Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1291
Revised: April 10, 2024
Accepted: April 24, 2024
Published online: May 27, 2024
Processing time: 142 Days and 17.6 Hours
The prognostic nutritional index (PNI), a marker of immune-nutrition balance, has predictive value for the survival and prognosis of patients with various cancers.
To explore the clinical significance of the preoperative PNI on the prognosis of ampullary adenocarcinoma (AC) patients who underwent curative pancreaticoduodenectomy.
The data concerning 233 patients diagnosed with ACs were extracted and analyzed at our institution from January 1998 to December 2020. All patients were categorized into low and high PNI groups based on the cutoff value determined by receiver operating characteristic curve analysis. We compared disease-free survival (DFS) and overall survival (OS) between these groups and assessed prognostic factors through univariate and multivariate analyses.
The optimal cutoff value for the PNI was established at 45.3. Patients with a PNI ≥ 45.3 were categorized into the PNI-high group, while those with a PNI < 45.3 were assigned to the PNI-low group. Patients within the PNI-low group tended to be of advanced age and exhibited higher levels of aspartate transaminase and total bilirubin and a lower creatinine level than were those in the PNI-high group. The 5-year OS rates for patients with a PNI ≥ 45.3 and a PNI < 45.3 were 61.8% and 43.4%, respectively, while the 5-year DFS rates were 53.5% and 38.3%, respectively. Patients in the PNI- low group had shorter OS (P = 0.006) and DFS (P = 0.012). In addition, multivariate analysis revealed that the PNI, pathological T stage and pathological N stage were found to be independent prognostic factors for both OS and DFS.
The PNI is a straightforward and valuable marker for predicting long-term survival after pancreatoduodenectomy. The PNI should be incorporated into the standard assessment of patients with AC.
Core Tip: In light of emerging evidence that has substantiated the correlation between malnutrition and immune suppression with poor prognosis across various cancer types, we examined the prognostic significance of the preoperative prognostic nutritional index (PNI) in patients with ampullary adenocarcinoma (AC) who underwent curative pancreaticoduodenectomy (PD). Our findings revealed that the PNI, pathological T stage and pathological N stage were independent prognostic factors for both overall survival and disease-free survival in AC patients who underwent curative PD.
- Citation: Sun CY, Zhang XJ, Li Z, Fei H, Li ZF, Zhao DB. Preoperative prognostic nutritional index predicts long-term outcomes of patients with ampullary adenocarcinoma after curative pancreatoduodenectomy. World J Gastrointest Surg 2024; 16(5): 1291-1300
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1291.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1291
Ampullary adenocarcinoma (AC) accounts for only 0.2% of all gastrointestinal malignancies and is exceedingly rare[1,2]. Owing to the distinctive anatomical structure and biliary obstruction of AC, discernible clinical symptoms often appear at an early stage in patients, making surgical interventions feasible. Ordinarily, pancreaticoduodenectomy (PD) is the preferred therapeutic approach[3]. Although ampullary carcinoma exhibits a higher rate of radical resection and a more favorable prognosis compared to other periampullary malignancies, the long-term survival rate beyond five years after radical resection remains modest, ranging from 30% to 53% for these patients[4,5].
The prognostic nutritional index (PNI), which is calculated from the albumin concentration and lymphocyte count, was initially proposed to evaluate the perioperative immune-nutritional status and surgical risk in patients undergoing gastrointestinal surgery[6]. Emerging evidence has shown that malnutrition and immune suppression, which are assessed by the PNI, serve as independent predictors of poor prognosis in various types of cancer[7-10]. Moreover, Sun et al[11] conducted a pooled analysis and revealed that a low PNI was associated with poor overall survival (OS) [pooled odds ratio (OR): 1.80; 95% confidence interval (95%CI): 1.59-2.04] and the presence of postoperative complications (pooled OR: 2.45; 95%CI: 1.31-4.58) in cancer patients. In the context of AC, however, only a limited number of studies have explored this aspect[12], leaving the clinical significance and prognostic value of this marker uncertain.
Therefore, the primary objective of this study was to evaluate the prognostic value of the PNI and investigate its correlation with clinicopathological characteristics in patients diagnosed with AC.
This retrospective study included patients with pathologically confirmed AC who underwent PD for curative resection at the China National Cancer Center between January 1998 and December 2020. Peripheral blood tests were conducted by the laboratory of the center preoperatively. The exclusion criteria were as follows: (1) Patients using anti-inflammatory or immunosuppressive medications; (2) patients with hematological disorders; (3) patients diagnosed with secondary tumors; (4) patients lacking clinicopathological information; and (5) patients lost to follow-up. According to these criteria, a cohort of 233 patients were enrolled in the study. The surgical informed consent forms of these patients were signed, and the study was approved by the institutional review board of the China National Cancer Center.
The clinicopathological characteristics were retrospectively obtained from the medical records and assessed as prognostic factors. These included patient age, sex, tumor size, tumor differentiation, vascular invasion status, TNM stage, number of dissected lymph nodes, postoperative complications, and postoperative adjuvant therapy. AC was classified according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM classification system. Additionally, data from preoperative blood tests, including platelet count, neutrophil cell count, lymphocyte cell count, monocyte count, and serum albumin level, were collated.
At hospital discharge, patients were followed up every 3 months for up to 2 years after surgery, every 6 months for up to 5 years, and thereafter every year or until death. Postoperative follow-up data were collected through telephone reviews, outpatient follow-up, and the death registry system. OS was defined as the duration from the date of surgery to death from any cause or censoring at the time of the last follow-up. Disease-free survival (DFS) was calculated from the date of surgery to the onset of tumor recurrence or death.
(1) PNI: Peripheral serum albumin level (g/L) + 5 × absolute lymphocyte count in peripheral blood (109/L); (2) neutrophil-to-lymphocyte ratio (NLR): Absolute neutrophil count in peripheral blood (109/L)/absolute lymphocyte count in peripheral blood (109/L); (3) platelet-to-lymphocyte ratio (PLR): Absolute platelet count in peripheral blood (109/L)/absolute lymphocyte count in peripheral blood (109/L); and (4) systemic immune-inflammation index (SII): Absolute neutrophil count in peripheral blood (109/L) × absolute platelet count in peripheral blood (109/L)/absolute lymphocyte count in peripheral blood (109/L).
Statistical analysis was performed using SPSS version 26 (SPSS Inc., Chicago, IL, United States) and R software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria). All categorical variables are expressed as frequencies (percentages), and the χ2 test or Fisher’s exact test was used for comparisons of different groups. DFS and OS curves were constructed using the Kaplan-Meier method. The optimal cutoff values of the Inflammatory indicators including PNI, NLR, PLR, and SII were determined by receiver operating characteristic (ROC) analysis. Furthermore, both univariate and multivariate Cox proportional hazard regression analyses were performed to ascertain the independent prognostic factors. Covariates demonstrating P < 0.05 in univariate analyses were incorporated into the subsequent multivariate analysis. All the statistical tests were two-sided, and P values < 0.05 were considered to indicate statistical significance.
After screening according to the inclusion and exclusion criteria, a total of 233 eligible AC patients were enrolled in the study. The median age of the patients was 57 years (range from 14 to 78 years), and the ratio of males to females was 1.33. Overall, lymphatic metastasis was observed in 71 patients (30.5%) and at least 17 lymph nodes were resected in 77 patients (33.0%). After surgery, complications were observed in 91 patients (39.1%), and postoperative adjuvant therapy was administered to 63 patients (27.0%). Analysis of these details revealed that biliary/pancreatic fistula (36.3%), gastric emptying disorder (28.5%), and hemorrhage (16.4%) were the most prevalent postoperative complications. In terms of postoperative therapy, the majority of patients (88.8%) received gemcitabine-based chemotherapy as an adjuvant treatment. The baseline characteristics of the patients are summarized in Table 1.
| Characteristic | Total (n = 233) | PNI-low (n = 100) | PNI-high (n = 133) | P value | |
| Gender | Male | 133 (57.1) | 56 (56.0) | 77 (57.9) | 0.772 |
| Female | 100 (42.9) | 44 (44.0) | 56 (41.1) | ||
| Age, yr | ≤ 60 | 141 (60.5) | 52 (52.0) | 89 (66.9) | 0.021 |
| > 60 | 92 (39.5) | 48 (48.0) | 44 (33.1) | ||
| Tumor size, cm | ≤ 2 | 112 (48.1) | 42 (42.0) | 70 (52.6) | 0.108 |
| > 2 | 121 (51.0) | 58 (58.0) | 63 (47.4) | ||
| Tumor differentiation | Poor differentiation | 88 (37.8) | 43 (43.0) | 45 (33.8) | 0.063 |
| Moderate differentiation | 96 (41.2) | 43 (43.0) | 53 (39.8) | ||
| Well differentiation | 49 (21.0) | 14 (14.0) | 35 (26.4) | ||
| Lymph nodes resection | < 17 | 156 (67.0) | 64 (41.0) | 92 (59.0) | 0.406 |
| ≥ 17 | 77 (33.0) | 36 (46.8) | 41 (53.2) | ||
| Vascular invasion | No | 182 (78.1) | 74 (74.0) | 108 (81.2) | 0.188 |
| Yes | 51 (21.9) | 26 (26.0) | 25 (18.8) | ||
| pT | T1 | 31 (13.3) | 13 (13.0) | 18 (13.5) | 0.8291 |
| T2 | 86 (36.9) | 34 (34.0) | 52 (39.1) | ||
| T3 | 110 (47.2) | 50 (50.0) | 60 (45.1) | ||
| T4 | 6 (2.6) | 3 (3.0) | 3 (2.3) | ||
| pN | N0 | 162 (69.5) | 64 (64.0) | 98 (73.7) | 0.193 |
| N1 | 58 (24.9) | 28 (28.0) | 30 (22.6) | ||
| N2 | 13 (5.6) | 8 (8.0) | 5 (3.7) | ||
| TNM stage | I | 97 (41.6) | 36 (36.0) | 61 (45.9) | 0.213 |
| II | 65 (27.9) | 28 (28.0) | 37 (27.8) | ||
| III | 71 (30.5) | 36 (36.0) | 35 (26.3) | ||
| Postoperative complication | No | 142 (60.9) | 40 (40.0) | 51 (38.3) | 0.798 |
| Yes | 91 (39.1) | 60 (60.0) | 82 (61.6) | ||
| Postoperative adjuvant therapy | No | 152 (65.2) | 29 (29.0) | 34 (25.6) | 0.597 |
| Yes | 63 (27.0) | 64 (64.0) | 88 (74.4) | ||
| Unknow | 18 (7.8) | ||||
| CA199, U/mL | ≤ 59.67 | 107 (45.9) | 38 (38.0) | 69 (51.9) | 0.053 |
| > 59.67 | 107 (45.9) | 52 (52.0) | 55 (48.1) | ||
| Unknow | 19 (8.2) | ||||
| ALT, U/L | ≤ 52.0 | 118 (50.6) | 47 (47.0) | 71 (53.4) | 0.335 |
| > 52.0 | 115 (49.4) | 53 (53.0) | 62 (46.6) | ||
| AST, U/L | ≤ 55.0 | 122 (52.4) | 44 (44.0) | 78 (58.6) | 0.027 |
| > 55.0 | 111 (47.6) | 56 (56.0) | 55 (41.4) | ||
| TBIL, μmol/L | ≤ 51.3 | 117 (50.2) | 42 (42.0) | 75 (56.4) | 0.030 |
| > 51.3 | 116 (49.8) | 58 (58.0) | 58 (43.6) | ||
| Cr, μmol/L | ≤ 62.0 | 115 (49.4) | 61 (61.0) | 54 (40.6) | 0.002 |
| > 62.0 | 118 (50.6) | 39 (39.0) | 79 (59.4) | ||
The predictive efficacy of the PNI was evaluated through ROC curve analysis, and the PNI was compared with other inflammatory biomarkers (PLR, NLR, and SII). The results showed that the PNI exhibited superior precision for OS, with an area under the curve (AUC) of 0.535, surpassing the AUC values of the PLR (0.518), NLR (0.522), and SII (0.501). The recommended PNI cutoff, optimized for both sensitivity and specificity, was determined to be 45.3. Patients were subsequently stratified into PNI-high (PNI ≥ 45.3) and PNI-low (PNI < 45.3) groups.
No statistically significant differences were observed in sex, tumor size, tumor differentiation, vascular invasion or adjuvant therapy between the two groups. Patients aged 60 years or older were more prevalent in the PNI-low group. The incidence of postoperative complications and the severity of the disease (pT, pN, and pTNM stage) were comparable between the two groups (P > 0.05). Patients in the PNI-low group were more likely to exhibit higher aspartate transaminase (AST) and total bilirubin (TBIL) levels and lower creatinine (Cr) levels than were those in the PNI-high group (Table 1).
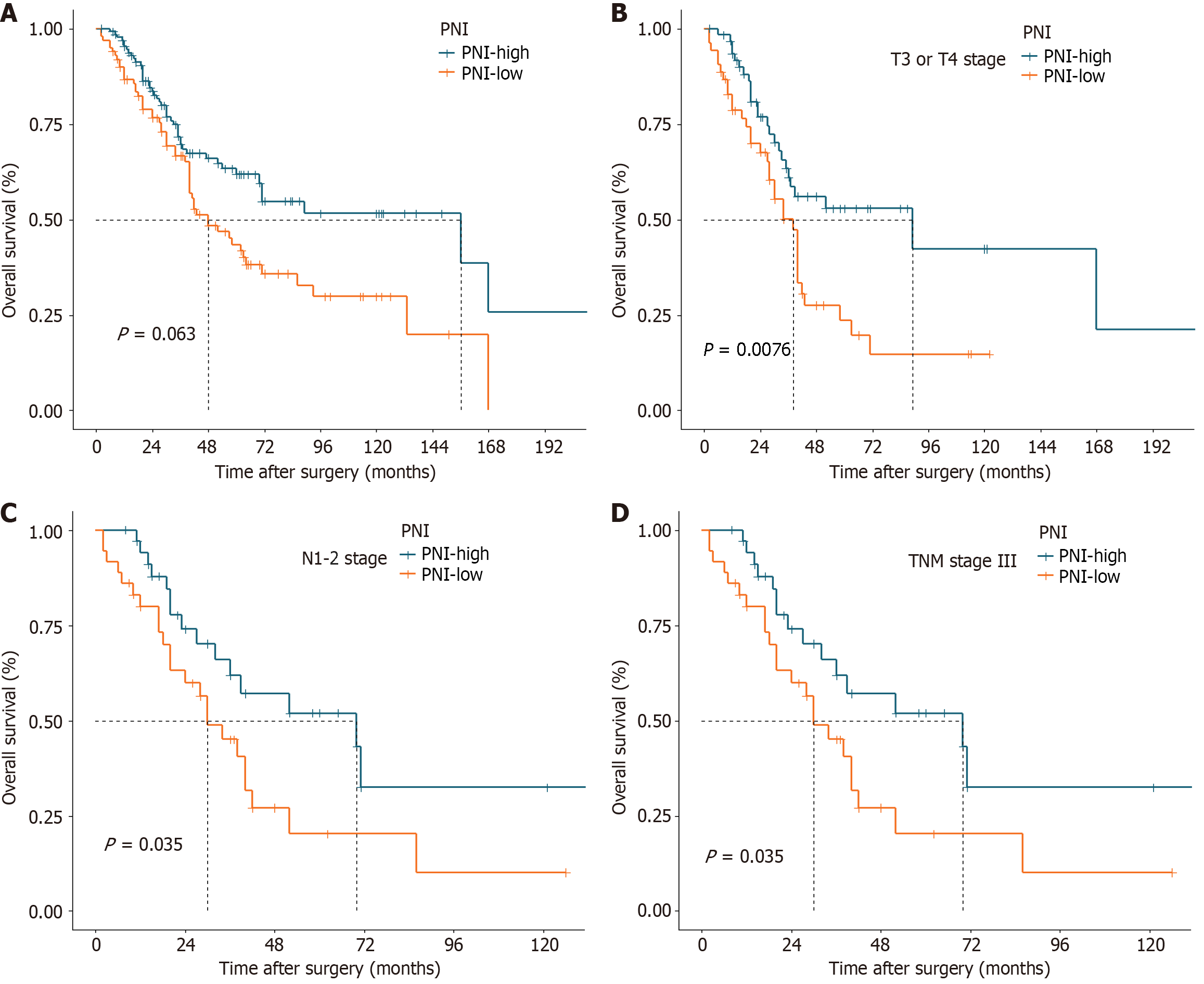
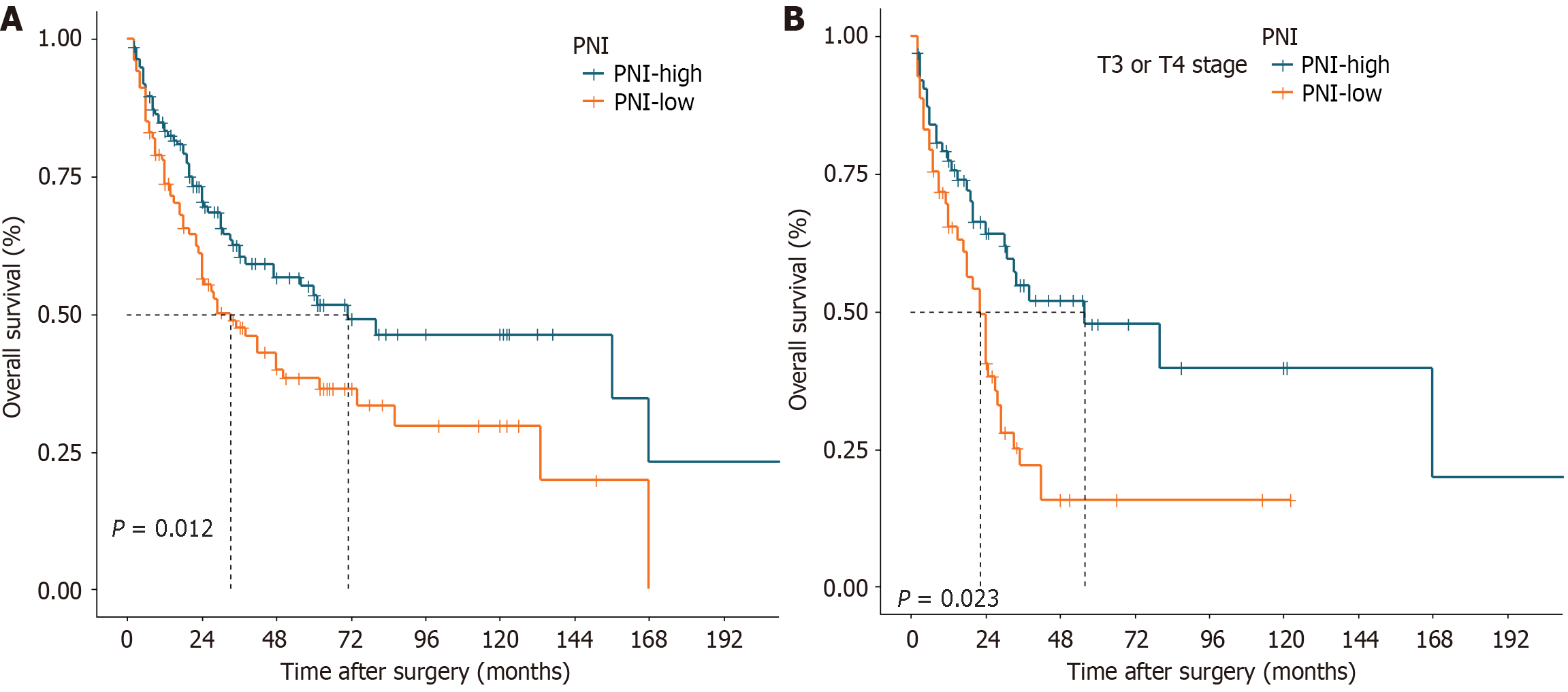
The median OS for the entire cohort was 70 months, with estimated 1-year, 3-year, and 5-year OS rates of 91.6%, 68.4%, and 53.5%, respectively. Patients with a PNI ≥ 45.3 had a significantly greater 5-year OS rate than did those with a PNI < 45.3 (61.8% vs 43.4%, P = 0.006; Figure 1A). According to the stratified analyses involving pT stage, pN stage, and TNM stage, patients with pT3 or pT4 tumors in the PNI-low group had a poorer prognosis (P = 0.008, Figure 1B). Similarly, patients with stage III disease, characterized by lymph node metastasis without distant spread, exhibited worse outcomes in the PNI-low group (P = 0.035, Figure 1C and D). Nonetheless, there were no statistically significant differences between the two groups in the remaining stratified analyses (Supplementary Figure 1).
Univariate analysis for OS showed that pT and pN, but not age, sex, postoperative complications, or adjuvant chemotherapy, were associated with OS. Multivariate analyses revealed that PNI [Hazard ratio (HR): 0.569; 95%CI: 0.383-0.846; P = 0.005], pT stage (HR: 1.901; 95%CI: 1.257-2.876; P =0.002), and pN stage (HR: 1.851; 95%CI: 1.209-2.834; P =0.005) were independent factors associated with OS (Table 2).
| Variate | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender (male vs female) | 0.882 | 0.593-1.313 | 0.537 | |||
| age (≤ 60 vs > 60) | 1.147 | 0.769-1.713 | 0.501 | |||
| Tumor size (≤ 2 vs > 2) | 1.142 | 0.773-1.689 | 0.504 | |||
| Tumor differentiation (non-well vs well) | 0.667 | 0.407-1.094 | 0.109 | |||
| Lymph nodes resection (< 17 vs ≥ 17) | 1.161 | 0.761-1.771 | 0.488 | |||
| Vascular invasion (No vs Yes) | 1.318 | 0.846-2.053 | 0.222 | |||
| pT (T1, T2 vs T3, T4) | 2.114 | 1.417-3.153 | < 0.001 | 1.901 | 1.257-2.876 | 0.002 |
| pN (N0 vs N1, N2) | 2.197 | 1.454-3.319 | < 0.001 | 1.851 | 1.209-2.834 | 0.005 |
| Postoperative complication (No vs Yes) | 1.043 | 0.695-1.567 | 0.838 | |||
| Postoperative adjuvant therapy (No vs Yes) | 1.432 | 0.934-2.195 | 0.100 | |||
| PNI (< 45.3 vs ≥ 45.3) | 0.584 | 0.393-0.866 | 0.007 | 0.569 | 0.383-0.846 | 0.005 |
The estimated 1-year, 3-year, and 5-year DFS rates for all patients were 81.0%, 59.8%, and 52.7%, respectively, with a median DFS of 71 months. Patients with a PNI ≥ 45.3 also had a significantly greater 5-year DFS rate than did those with a PNI < 45.3 (53.5% vs 38.3%, P = 0.012; Figure 2A). After stratified analyses were performed, it was observed that among patients with stage pT3 or pT4 disease, those with a lower PNI tended to experience shorter DFS (P = 0.002; Figure 2B). No statistically significant differences were observed between the two groups in the remaining stratified analyses (Supplementary Figure 2).
The results of univariate survival analysis for DFS indicated associations with vascular invasion, pT stage, pN stage, and postoperative adjuvant therapy. Multivariate analysis identified PNI (HR: 0.674; 95%CI: 0.464-0.980; P = 0.039), pT stage (HR: 1.819; 95%CI: 1.215-2.723; P = 0.004) and pN stage (HR: 1.793; 95%CI: 1.130-2.846; P = 0.013) as independent predictors of DFS (Table 3).
| Variate | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender (male vs female) | 0.945 | 0.653-1.367 | 0.764 | |||
| Age (≤ 60 vs > 60) | 1.048 | 0.723-1.518 | 0.806 | |||
| Tumor size (≤ 2 vs > 2) | 1.213 | 0.844-1.745 | 0.297 | |||
| Tumor differentiation (non-well vs well) | 0.666 | 0.420-1.056 | 0.084 | |||
| Lymph nodes resection (< 17 vs ≥ 17) | 1.061 | 0.711-1.583 | 0.771 | |||
| Vascular invasion (No vs Yes) | 1.523 | 1.012-2.291 | 0.044 | 1.063 | 0.677-1.668 | 0.792 |
| pT (T1, T2 vs T3, T4) | 1.975 | 1.363-2.861 | < 0.001 | 1.819 | 1.215-2.723 | 0.004 |
| pN (N0 vs N1, N2) | 2.113 | 1.440-3.101 | < 0.001 | 1.793 | 1.130-2.846 | 0.013 |
| Postoperative complication (No vs Yes) | 1.022 | 0.701-1.491 | 0.909 | |||
| Postoperative adjuvant therapy (No vs Yes) | 1.591 | 1.072-2.360 | 0.021 | 0.958 | 0.590-1.557 | 0.863 |
| PNI (< 45.3 vs ≥ 45.3) | 0.631 | 0.438-0.909 | 0.013 | 0.674 | 0.464-0.980 | 0.039 |
The prognostic and clinicopathological significance of the PNI has been investigated in various malignancies. However, its specific role in patients with AC remains unclear. To the best of our knowledge, this is the first retrospective study in which the prognostic significance of the PNI and its correlation with clinicopathological characteristics in AC patients has been comprehensively examined. Our findings indicated that a high preoperative PNI was a significant predictor of improved OS and DFS, which might have a favorable impact on AC patients who underwent curative surgical resection.
The PNI serves as a biomarker for evaluating the nutritional and inflammatory status of patients. It was initially developed by Onodera et al[6] to evaluate the nutritional status of surgical patients, predict surgical risk, and determine prognosis. Subsequent studies have illuminated the predictive utility of the PNI across diverse tumor types. Okadome et al[13] reported that the PNI was valuable for assessing survival in patients with esophageal cancer. Yang et al[14] indicated that the preoperative PNI serves as a valuable predictor of postoperative complications and survival outcomes in patients diagnosed with gastric cancer. Park et al[15] also substantiated that the PNI, an indicator of immune-nutritional status, could predict the long-term outcome of non-small cell lung cancer patients. Our present study revealed that the OS and DFS rates of patients in the PNI-low group were significantly lower than those in the PNI-high group (both P < 0.05) and the predictive efficacy of the PNI was superior to that of other inflammatory biomarkers (PLR, NLR, and SII), with an AUC value of 0.535. Multivariate analysis revealed that the prognostic significance of the PNI paralleled that of lymph node metastasis (N stage) and infiltration depth (T stage). A diminished PNI was independently linked to a less favorable prognosis for individuals afflicted with AC. According to the stratified analysis, the PNI-low group exhibited significantly lower OS and DFS rates than did the PNI high-group among patients with T3 or T3 stage disease, while only an OS rate difference was observed between the PNI-high and PNI-low groups with lymph node metastasis.
Several factors contribute to the association between a low PNI and poor prognosis in patients with AC. First, the serum albumin concentration not only reflects nutritional status but also serves as a biomarker for systemic inflammation[16]. Some inflammatory factors may impede albumin synthesis, while oxidative stress can lead to the denaturation of albumin, both of which contribute to the swift reduction in serum albumin levels among patients in an inflammatory state[17-19]. Malnutrition and systemic inflammation are important factors driving tumor progression and metastasis[20-22]. Lymphocytes are essential components of the immune system with the capacity to eradicate cancer cells, making them indicative of immunological status. Research has indicated a significant association between reduced serum lymphocytes and adverse prognosis among cancer patients[23-25]. Therefore, the PNI reflects both the nutritional and immunological status of the host and can be a predictor of prognosis in patients with cancer. Second, we noted a close correlation between the PNI and age, TBIL, and AST. This observation aligns with the findings of Konishi et al[7], who noted a significantly higher PNI among younger patients undergoing gastrectomy compared to older patients. In our investigation, we found that the proportion of patients aged 60 years or older was higher in the PNI-low group than in the PNI-high group (P = 0.021). Numerous studies have corroborated that advanced age serves as an independent adverse prognostic factor for cancer patients[26,27]. In addition, the elevated levels of TBIL and AST in the PNI-high group may be attributed to biliary obstruction and impaired liver function, which have also been confirmed to be associated with poor prognosis in colorectal[28] and breast cancer patients[29]. Third, considering the established adverse effect of severe postoperative complications on long-term outcomes[30], the poorer OS and DFS in the low PNI group could be attributed to a greater incidence of postoperative complications. However, our present study revealed comparable incidences of severe postoperative complications between the two groups, with no statistically significant difference. Sakurai et al[31] reported a lack of significant correlation between the preoperative PNI and postoperative complications, probably because recent improvements in perioperative management have enhanced the safety of surgery.
Therefore, maintaining and/or increasing the preoperative PNI appears to be crucial for improving the outcomes of AC patients. Migita et al[32] found that oral nutritional supplementation did not increase the PNI in gastric cancer patients with low PNI values (baseline vs before surgery: 44.0 ± 3.9 vs 43.0 ± 4.4, P = 0.049). Similarly, Gunsel-Yildirim et al[33] observed that despite providing lung cancer patients with oral immunonutritional support twice daily, PNI levels significantly decreased in the postoperative period compared to those in the preoperative period. However, in a study involving patients undergoing PD, Tsukagoshi et al[34] reported that preoperative nutritional support (enteral nutrition) and prehabilitation (resistance or aerobic exercises) prevented a decrease in the PNI in patients with skeletal muscle loss. Paccagnella et al[35] also found that perioperative supplementation with arginine can reduce the incidence of complications and significantly increase long-term survival. Due to potential differences in patient characteristics and interventions across various studies, the results may exhibit heterogeneity. Nevertheless, it remains important for physicians to pay special attention to perioperative care for patients with low PNI values.
Furthermore, the optimal cutoff value for the PNI to effectively predict long-term outcomes remains uncertain. According to a meta-analysis focused on gastric cancer, the PNI cutoff value in the included studies varied between 40.0 and 49.7[14]. Okamura et al[36] found that the optimal cutoff value for the PNI for prognosis differs among TNM stages. Initially, this value was established at 45 because resection and anastomosis of the gastrointestinal tract can be safely performed when the PNI is > 45[6]. In our present study, we conducted an ROC curve analysis and identified the optimal PNI cutoff value as 45.3, where the Youden index was maximal. However, whether this cutoff value is the optimal prognostic value remains unknown, requiring further well-designed studies for clarification.
The strength of our study lies in being the first to establish a significant correlation between the PNI and the prognosis of patients with ampullary carcinoma following PD. The use of perioperative immunonutrition may improve early postoperative nutritional status and reduce postoperative complications for patients with ampullary carcinoma. Furthermore, the inclusion of data on DFS in our follow-up strengthens the validity of our research findings. Nevertheless, we must admit the limitations of the current study. First, this retrospective study is subject to selection bias due to inherent limitations in sample selection and data collection. Second, the follow-up time of some patients was relatively short, which is a limitation when pushing the results into clinical practice. Third, due to the lack of postoperative peripheral blood testing, we were unable to further assess the impact of dynamic changes in PNI on prognosis.
In this study, the preoperative PNI could be clinically utilized as a straightforward and valuable marker for predicting long-term survival after surgery. Physicians should enhance perioperative management for patients with low preoperative PNI.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Sperti C, Italy S-Editor: Chen YL L-Editor: A P-Editor: Guo X
| 1. | Hester CA, Dogeas E, Augustine MM, Mansour JC, Polanco PM, Porembka MR, Wang SC, Zeh HJ, Yopp AC. Incidence and comparative outcomes of periampullary cancer: A population-based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J Surg Oncol. 2019;119:303-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Nagino M, Hirano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, Ebata T, Konishi M, Sano K, Shimada K, Shimizu H, Higuchi R, Wakai T, Isayama H, Okusaka T, Tsuyuguchi T, Hirooka Y, Furuse J, Maguchi H, Suzuki K, Yamazaki H, Kijima H, Yanagisawa A, Yoshida M, Yokoyama Y, Mizuno T, Endo I. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J Hepatobiliary Pancreat Sci. 2021;28:26-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 4. | Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014;112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 5. | Moekotte AL, Lof S, Van Roessel S, Fontana M, Dreyer S, Shablak A, Casciani F, Mavroeidis VK, Robinson S, Khalil K, Gradinariu G, Mowbray N, Al-Sarireh B, Fusai GK, Roberts K, White S, Soonawalla Z, Jamieson NB, Salvia R, Besselink MG, Abu Hilal M. Histopathologic Predictors of Survival and Recurrence in Resected Ampullary Adenocarcinoma: International Multicenter Cohort Study. Ann Surg. 2020;272:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-1005. [PubMed] |
| 7. | Konishi T, Kosuga T, Inoue H, Konishi H, Shiozaki A, Kubota T, Okamoto K, Fujiwara H, Otsuji E. Significance of Preoperative Prognostic Nutritional Index in the Perioperative Management of Gastric Cancer. J Gastrointest Surg. 2022;26:558-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 8. | Lee J, Weng CS, Chang CL, Hsu WH, Jan YT, Wu KP. Association of prognostic nutritional index with muscle loss and survival in patients with ovarian cancer treated with primary debulking surgery and chemotherapy. Support Care Cancer. 2023;31:267. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Kazi M, Gori J, Sasi S, Srivastava N, Khan AM, Mukherjee S, Garg V, Singh L, Mundhada R, Patil P, Desouza A, Saklani A. Prognostic Nutritional Index Prior to Rectal Cancer Resection Predicts Overall Survival. Nutr Cancer. 2022;74:3228-3235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Salati M, Filippi R, Vivaldi C, Caputo F, Leone F, Salani F, Cerma K, Aglietta M, Fornaro L, Sperti E, Di Maio M, Ortega C, Fenocchio E, Lombardi P, Cagnazzo C, Depetris I, Gelsomino F, Spallanzani A, Santini D, Silvestris N, Aprile G, Roviello G, Scartozzi M, Cascinu S, Casadei-Gardini A. The prognostic nutritional index predicts survival and response to first-line chemotherapy in advanced biliary cancer. Liver Int. 2020;40:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 12. | Sun S, He C, Wang J, Huang X, Wu J, Li S. The prognostic significance of inflammation-based scores in patients with ampullary carcinoma after pancreaticoduodenectomy. BMC Cancer. 2020;20:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann Surg. 2020;271:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, Ma B, Wang Z. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. 2016;42:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 15. | Park S, Ahn HJ, Yang M, Kim JA, Kim JK, Park SJ. The prognostic nutritional index and postoperative complications after curative lung cancer resection: A retrospective cohort study. J Thorac Cardiovasc Surg. 2020;160:276-285.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, Jensen GL; ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr Clin Pract. 2021;36:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 17. | Bito R, Hino S, Baba A, Tanaka M, Watabe H, Kawabata H. Degradation of oxidative stress-induced denatured albumin in rat liver endothelial cells. Am J Physiol Cell Physiol. 2005;289:C531-C542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59:945-952. [PubMed] |
| 19. | Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 916] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 20. | Salas S, Cottet V, Dossus L, Fassier P, Ginhac J, Latino-Martel P, Romieu I, Schneider S, Srour B, Touillaud M, Touvier M, Ancellin R. Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1623] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 22. | Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, Zhou L. Prognostic Nutritional Index and Systemic Immune-Inflammation Index Predict the Prognosis of Patients with HCC. J Gastrointest Surg. 2021;25:421-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 23. | Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 2228] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 24. | Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, Vanella V, Simeone E, Paone M, Palmieri G, Cavalcanti E, Caracò C, Ascierto PA. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 25. | Kim EY, Song KY. The preoperative and the postoperative neutrophil-to-lymphocyte ratios both predict prognosis in gastric cancer patients. World J Surg Oncol. 2020;18:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Kung CY, Fang WL, Wang RF, Liu CA, Li AFY, Wu CW, Shyr YM, Chou SC, Huang KH. Prognosis and clinicopathologic features in patients with gastric stump cancer after curative surgery. Curr Oncol. 2020;27:e259-e264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Yao L, Luo J, Liu L, Wu Q, Zhou R, Li L, Zhang C. Risk factors for postoperative pneumonia and prognosis in lung cancer patients after surgery: A retrospective study. Medicine (Baltimore). 2021;100:e25295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Cao Y, Deng S, Yan L, Gu J, Yang J, Yang M, Liu L, Cai K. A nomogram based on pretreatment levels of serum bilirubin and total bile acid levels predicts survival in colorectal cancer patients. BMC Cancer. 2021;21:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Liu X, Meng QH, Ye Y, Hildebrandt MA, Gu J, Wu X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. 2015;36:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Scaife CL, Hartz A, Pappas L, Pelletier P, He T, Glasgow RE, Mulvihill SJ. Association between postoperative complications and clinical cancer outcomes. Ann Surg Oncol. 2013;20:4063-4066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, Tanaka H, Muguruma K, Yashiro M, Maeda K, Hirakawa K. Predictive Potential of Preoperative Nutritional Status in Long-Term Outcome Projections for Patients with Gastric Cancer. Ann Surg Oncol. 2016;23:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Migita K, Matsumoto S, Wakatsuki K, Kunishige T, Nakade H, Miyao S, Sho M. Effect of Oral Nutritional Supplementation on the Prognostic Nutritional Index in Gastric Cancer Patients. Nutr Cancer. 2021;73:2420-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Gunsel-Yildirim G, Ceylan KC, Dikmen D. The effect of perioperative immunonutritional support on nutritional and inflammatory status in patients undergoing lung cancer surgery: a prospective, randomized controlled study. Support Care Cancer. 2023;31:365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Tsukagoshi M, Harimoto N, Araki K, Kubo N, Watanabe A, Igarashi T, Ishii N, Yamanaka T, Hagiwara K, Hoshino K, Muranushi R, Yajima T, Wada N, Shirabe K. Impact of preoperative nutritional support and rehabilitation therapy in patients undergoing pancreaticoduodenectomy. Int J Clin Oncol. 2021;26:1698-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol. 2011;23:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Okamura Y, Sugiura T, Ito T, Yamamoto Y, Ashida R, Uesaka K. The optimal cut-off value of the preoperative prognostic nutritional index for the survival differs according to the TNM stage in hepatocellular carcinoma. Surg Today. 2017;47:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |