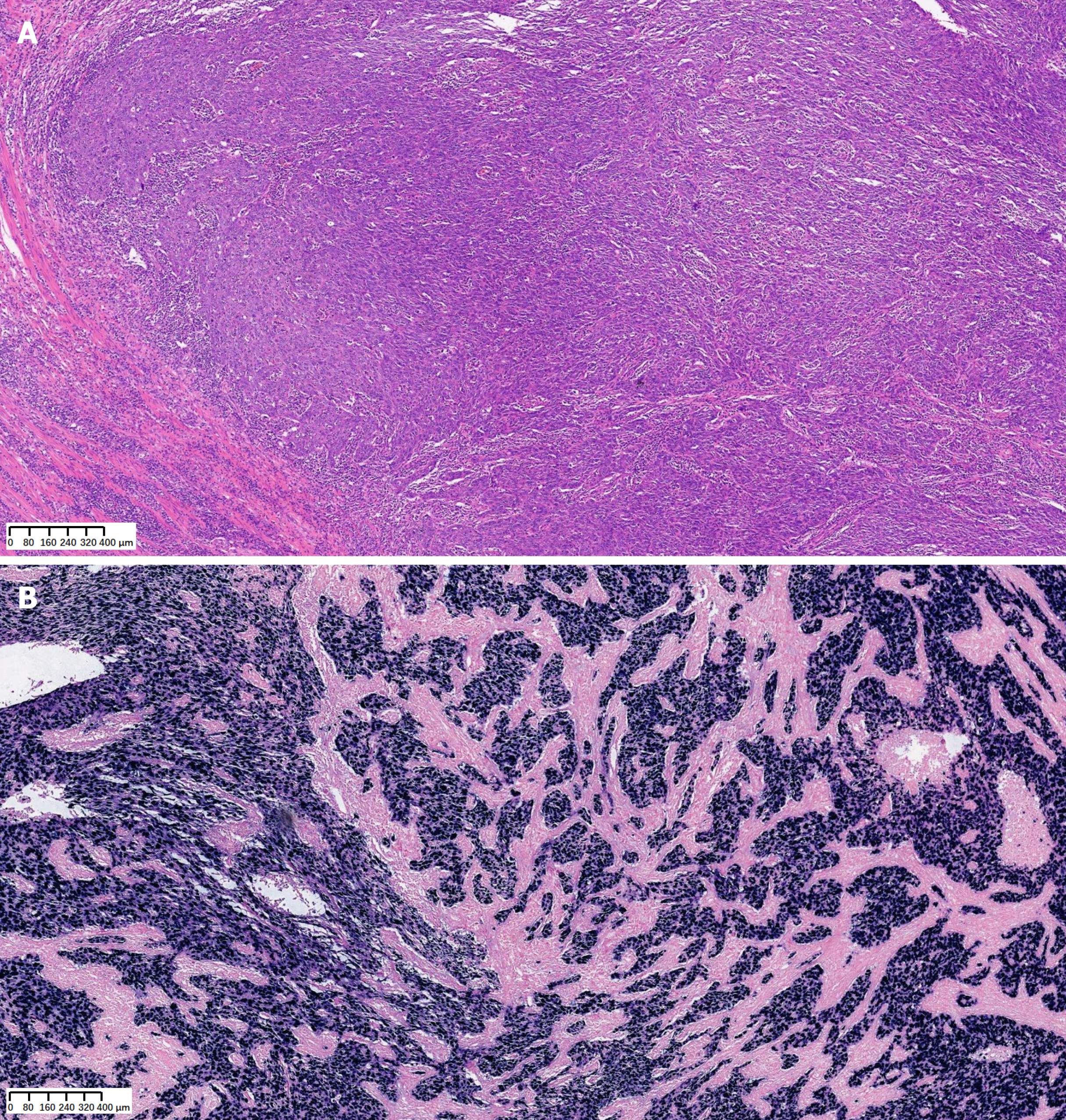
Based on all the findings, the patient was diagnosed with metastatic LELC of the stomach.
Based on the above diagnosis, we performed laparoscopic exploration, proximal gastrectomy, and double-channel ana
The patient underwent immune checkpoint inhibitor (ICI) treatment with a PD-1 inhibitor (toripalimab, 240 mg, in
Pulmonary LELC is a rare type of non-small-cell lung cancer. Cases of pulmonary or metastatic LELC have been reported recently, but to our knowledge, this is the first report of stomach LELC metastasis secondary to a pulmonary tumor.
The most common approach for treating LELC is multimodal therapy. The expression of PD-1/PD-L1 may be related to the prognosis of LELC[10]. Growing evidence shows that ICIs are effective against pulmonary LELC[5]. One study reviewed 36 patients with PLELC treated with PD-1/PD-L1 inhibitors[6]. The objective response rate of all 36 patients was 57.6%, and the patients with higher PD-L1 expression were more likely to have a tumor response. In another study in which patients received multiple treatments that were ineffective, including surgery, chemotherapy and radiotherapy, ICIs proved to be a feasible option[11]. The efficacy of ICI therapy in patients with metastatic stomach LELC is unknown.
In one metastasis study[9], the patient was diagnosed with PLELC as well as metastasis to the mediastinal lymph nodes and liver. After five cycles of nivolumab, the tumor and the lesions in the liver became smaller. The values of CYFRA21-1 and NSE dramatically decreased. Another study found advanced thymic LELC with bone marrow meta
For this case, two questions are worth considering. First, what is the pathway through which lung cancer metastasizes to the stomach? Although gastric metastasis from lung cancer is rare, it can spread to the gastrointestinal tract through hematogenous and lymphatic routes[13]. Based on the findings in the positive LN, which was consistent with those in the stomach lesions, we believe that this patient’s gastric metastasis was through the lymphatic pathway. Second, do we have better treatment options, such as PD-1 inhibitor treatment, before surgery? Because of the growing evidence about PD-1 inhibitors in pulmonary or metastatic LELC, we may prioritize PD-1 inhibitors in later-stage cases, especially for patients who cannot undergo surgical resection.
In conclusion, the present case presents a rare type of stomach tumor secondary to pulmonary LELC. This case de
The case suggested that for metastatic gastric LELC, PD-1 inhibitor therapy can become a potential therapeutic approach. However, there is still a lack of evidence from large data and large samples to support this.
We greatly thank the Department of Gastroenterology Surgery, the Second Affiliated Hospital, Zhejiang University School of Medicine, for providing technical advice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Jabbarpour Z, United Kingdom S-Editor: Chen YL L-Editor: A P-Editor: Xu ZH
| 1. | Sathirareuangchai S, Hirata K. Pulmonary Lymphoepithelioma-like Carcinoma. Arch Pathol Lab Med. 2019;143:1027-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Chen B, Zhang Y, Dai S, Zhou P, Luo W, Wang Z, Chen X, Cheng P, Zheng G, Ren J, Yang X, Li W. Molecular characteristics of primary pulmonary lymphoepithelioma-like carcinoma based on integrated genomic analyses. Signal Transduct Target Ther. 2021;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Fan Y, Li C, Qin J, Lu H. Primary pulmonary lymphoepithelioma-like carcinoma. Med Oncol. 2020;37:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Hong S, Liu D, Luo S, Fang W, Zhan J, Fu S, Zhang Y, Wu X, Zhou H, Chen X, Chen G, Zhang Z, Zheng Q, Li X, Chen J, Liu X, Lei M, Ye C, Wang J, Yang H, Xu X, Zhu S, Yang Y, Zhao Y, Zhou N, Zhao H, Huang Y, Zhang L, Wu K. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat Commun. 2019;10:3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Archwamety A, Ruangchira-Urai R, Akewanlop C, Korphaisarn K. Primary pulmonary lymphoepithelioma-like carcinoma treated with immunotherapy: A case report and literature review. Thorac Cancer. 2022;13:2539-2541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Zhou N, Tang H, Yu S, Lin Y, Wang Y. Anti-PD-1 antibodies, a novel treatment option for advanced chemoresistant pulmonary lymphoepithelioma carcinoma. Front Immunol. 2022;13:1001414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Shima T, Taniguchi K, Kobayashi Y, Kakimoto S, Fujio N, Uchiyama K. Clinical silence of pulmonary lymphoepithelioma-like carcinoma with subcutaneous metastasis: a case report. World J Surg Oncol. 2019;17:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Mlika M, Hamdi B, Marghli A, El Mezni F. About a lymphoepithelioma-like carcinoma of the lung with an endotracheal localization. Tunis Med. 2018;96:451-453. [PubMed] |
| 9. | Qiu ZX, Zhou P, Wang K. Primary Pulmonary Lymphoepithelioma-Like Carcinoma Response Favorably To Nivolumab: A Case Report. Onco Targets Ther. 2019;12:8595-8600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Sha Z, Wei Y, Gao T, Luo Y, Chen J, Li T, Hu L, Niu X, Lin Z, Lv W, Pei X. Clinical observation of pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis. 2021;13:5683-5690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Wu Z, Xian X, Wang K, Cheng D, Li W, Chen B. Immune Checkpoint Blockade Therapy May Be a Feasible Option for Primary Pulmonary Lymphoepithelioma-like Carcinoma. Front Oncol. 2021;11:626566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Li YJ, Li YW, Cui GH, Li SH, Deng YW, Lu D. Advanced thymic lymphoepithelioma-like carcinoma with bone marrow metastases treated by immunotherapy combined with antiangiogenesis therapy: a case report. Anticancer Drugs. 2022;33:686-690. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Tang D, Lv J, Liu Z, Zhan S, Gao Y. Gastric Metastasis of Primary Lung Cancer: Case Report and Systematic Review With Pooled Analysis. Front Oncol. 2022;12:922016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |