Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.944
Peer-review started: November 29, 2023
First decision: December 6, 2023
Revised: December 17, 2023
Accepted: February 22, 2024
Article in press: February 22, 2024
Published online: March 27, 2024
Processing time: 113 Days and 20 Hours
Appendiceal mucinous neoplasms (AMNs), although not classified as rare, are relatively uncommon tumors most often discovered incidentally during colorectal surgery. Accurate identification of AMNs is difficult due to non-specific sym
This case report describes the unexpected discovery and treatment of a low-grade AMN (LAMN) in a 74-year-old man undergoing laparoscopic hemicolectomy for transverse colon adenocarcinoma (AC). Preoperatively, non-specific gastroin
This case highlights the diagnostic complexity of AMNs, emphasizing the need for vigilant identification to avert potential complications, such as pseudomyxoma peritonei.
Core Tip: Here we present a case of an appendiceal mucinous neoplasm (AMN) incidentally identified during a laparoscopic hemicolectomy performed to treat adenocarcinoma in the transverse colon of a 74-year-old male. This presentation underscores the diagnostic complexity posed by AMNs, which often manifest nonspecifically and may be mistaken for other malignancies. This case highlights the critical role of immunohistochemical analysis in establishing a definitive diagnosis and informing surgical strategy, with a particular focus on averting misdiagnosis and potential complications, such as pseudomyxoma peritonei.
- Citation: Chang HC, Kang JC, Pu TW, Su RY, Chen CY, Hu JM. Mucinous neoplasm of the appendix: A case report and review of literature. World J Gastrointest Surg 2024; 16(3): 944-954
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/944.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.944
Appendiceal mucinous neoplasm (AMN) is an uncommon malignancy originating from the epithelium of the appendix, often incidentally discovered during surgical procedures. Symptoms may be absent or nonspecific, making preoperative identification difficult. In females, elevated tumor markers [carbohydrate antigen 19-9 (CA19-9), CA 125, or carcinoembryonic antigen (CEA)] can lead to misdiagnosis, particularly as ovarian cancer[1-3]. As such, AMN should be considered in the differential diagnosis of asymptomatic right adnexal cystic masses, particularly in postmenopausal women with increased tumor marker levels. Surgical removal, prioritizing the prevention of appendiceal rupture to avoid mucin spr
Herein, we report the case of a 74-year-old male in whom a low-grade AMN (LAMN), initially misdiagnosed as a peritoneal cyst, was incidentally discovered during laparoscopic extended right hemicolectomy for transverse colon ade
A 74-year-old Chinese man presented the gastrointestinal outpatient clinic with gastrointestinal discomfort and occult blood in the stool.
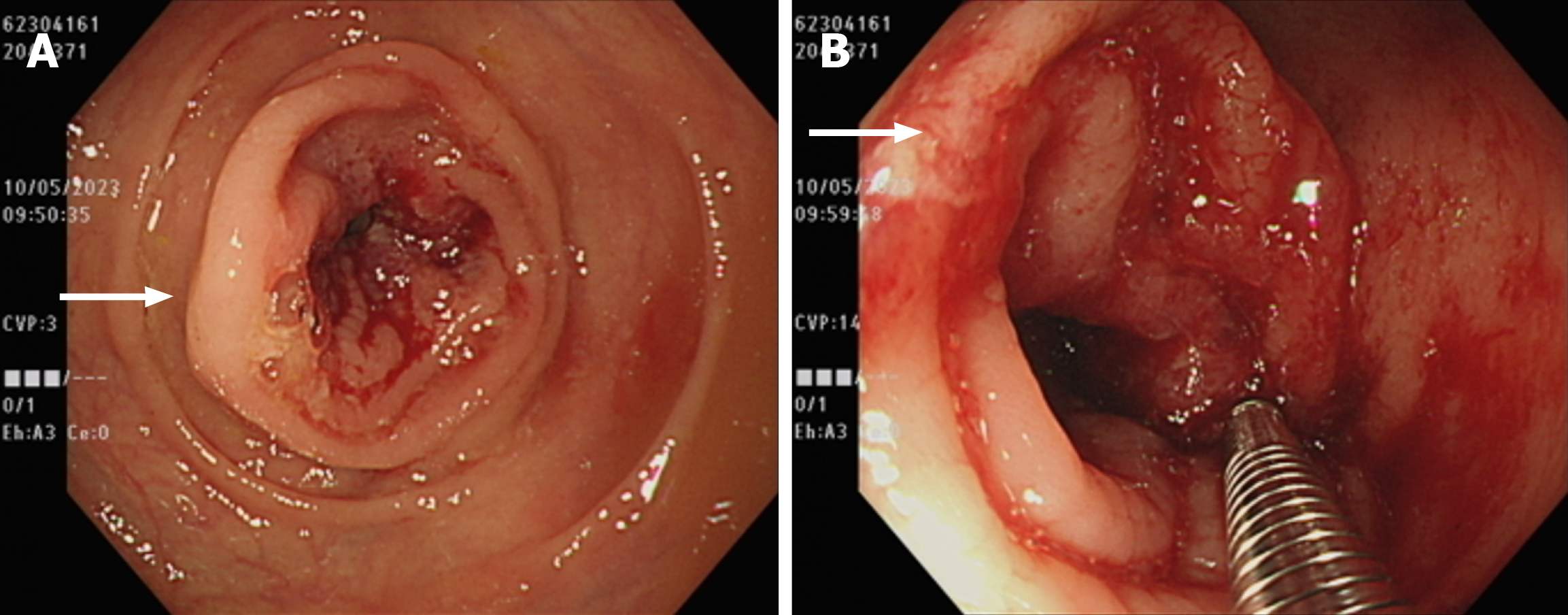
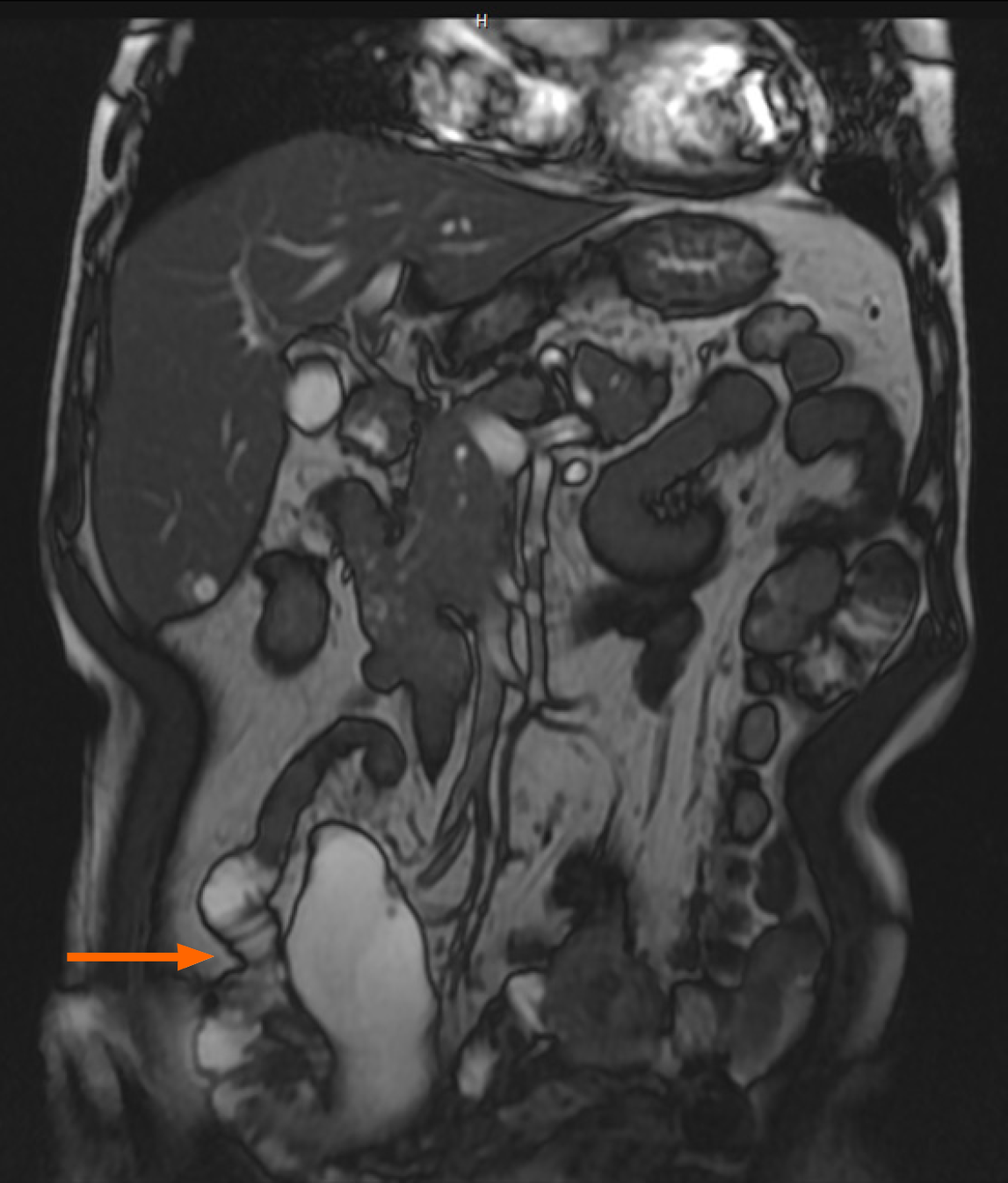
The patient’s condition was characterized by an unexplained weight loss and hematochezia for 6 months. Colonoscopy revealed obstructive AC in the transverse colon, causing further investigative challenges (Figures 1 and 2).
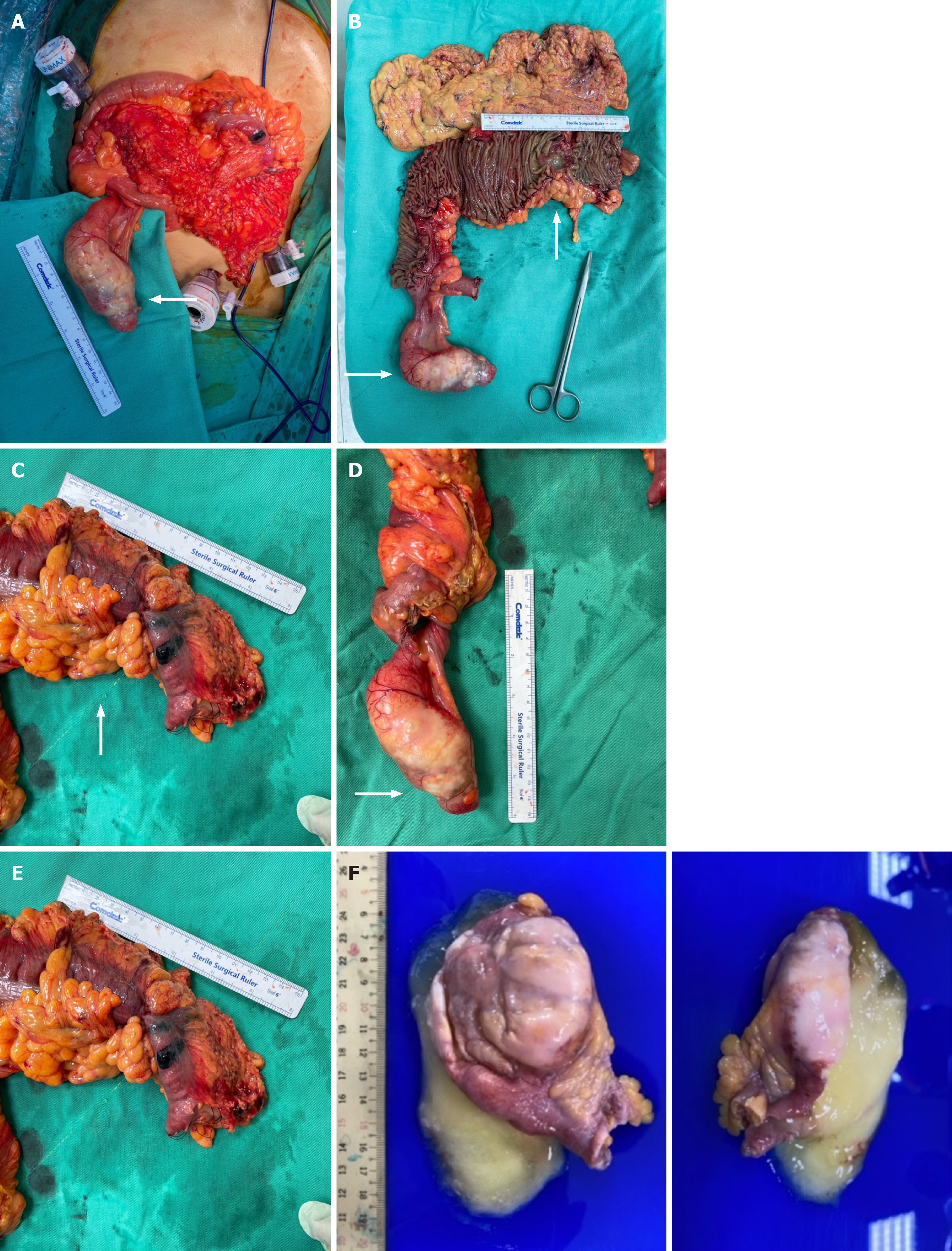
The patient underwent extended right hemicolectomy during surgery. A 6 cm transverse colon tumor with regional lymph nodes was excised, along with a 9 cm encapsulated appendiceal mass. Histopathological analysis revealed a 4 cm × 4 cm ulcerative colon tumor infiltrating the muscularis layer and a diffusely enlarged 6 cm × 9 cm appendix with a significantly thinned mucin-filled wall with no solid components (Figure 3A-E). Postoperatively, the patient was extu
The patient denied any family history of malignant tumors.
During physical assessment, the patient's vital signs were recorded as follows: Body temperature of 36.0 ℃, blood pre
Preoperative laboratory evaluations revealed a normal complete blood count, including a white blood cell count of 7830/μL, red blood cell count of 4250/μL, mean corpuscular volume of 87.8 fL, mean corpuscular hemoglobin of 27.5 pg, and mean corpuscular hemoglobin concentration of 31.4 g/dL. The coagulation panel revealed an international normalized ratio of 1.0 and an activated partial thromboplastin time of 34.4 s. Overall, the patient’s blood work demonstrated no significant abnormalities. Tumor marker analysis revealed normal alpha-fetoprotein levels (2.80 ng/mL), elevated CEA levels (9.05 ng/mL), and normal CA19-9 levels (12.2 U/mL). Squamous cell carcinoma antigen (0.5 ng/mL) and prostate-specific antigen (0.702 ng/mL) remained within the normal ranges. Postoperative immunohistochemical (IHC) analysis revealed positive expression of epidermal growth factor receptor and intact mismatch repair (MMR) proteins.
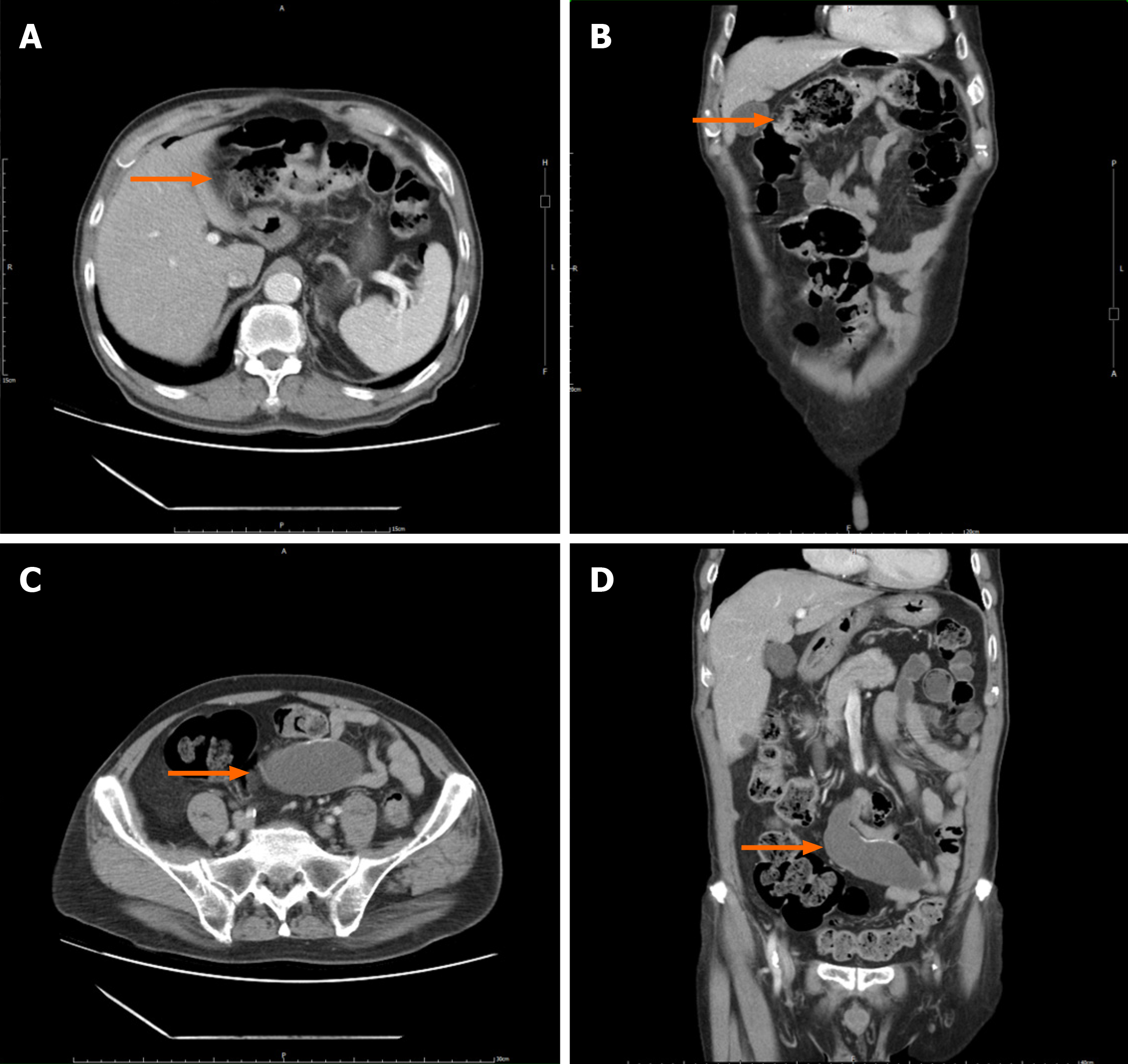
Computed tomography (CT) and magnetic resonance imaging (MRI) confirmed transverse colon wall thickening and regional lymphadenopathy and identified a 9 cm mesenteric cystic mass, supporting the clinical diagnosis of transverse colon AC (Figures 4 and 5).
Pathological examination of the transverse colon revealed a 35.0 cm segment harboring three ulcerative tumors invading the muscularis propria. Two of these tumors were well-differentiated, while the third showed only moderate differentiation. Metastasis was identified in only one of the 26 examined lymph nodes (pT3N1aMx, mpStage IIIB). IHC analysis confirmed epidermal growth factor receptor positivity (1+) and intact expression of MMR proteins, suggesting a low pro
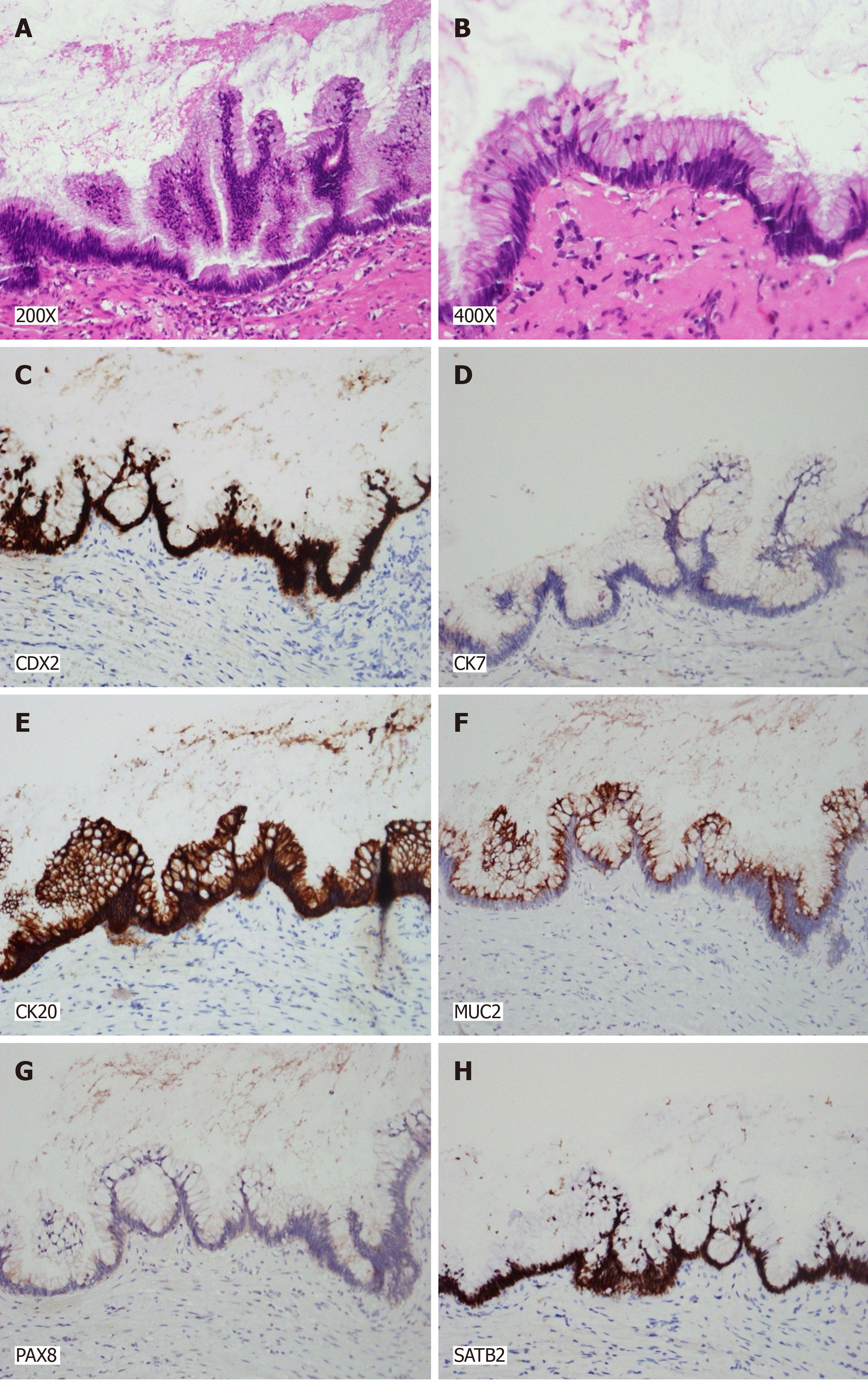
Examination of the appendiceal lesion revealed a 10.6 cm × 4.0 cm × 3.3 cm LAMN filled with mucinous material (Figure 3F). The neoplasm was lined by tall columnar epithelial cells exhibiting low-grade dysplastic alterations, em
The patient was finally diagnosed with transverse colon AC, classical subtype, with lymphovascular invasion (mpStage IIIB), and concurrent LAMN, stage TisN0M0.
In the postoperative phase, the patient exhibited a favorable recovery, culminating in discharge on the seventh day following the surgical procedure. Four weeks after surgery, the patient commenced a chemotherapeutic regimen com
One month following surgery, the patient’s CEA levels presented a slight increase (7.81 ng/mL), albeit with a significant decrease compared to the initial reference point. Concurrent chest and abdominal radiographic evaluations showed no abnormalities, and the surgical wound exhibited satisfactory healing.
AMN is a relatively rare pathological condition characterized by the cystic dilation of the appendiceal lumen filled with mucinous content. Clinically, AMNs can present with various syndromes, or may be detected incidentally. Epidemiological data suggest that AMNs occur in approximately 0.2%-0.4% of appendectomy specimens and constitute 0.4%-1% of all gastrointestinal malignancies in the United States, with an estimated 1500 new cases each year[1-5].
Individuals with appendiceal tumors may present with non-specific clinical symptoms, leading to potential delays in diagnosis[6,7]. In the early stages of the disease, symptoms may resemble acute appendicitis, often manifesting as right lower quadrant pain, which can be attributed to mucin-induced distension of the appendix[8]. Complications such as appendicitis or perforation of the appendix can occur if the neoplasm obstructs the appendiceal lumen. A previous study reported that 32% of patients with appendiceal neoplasms are preliminarily diagnosed with acute appendicitis before sur
In the advanced stages of the disease, patients may exhibit increased abdominal girth, often due to the accumulation of mucinous ascitic fluid within the peritoneal cavity. Other clinical features at this stage may include chronic abdominal pain, significant weight loss, anemia, infertility, and the development of umbilical or inguinal hernias[6,10]. Although mucinous implants are frequently found on peritoneal, serosal, and omental layers, the initial presentation involving in
Preoperative diagnosis of appendiceal mucoceles poses significant challenges owing to its typically asymptomatic nature and non-specific clinical manifestations. Diagnostic imaging techniques, such as ultrasound and CT, are com
Historically, the taxonomy of appendiceal ACs has been debated, yielding disparate conclusions. The Peritoneal Sur
In the realm of AMNs, the TNM classification system delineated by the AJCC serves as a preliminary framework for staging. This system involves methodical evaluation of the dimensions and invasion depth of the primary tumor, the extent of lymph node involvement, presence of distant metastasis, and histological grade[25]. Distinctions in survival rates are apparent when comparing non-mucinous tumors with mucinous histology.
In a previous study, in the cohort with non-mucinous histologic types, 5-year survival rates were 90.34%, 87.97%, 75.47%, and 59.84% for stages I, IIA, IIB, and IIC, respectively. Notably, patients with stages IIIA and IIIB disease ex
Despite the in-situ nature of the AMN, the possibility of metastasis must still be considered. In our case, the patient had coexisting transverse colon cancer, specifically the classical AC subtype, which virtually eliminated the possibility of a metastatic origin for the AMN. However, it is important to remember that colon tumors can exhibit a range of subtypes. Currently, four main subtypes are recognized: Classical AC, mucinous AC (MAC), signet-ring cell carcinoma, and mixed subtypes[27-29]. Of note, MAC may also develop in the colon, meaning that metastatic concerns should not be excluded. To date, no studies have yet reported a double tumor of MACs due to its rarity.
In contrast to patients with non-mucinous tumors, patients with mucinous and signet ring cell appendiceal cancers demonstrated a more systematic hierarchical order in 5-year overall survival rates when grouped by pathological stage. Specifically, individuals with mucinous histology at stage I had a 5-year survival rate of 92.80%, with a sequential decrease noted across advancing stages: 84.32% for stage IIA, 79.71% for stage IIB, 72.07% for stage IIC, 58.01% for stage IIIA to IIIC, 63.25% for stage IVA to IVB, and 46.81% for stage IVC[26]. Moreover, the survival metrics for patients presenting with signet ring cell histology were as follows: 86.67% for stage I, which decreased to 60.82% for stages IIA through IIIC, and markedly lower for stages IVA through IVB at 10.26%, with stage IVC disease showing a survival rate of 12.81%[26].
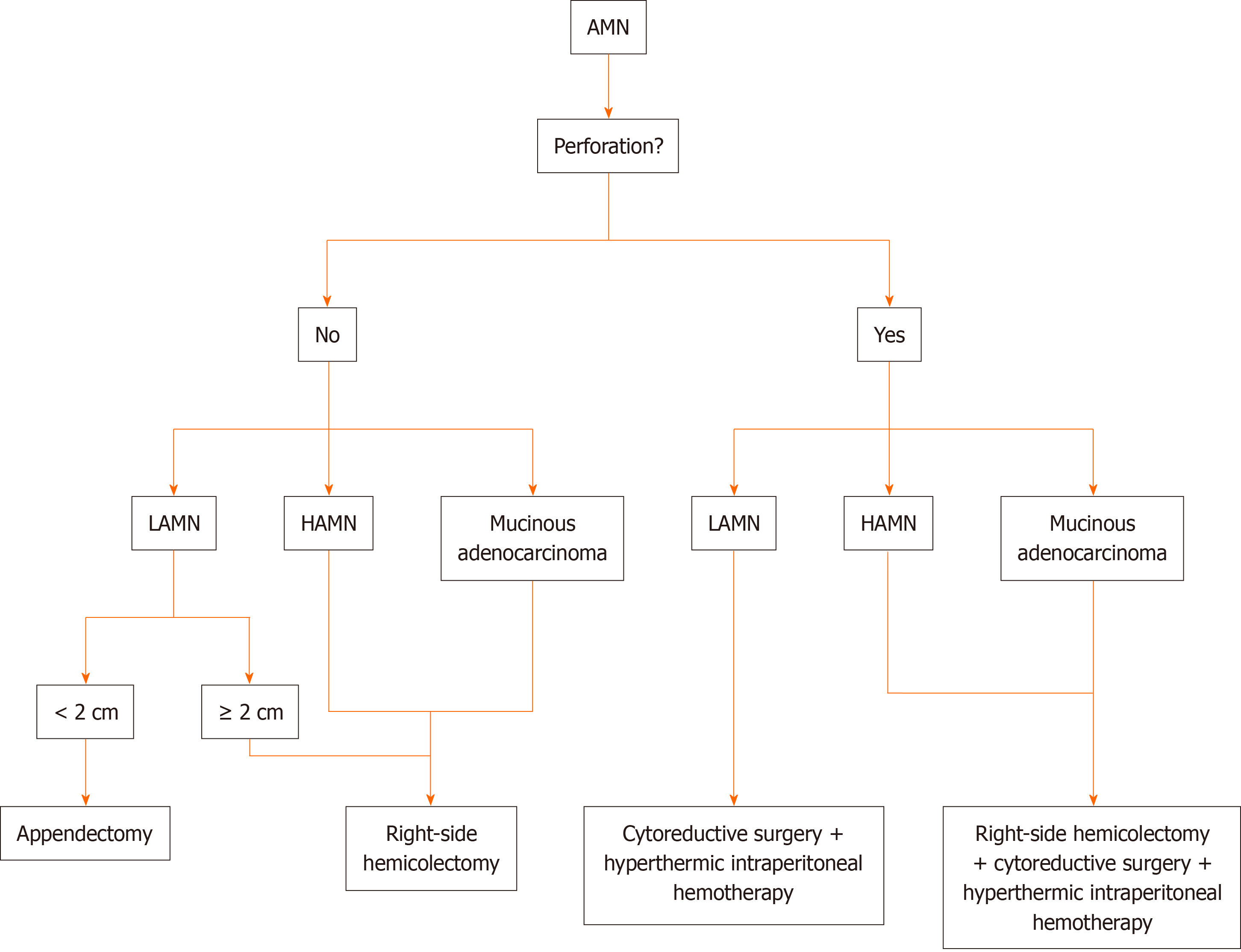
The management strategy for AMN is contingent upon the initial diagnostic findings, particularly the presence of perforation. A treatment algorithm for AMNs, as proposed by Govaerts et al[30] and Shaib et al[31], is depicted in Figure 7. Appendectomy is generally prescribed for straightforward mucoceles. To rule out mucinous AC, histopathological evaluation of frozen sections of the appendiceal base is recommended[18,30]. In cases of mucinous AC without perforation and mesenteric lymph node involvement or encroachment on surrounding tissues, a simple appendectomy coupled with mesenteric excision is often deemed adequate[30,31]. In contrast, in instances where diagnostic ambiguity prevails, a more aggressive surgical approach, such as cecal resection, right hemicolectomy, or cytoreductive surgery, may be recommended[31]. A thorough abdominal assessment is considered crucial for all patients undergoing surgical treatment for AMN[18,30-32].
Surgical excision of benign mucocele, mucosal hyperplasia, and mucinous AC typically results in excellent outcomes, with 5-year survival rates reported ranging between 90% and 100%[18,26]. The development of recurrent disease fo
Postoperative histopathological assessment of AMN relies heavily on mucin detection. AMNs characteristically exhibit consistent positivity for CK20 in all cases (100%), with CK7 negativity in 71% of cases. Additionally, these neoplasms frequently express Mucin-5AC (86%) and deleted in Pancreatic Cancer-4/Mothers against decapentaplegic homolog 4 (100%). It is worth noting that both colorectal carcinoma and appendiceal tumors typically present with a patchy CK7 and a diffuse CK20 staining pattern[33,34]. However, distinguishing primary ovarian mucinous tumors from AMNs that have metastasized to the ovary presents diagnostic difficulties owing to similarities in the morphological characteristics and IHC profiles of conventional markers. Strickland et al[35] suggested the utility of additional markers, including SATB2, CK20, MUC2, and PAX8, in the differential diagnosis. SATB2 is positive in 93.8% of appendiceal tumors and is rarely positive in ovarian tumors, demonstrating 97.5% specificity for the appendiceal origin. CK20, CDX2, and MUC2 are typically strongly and diffusely positive in appendiceal tumors, whereas ovarian tumors may exhibit positivity, but with a patchy distribution and less intensity. CK7 expression was observed in 97.5% of ovarian tumors compared to 31.2% of appendiceal tumors. PAX8 is positive in 70% of ovarian tumors but is consistently negative in appendiceal tumors. These markers are recommended as markers for the evaluation of ovarian mucinous tumors, particularly when clinical or pathological indications suggest a secondary origin.
Our case serves as a pertinent example of the diagnostic intricacies associated with AMNs and underscores the importance of their consideration in differential diagnoses, especially when a right adnexal or mesenteric mass is detected via imaging. Initially, the rarity of AMNs led to the misinterpretation of the cystic fluid observed on CT and MRI as a simple cyst, inadvertently posing the risk of PMP. Furthermore, the patient's elevated tumor marker levels and gastro
The histopathological evaluation of the tumor in the present case demonstrated IHC positivity for CDX2, CK20, SATB2, and MUC2 and negativity for CK7 and PAX8, confirming the diagnosis of a LAMN. This immunoprofile is emblematic of neoplasms emanating from the appendiceal epithelium, providing a critical distinction between gastrointestinal and ovarian mucinous tumors that exhibit variant marker expression. As such, this case report details the innovative app
The strategic application of IHC analysis, as demonstrated in this case report, significantly enhances our ability to accurately identify the origin of and to classify neoplasms. This improved accuracy is crucial for optimal patient mana
AMNs, a heterogeneous group of tumors, are rarely diagnosed before elective surgery, while treatment is guided by both stage and histopathology. Early-stage, low-grade tumors typically require surgical excision, while the optimal mana
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S, Russia; Xu HT, China S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | Yakan S, Caliskan C, Uguz A, Korkut M, Çoker A. A Retrospective Study on Mucocele of the Appendix Presented with Acute Abdomen or Acute Appendicitis. Hong Kong J Emerg Med. 2011;18:144-149. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Minni F, Petrella M, Morganti A, Santini D, Marrano D. Giant mucocele of the appendix: report of a case. Dis Colon Rectum. 2001;44:1034-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Fujiwara T, Hizuta A, Iwagaki H, Matsuno T, Hamada M, Tanaka N, Orita K. Appendiceal mucocele with concomitant colonic cancer. Report of two cases. Dis Colon Rectum. 1996;39:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94:3307-3312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 404] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | COLLINS DC. 71,000 HUMAN APPENDIX SPECIMENS. A FINAL REPORT, SUMMARIZING FORTY YEARS' STUDY. Am J Proctol. 1963;14:265-281. [PubMed] |
| 6. | Ito H, Osteen RT, Bleday R, Zinner MJ, Ashley SW, Whang EE. Appendiceal adenocarcinoma: long-term outcomes after surgical therapy. Dis Colon Rectum. 2004;47:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 510] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 8. | Bradley RF, Stewart JH 4th, Russell GB, Levine EA, Geisinger KR. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995;75:757-768. [PubMed] [DOI] [Full Text] |
| 10. | Garg PK, Prasad D, Aggarwal S, Mohanty D, Jain BK. Acute intestinal obstruction: an unusual complication of mucocele of appendix. Eur Rev Med Pharmacol Sci. 2011;15:99-102. [PubMed] |
| 11. | Rutledge RH, Alexander JW. Primary appendiceal malignancies: rare but important. Surgery. 1992;111:244-250. [PubMed] |
| 12. | Young RH. Pseudomyxoma peritonei and selected other aspects of the spread of appendiceal neoplasms. Semin Diagn Pathol. 2004;21:134-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Isaacs KL, Warshauer DM. Mucocele of the appendix: computed tomographic, endoscopic, and pathologic correlation. Am J Gastroenterol. 1992;87:787-789. [PubMed] |
| 14. | Pickhardt PJ, Levy AD, Rohrmann CA Jr, Kende AI. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation. Radiographics. 2003;23:645-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Chiou YY, Pitman MB, Hahn PF, Kim YH, Rhea JT, Mueller PR. Rare benign and malignant appendiceal lesions: spectrum of computed tomography findings with pathologic correlation. J Comput Assist Tomogr. 2003;27:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ruiz-Tovar J, Teruel DG, Castiñeiras VM, Dehesa AS, Quindós PL, Molina EM. Mucocele of the appendix. World J Surg. 2007;31:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol. 2005;12:291-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Aho AJ, Heinonen R, Laurén P. Benign and malignant mucocele of the appendix. Histological types and prognosis. Acta Chir Scand. 1973;139:392-400. [PubMed] |
| 19. | Noritake N, Ito Y, Yamakita N, Azuma S, Shimokawa K, Miura K. A case of primary mucinous cystadenocarcinoma of the appendix with elevated serum carcinoembryonic antigen (CEA). Jpn J Med. 1990;29:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Ponsky JL. An endoscopic view of mucocele of the appendix. Gastrointest Endosc. 1976;23:42-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Hamilton DL, Stormont JM. The volcano sign of appendiceal mucocele. Gastrointest Endosc. 1989;35:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González-Moreno S, Taflampas P, Chapman S, Moran BJ; Peritoneal Surface Oncology Group International. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. 2016;40:14-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 515] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 23. | Carr NJ, Bibeau F, Bradley RF, Dartigues P, Feakins RM, Geisinger KR, Gui X, Isaac S, Milione M, Misdraji J, Pai RK, Rodriguez-Justo M, Sobin LH, van Velthuysen MF, Yantiss RK. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology. 2017;71:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 24. | Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation. Radiographics. 2017;37:1059-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Overman MJ, Kakar S, Carr NJ, Hanna N, Holowatyj AN, Gress D, Willianms A, Goldberg RM, Kitterman AC, Washington MK. AJCC Cancer Staging System: Appendix: Version 9 of AJCC Cancer Staging System (Version 9 of the AJCC Cancer Staging System). Am Joint Committee Cancer 2022. Available from: https://www.facs.org/quality-programs/cancer-programs/american-joint-committee-on-cancer/. |
| 26. | Janczewski LM, Browner AE, Cotler JH, Nelson H, Kakar S, Carr NJ, Hanna NN, Holowatyj AN, Goldberg RM, Washington MK, Asare EA, Overman MJ; American Joint Committee on Cancer Expert Panel on Cancers for the Lower Gastrointestinal Appendix Disease Site. Survival outcomes used to validate version 9 of the American Joint Committee on Cancer staging system for appendiceal cancer. CA Cancer J Clin. 2023;73:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, Slotta-Huspenina J, Käser SA, Michalski CW, Janssen KP, Friess H, Rosenberg R, Bader FG. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258:775-82; discussion 782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Sheng H, Wei X, Mao M, He J, Luo T, Lu S, Zhou L, Huang Z, Yang A. Adenocarcinoma with mixed subtypes is a rare but aggressive histologic subtype in colorectal cancer. BMC Cancer. 2019;19:1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012;3:153-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 274] [Reference Citation Analysis (0)] |
| 30. | Govaerts K, Lurvink RJ, De Hingh IHJT, Van der Speeten K, Villeneuve L, Kusamura S, Kepenekian V, Deraco M, Glehen O, Moran BJ; PSOGI. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol. 2021;47:11-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (1)] |
| 31. | Shaib WL, Assi R, Shamseddine A, Alese OB, Staley C 3rd, Memis B, Adsay V, Bekaii-Saab T, El-Rayes BF. Appendiceal Mucinous Neoplasms: Diagnosis and Management. Oncologist. 2017;22:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (3)] |
| 32. | Higa E, Rosai J, Pizzimbono CA, Wise L. Mucosal hyperplasia, mucinous cystadenoma, and mucinous cystadenocarcinoma of the appendix. A re-evaluation of appendiceal "mucocele". Cancer. 1973;32:1525-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3455] [Cited by in RCA: 3346] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 35. | Strickland S, Parra-Herran C. Immunohistochemical characterization of appendiceal mucinous neoplasms and the value of special AT-rich sequence-binding protein 2 in their distinction from primary ovarian mucinous tumours. Histopathology. 2016;68:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Schmoeckel E, Kirchner T, Mayr D. SATB2 is a supportive marker for the differentiation of a primary mucinous tumor of the ovary and an ovarian metastasis of a low-grade appendiceal mucinous neoplasm (LAMN): A series of seven cases. Pathol Res Pract. 2018;214:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |