Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.740
Peer-review started: November 30, 2023
First decision: December 25, 2023
Revised: January 4, 2024
Accepted: January 30, 2024
Article in press: January 30, 2024
Published online: March 27, 2024
Processing time: 112 Days and 11.9 Hours
Evidence suggests inflammatory mesenteric fat is involved in post-operative recurrence (POR) of Crohn’s disease (CD). However, its prognostic value is uncertain, in part, due to difficulties studying it non-invasively.
To evaluate the prognostic value of pre-operative radiographic mesenteric parameters for early endoscopic POR (ePOR).
We conducted a retrospective cohort study of CD subjects ≥ 12 years who underwent ileocecal or small bowel resection between 1/1/2007 to 12/31/2021 with computerized tomography abdomen/pelvis ≤ 6 months pre-operatively and underwent ileocolonoscopy ≤ 15 months post-operatively. Visceral adipose tissue (VAT) volume (cm3), ratio of VAT:subcutaneous adipose tissue (SAT) volume, VAT radiodensity, and ratio of VAT:SAT radiodensity were generated semiautomatically. Mesenteric lymphadenopathy (LAD, largest lymph node > 10 mm) and severe vasa recta (VR) engorgement (diameter of the VR supplying diseased bowel ≥ 2 × VR supplying healthy bowel) were derived manually. The primary outcome was early ePOR (Rutgeert’s score ≥ i2 on first endoscopy ≤ 15 months post-operatively) and the secondary outcome was ePOR severity (Rutgeert’s score i0-4). Regression analyses were performed adjusting for demographic and disease-related characteristics to calculate adjusted odds ratio (aOR) and 95% confidence interval (CI).
Of the 139 subjects included, 45% of subjects developed early ePOR (n = 63). VAT radiodensity (aOR 0.59, 95%CI: 0.38-0.90) and VAT:SAT radiodensity (aOR 8.54, 95%CI: 1.48-49.28) were associated with early ePOR, whereas, VAT volume (aOR 1.23, 95%CI: 0.78-1.95), VAT:SAT volume (aOR 0.80, 95%CI: 0.53-1.20), severe VR engorgement (aOR 1.53, 95%CI: 0.64-3.66), and mesenteric LAD (aOR 1.59, 95%CI: 0.67-3.79) were not. Similar results were observed for severity of ePOR.
VAT radiodensity is potentially a novel non-invasive prognostic imaging marker to help risk stratify CD patients for POR.
Core Tip: Risk stratification for post-operative recurrence of Crohn’s disease (CD) remains a clinical challenge. We evaluated the prognostic value of pre-operative radiographic mesenteric parameters for post-operative recurrence of CD. Of the parameters evaluated, lower visceral adipose tissue radiodensity was independently associated with post-operative recurrence. Visceral adipose tissue radiodensity may be a novel imaging prognostic marker to help risk stratify for post-operative recurrence of CD during pre-operative work up.
- Citation: Gu P, Dube S, Gellada N, Choi SY, Win S, Lee YJ, Yang S, Haritunians T, Melmed GY, Vasiliauskas EA, Bonthala N, Syal G, Yarur AJ, Ziring D, Rabizadeh S, Fleshner P, Kallman C, Devkota S, Targan SR, Li D, McGovern DP. Pre-operative visceral adipose tissue radiodensity is a potentially novel prognostic biomarker for early endoscopic post-operative recurrence in Crohn’s disease. World J Gastrointest Surg 2024; 16(3): 740-750
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/740.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.740
Crohn’s disease (CD) is a debilitating chronic immune-mediated inflammatory disease (IMID) of the gastrointestinal tract that is increasing in incidence and prevalence globally[1]. CD patients often undergo surgery for disease-related complications and/or medically refractory disease. Unfortunately, surgery is not curative, and many patients develop post-operative recurrence (POR) of CD with a significant proportion eventually requiring additional surgeries. With advances in early detection and therapeutics, the contemporary 10-year risk of surgery has improved from 50% to 26%, but the risk of recurrent surgery has remained unchanged at 30%, suggesting a need to improve post-operative management strategies[2].
Presently, there are two accepted strategies to mitigate POR, but each have potential limitations. Firstly, patients start early post-operative pharmacologic prophylaxis within 4-6 wk after surgery. This strategy can potentially overtreat a subset of patient who may not develop long-term disease recurrence off therapy. Consequently, these patients are at risk of medication-related adverse events and the direct and indirect costs associated with therapy with little or no benefit[3]. The second strategy is performing early colonoscopy within 6-12 months after surgery and escalating therapy based on evidence of endoscopic POR. This strategy risks delaying treatment if CD recurs before 6-12 months. Furthermore, in those who received an anti-TNF agent before surgery, the gap in drug exposure may increase the risk of drug reaction from re-exposure and/or failure from immunogenicity[4,5]. Because data about the comparative effectiveness of each strategy is lacking[6], societal guidelines recommend strategy selection based on stratifying patients into high or low risk for POR[7,8]. However, aside from active smoking, internal penetrating disease, and prior surgeries, many risk factors for POR are variably supported by the literature[9]. Also, many patients who require surgery for medically refractory disease do not strictly fit the high risk category. The pitfalls of our current POR risk-stratification models is highlighted by a recent study that found that CD patients at high-risk for POR (defined by ≥ 1 risk factor: Smoking, internal penetrating disease, and prior surgery) and low-risk for POR (no risk factors) had similar rates of early endoscopic POR at 36%[10]. These gaps in knowledge emphasize the need to identify additional risk factors. Finally, since most established POR risk factors reflect luminal disease, work is needed to evaluate if there are also extraintestinal components associated with or contributing to POR.
Historically, inflammatory mesenteric adipose tissue (MAT) was perceived as an inert pathognomonic feature of CD, but increasing evidence supports its active involvement in the pathophysiology of POR[11,12]. In fact, emerging data suggest intestinal resection with extended mesenteric excision can mitigate POR[13-16]. However, the prognostic utility of MAT is uncertain, in part, due to difficulties studying it non-invasively[12]. Most studies evaluating MAT rely on surgical resection specimens, which also limits the applicability of the findings. Several radiologic studies have provided indirect evidence that radiographic mesenteric parameters may harbor prognostic markers. For example, severe mesenteric lymphadenopathy and vasa recta engorgement (i.e., Comb’s sign) on CT enterography have been associated with large ulcers and greater proportion of ulcerated surfaces on endoscopy[17], which are risk factors for internal penetrating and medically recalcitrant CD[18]. Furthermore, increased visceral adipose tissue (VAT) on imaging, which is often used as a surrogate marker of MAT hypertrophy[12], is associated with anti-TNF response, greater anti-TNF dose requirements, and risk of surgery[19-21]. Two small studies also found increased VAT is associated with POR, but methodological limitations, such as sample size and study design, require that further investigation is necessary[22,23]. Additionally, these studies did not evaluate other radiographic mesenteric parameters such as mesenteric lymphadenopathy, vasa recta engorgement, or VAT radiodensity. Considering surgical CD patients very often require diagnostic imaging and our current POR risk-stratification models are suboptimal, establishing the prognostic value of radiographic mesenteric parameters for POR has important implications for post-operative management of CD. Thus, we aim to establish the prognostic value of pre-operative radiographic mesenteric parameters for early endoscopic POR (ePOR) as well as the severity of ePOR.
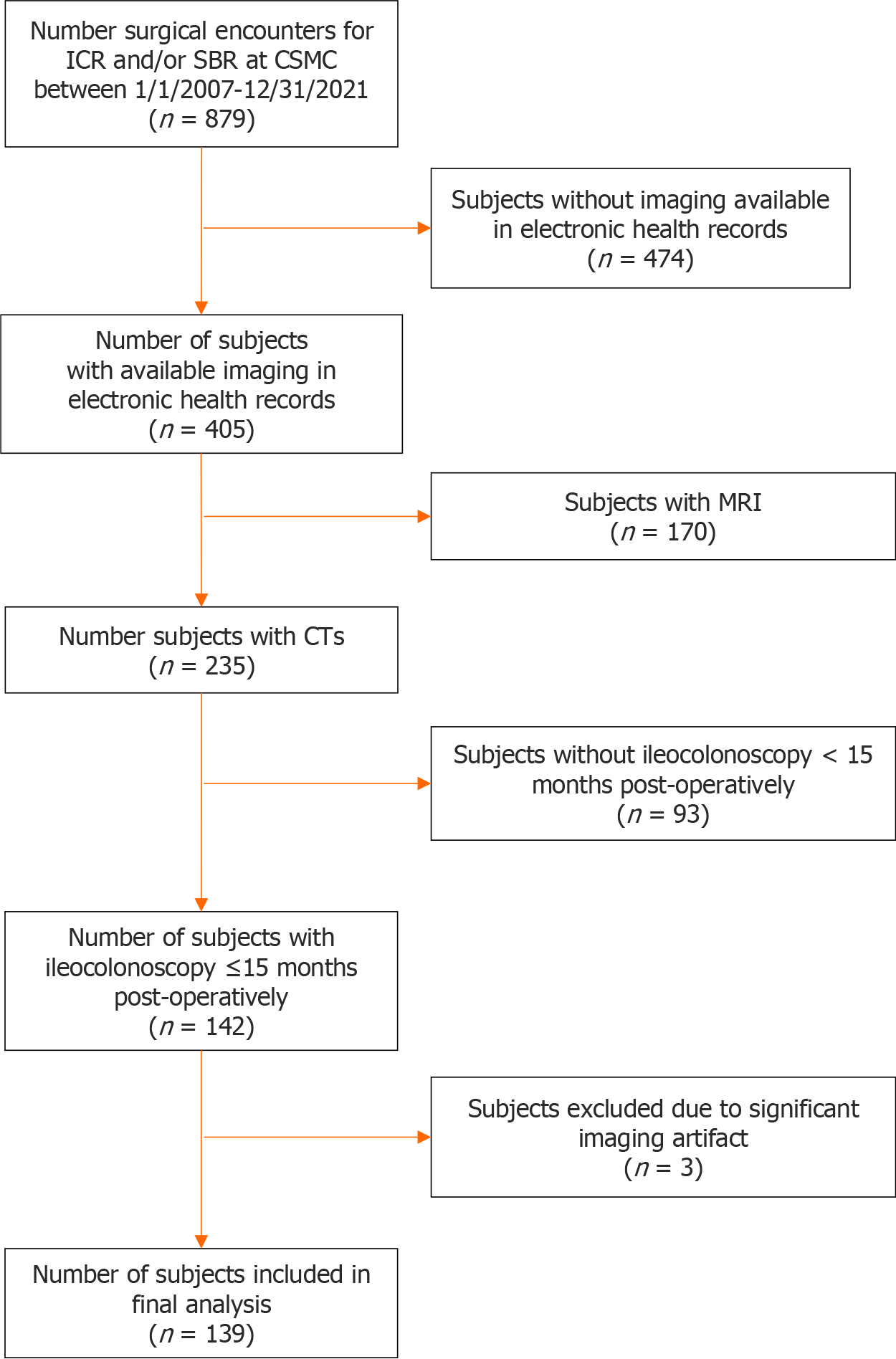
We conducted a retrospective, single center study of adult and pediatric CD subjects who underwent ileocecal or small bowel resection at Cedars-Sinai Medical Center (CSMC) between 1/1/2007 and 12/31/2021. Subjects with available computerized tomography (CT) abdomen/pelvis performed for clinical indications ≤ 6 months pre-operatively and an endoscopic evaluation for POR performed ≤ 15 months post-operatively were included. For small bowel anastomoses that could not be evaluated via ileocolonoscopy, endoscopic evaluation for recurrence was performed by wireless capsule endoscopy or double balloon enteroscopy (n = 9). Figure 1 summarizes inclusion and inclusion criteria. To align with clinical practice, CTs performed within 6 months of surgery were selected because imaging within 6 months is clinically sufficient to make surgical decisions. Furthermore, magnetic resonance imaging was excluded because CT scans offer additional radiographic parameters, such as radiodensity, that is not possible with magnetic resonance imaging. All intestinal resections were performed with same standard surgical techniques across the study period using functional end-to-end anastomosis and preservation of as much mesentery as possible. Extended mesenteric resection was not performed during the study period. This study was approved by the CSMC IRB (IRB #3358).
Baseline demographics, disease-related characteristics, and CD medications at the time of surgery were recorded via manual chart review. Disease location and disease behavior were classified by the Montreal classification system. Post-operatively prophylaxis status was also recorded and was defined as CD-directed therapy started after the primary anastomosis or ileostomy reversal with restoration of bowel continuity but before the first post-operative endoscopic evaluation. Rutgeert’s scores were recorded from endoscopy procedure reports for both ileocecal and small bowel anastomoses as previously described[24]. When a Rutgeert’s score was not recorded, text and images of endoscopy reports were reviewed by a board-certified gastroenterologist with advanced IBD training (PG) to retrospectively assign a Rugeert’s score. Rutgeert’s score i2 was not differentiated into i2a and i2b because this was not standard of practice during the early part of the study period and could not be reliably distinguished retrospectively based on text or images.
The primary exposures were the radiographic mesenteric parameters of interest, which included visceral adipose tissue (VAT) volume (cm3), ratio of VAT:subcutaneous adipose tissue (SAT) volume, VAT radiodensity [Hounsfield units (HU)], ratio of VAT:SAT ratio, severity of vasa recta engorgement (VR), and mesenteric lymphadenopathy.
VAT parameters: To generate VAT parameters, all CT scans were processed with Vitrea® Advanced Visualization Platform (Canon Medical Systems), which performs semiautomated segmentation of the abdominal VAT and SAT compartments using attenuation thresholds between -50 and -150 HU. Supplementary Figure 1 illustrates an example of the segmentation. VAT and SAT parameters were measured from the first lumbar vertebra (L1) to the fifth (L5), as previously described[19]. The boundaries of automated CT outlined adipose tissue compartments were manually reviewed and corrected for any errors in each slice. After segmentation, VAT and SAT volumes and radiodensity were automatically calculated in Vitrea®, which were then used to calculate VAT:SAT volume and radiodensity. All VAT and SAT segmentation were performed by a certified advanced imaging analyst (NG) who was blinded to the clinical data and outcomes. Three subjects had significant image artifact that precluded VAT and SAT analysis and were excluded from the final analysis.
Severe vasa recta engorgement: Severity of vasa recta engorgement was determined by calculating the ratio of the diameter of the vasa recta supplying the inflamed bowel intended for resection to the diameter of the vasa rectal supplying health bowel. Severe vasa recta engorgement was defined as diameter of vasa recta of the inflamed bowel ≥ 2 × the diameter of the vasa recta of the healthy bowel as previously described[17]. Vasa recta diameters were manually measured in the transverse plane of the CT by a study investigator (PG).
Mesenteric lymphadenopathy: Each CT scan was reviewed by a study investigator (PG) to locate and measure the largest mesenteric lymph node in the region of the inflamed bowel intended for resection. Mesenteric lymphadenopathy was defined as the largest lymph node measuring > 10 mm along the short axis in the transverse plan of the CT as previously described[17].
Radiographic vasa recta and lymph node measurements from 48 images (32% of cohort) were reviewed and confirmed with a board-certified body imaging radiologist with over 20 years of experience (CK) to ensure accuracy and consistency.
The primary outcome was early ePOR defined as Rutgeert’s ≥ i2 on endoscopy performed ≤ 15 months post-operatively. The secondary outcome was severity of ePOR as determined by the Rutgeert’s score i0-i4.
Descriptive statistics were used to examine baseline cohort demographics and disease-related characteristics. For variables not normally distributed based on visual assessment, a rank-based inverse normalized transformation was applied to the variable. Univariate regression was used to assess demographic and disease-related associations with radiographic mesenteric parameters. To determine independent associations between the radiographic mesenteric parameters and early ePOR and severity of ePOR, multivariable binomial and ordinal logistic regressions were performed, respectively. Adjustment for well-established POR risk factors were determined a priori and included age at surgery, sex, time from CD diagnosis to surgery, internal penetrating disease behavior, post-operative prophylaxis status, and prior CD-related surgery[6]. BMI was not included in the multivariate regression models because BMI is not an established risk factor for POR. Active smoking was not included in the models because only 4.4% subjects (n = 6) were active smokers in the cohort. The accuracy of regression models that included radiographic mesenteric parameters significantly associated with ePOR and were assessed by receiver operating characteristic (ROC) curve analysis and calculating the area under the curve (AUC). Subsequently, the thresholds of these radiographic mesenteric parameters to classify early ePOR were identified using hierarchical Bayes method[25]. P-value < 0.05 was considered statistically significant[25,26]. All statistical analyses were performed using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) and R version 4.2.2 (R Core Team, 2022, R: A Language and Environ
A total of 139 subjects were included in the final analysis (Figure 1). The median amount of time between CT and surgery was 0.93 (interquartile range 0.33-2.43) months. Table 1 summarizes cohort demographics. Only 4.4% (n = 6) were active smokers at the time of surgery. Pre-operatively, majority of patients had evidence of stricturing disease (69.5%, n = 96), and 52.5% (n = 73) were on a biologic agent before surgery. Additionally, 28.1% (n = 39) had at least one prior CD-related surgery. Post-operatively, 86.3% (n = 120) of subjects started post-operative prophylaxis with a biologic agent being the most common (78.3%, n = 94).
| Total (n = 139) | |
| Mean age (years, SD) | 39.1 (17.1) |
| Female, n (%) | 66 (47.5) |
| European Ancestry, n (%) | 121 (88.3) |
| Mean disease duration, (months, SD) | 151.8 (136.3) |
| Mean body mass index (kg/m2, SD) | 23.3 (4.8) |
| Active smoking, n (%) | 6 (4.4) |
| Disease location, n (%) | |
| Ileal (L1) | 61 (43.9) |
| Ileocolonic (L3) | 78 (56.1) |
| Upper GI (L4) | 12 (8.6) |
| Disease behavior, n (%) | |
| Non-fibrostenosing, non-penetrating (B1) | 7 (5.1) |
| Fibrostenosing (B2) | 54 (39.2) |
| Internal penetrating (B3) | 35 (25.4) |
| Stricturing and internal penetrating (B2/3) | 42 (30.4) |
| Perianal disease, n (%) | 23 (16.5) |
| Pre-operative medication, n (%) | |
| None | 33 (23.7) |
| Corticosteroid | 48 (34.5) |
| 5-aminosalicylic acid | 21 (15.1) |
| Immunomodulator | 32 (23.0) |
| Biologic | 73 (52.5) |
| Prior surgery, n (%) | 39 (28.1) |
| Post-operative prophylaxis, n (%) | 120 (86.3) |
| 5-aminosalicylic acid | 7 (5.8) |
| Immunomodulator | 23 (19.2) |
| Biologic | 94 (78.3) |
On univariate analysis, there were several notable associations with the radiographic mesenteric parameters of interest (Supplemental Table 1). VAT volume was associated with older age (β: 0.49, P = 2.10e-9), longer time to surgery from CD diagnosis (β: 0.23, p=0.008), and BMI (β: 0.68, P = 7.32e-18). VAT:SAT volume was associated with older age (β:0.26, p=0.002) and negatively associated with female sex (β: -0.26, P = 0.003). VAT radiodensity was not associated with age (P = 0.46) or sex (P = 0.66) but negatively associated with BMI (β: -0.21, P = 0.02). Finally, severe vasa recta engorgement was associated with prior surgery (OR: 0.43, P = 0.04). Finally, stricturing/penetrating disease behavior (B2/3) was associated with VAT radiodensity (β: -0.38, P = 0.01) and VAT:SAT radiodensity (β: 0.31, p=0.04). Active smoking and disease location were not associated with any of the radiographic mesenteric parameters. Similarly, mesenteric lymphadenopathy was not associated with any demographics or disease-related factors.
Of the entire cohort, 45.3% (n = 63) developed early ePOR, and the median time between surgery and first post-operative ileocolonoscopy was 32.86 (IQR 26.14-43.29) weeks. On binomial logistic regression (Table 2), VAT radiodensity [adjusted odds ratio (aOR): 0.59, 95% confidence interval (CI): 0.38-0.90] and VAT:SAT radiodensity (aOR 8.54, 95%CI: 1.48-49.28) were associated with early ePOR. VAT volume (aOR: 1.23, 95%CI: 0.78-1.95), VAT:SAT volume (aOR: 0.80, 95%CI: 0.53-1.20), severe vasa recta engorgement (aOR: 1.53, 95%CI: 0.64-3.66), and mesenteric lymphadenopathy (aOR: 1.59, 95%CI: 0.67-3.79) were not associated with early ePOR.
| Early endoscopic POR | Severity of endoscopic POR | |
| aOR (95%CI) | aOR (95%CI) | |
| VAT volume (cm3) | 1.23 (0.78-1.95) | 1.08 (0.72-1.53) |
| VAT:SAT volume | 0.80 (0.53-1.20) | 0.80 (0.56-1.14) |
| VAT Radiodensity (HU) | 0.59 (0.38-0.90) | 0.60 (0.42-0.87) |
| VAT:SAT Radiodensity | 8.54 (1.48-49.28) | 6.26 (1.43-27.42) |
| Severe vasa recta engorgement | 1.53 (0.64-3.66) | 1.61 (0.75-3.46) |
| Mesenteric lymphadenopathy | 1.59 (0.67-3.79) | 2.10 (0.99-4.47) |
The overall AUC for the multivariable regression models including the VAT radiodensity and VAT:SAT radiodensity was 0.72 (95%CI: 0.63-0.81) and 0.73 (95%CI: 0.64-0.81), respectively, showing the similar performance of classification (Supplementary Figure 2). Using the hierarchical Bayes method accounting for covariates, the estimated normalized VAT radiodensity to classify early ePOR was ≤ -0.23 (95% credible interval: -0.53 to 0.03]) and the threshold for VAT:SAT radiodensity was ≥ 0.54 (95% credible interval: 0.50-0.59).
Of the subjects who did not develop ePOR, 34.5% (n = 48) and 20.1% (n = 28) had Rugeert’s i0 and i1, respectively. Of the subjects who developed early ePOR, 30.2% had Rugeert’s i2 (n = 42) while 9.4% (n = 13) and 5.8% (n = 8) had Rutgeert’s i3 and i4, respectively. On ordinal logistic regression (Table 2), VAT radiodensity (aOR 0.60, 95%CI: 0.42-0.87) and VAT:SAT radiodensity (aOR: 6.26, 95%CI: 1.43-27.42) were associated with severity of ePOR. VAT volume (aOR: 1.08, 95%CI: 0.72-1.53), VAT:SAT volume (aOR: 0.80, 95%CI: 0.56-1.14), severe vasa recta engorgement (aOR: 1.61, 95%CI: 0.75-3.46), and mesenteric lymphadenopathy volume (aOR: 2.10, 95%CI: 0.99-4.47) were not associated with ePOR severity.
POR of CD is a persistent clinical challenge, and current risk stratification models for POR remain suboptimal. Aside from active smoking, internal penetrating CD, and prior surgeries, many risk factors for POR are variably supported by the literature[9]. Emerging evidence indicates MAT is involved in POR, but its prognostic value is uncertain, in part, due to difficulties studying it non-invasively[12]. In the largest cohort of surgical CD subjects with available pre-operative CT scans and early post-operative endoscopic evaluation, we established the prognostic value of pre-operative radiographic mesenteric parameters for early ePOR and severity of ePOR and found pre-operative VAT radiodensity is associated with ePOR and ePOR severity.
Radiodensity is an imaging parameter that can quantify the degree of attenuation of different types of tissue. With current technological capabilities, radiodensity can be measured on CT but not magnetic resonance imaging (MRI). On CT, adipose tissue conventionally has lower radiodensity relative to other tissue types, and organs with greater adiposity have lower radiodensity. For example, one of the radiographic criteria for severe fatty liver is hepatic radiodensity < 40 HU[27]. VAT radiodensity has been well-studied in cardiometabolic diseases, and decreased VAT radiodensity is associated with increased risk of cardiovascular disease, metabolic syndrome, and hypertension[28,29]. Additionally, in non-IBD subjects, VAT radiodensity is negatively associated with IL-6, TNF-α, C-reactive protein, adiponectin, and resistin serum levels, which have also been implicated in CD[12,30]. The literature suggests VAT radiodensity is a imaging biomarker the reflects “fat quality,” and poor fat quality could entail a variety of histologic alterations including macrophage infiltration[31-33], decreased vascularity[34], fibrosis[35-37], and adipocyte hypertrophy[38]. Interestingly, these histologic features have also been described in CD, supporting an overlap with metabolic diseases[12].
Since MAT is the largest component of VAT, imaging studies have used VAT as a surrogate marker for inflammatory alterations of MAT in CD because MAT is challenging to segment on imaging[12]. In our cohort, decreased VAT radiodensity on pre-operative CT images was associated with early ePOR as well as increased severity of ePOR. Our findings suggest MAT quality has important prognostic and mechanistic implications for POR in CD. Since surgical CD patients often require imaging, pre-operative imaging offers an accessible and cost-efficient opportunity to extract additional prognostic information to better inform post-operative management. VAT radiodensity can be semi-automatically calculated by many imaging analysis tools, so our finding potentially identifies a novel prognostic imaging biomarker for POR that can be rapidly employed into clinical practice if validated by larger, prospective studies. In addition to its potential as a new prognostic imaging biomarker for POR, VAT radiodensity may provide insight into the biologic underpinnings of POR. Preliminary data by our group suggest certain histomorphometric features of the MAT associated with resected uninvolved ileum is associated with POR, including adipocyte size and distance between adipocytes[38]. These histologic features are potentially reflected by VAT radiodensity on imaging but requires further data to confirm. Interestingly, on univariate analysis, VAT radiodensity was not associated with CD behavior or established clinical risk factors for POR, except prior surgery. This finding supports extra-luminal processes are likely involved in POR and provides further evidence that MAT could be a therapeutic surgical target for mitigating POR as implicated by recent studies evaluating extended mesenteric resection[13-16]. Moreover, given safety concerns with manipulating inflamed MAT, identifying pre-operative radiographic prognostic markers like VAT radiodensity can help identify candidates at high risk for POR and would derive the most benefit from extended mesenteric resection. This would also help avoid exposing patients at low risk for POR to potential complications of extended mesenteric resection. Finally, we also provide preliminary data demonstrating a multivariable regression model including radiographic mesenteric parameters yields an acceptable AUC and a possible VAT radiodensity threshold to identify high-risk POR patients, supporting larger studies to confirm our findings.
Conversely, we observed several radiographic mesenteric parameters that did not have prognostic value for POR. Contrary to two prior small studies[22,23], VAT and VAT:SAT volume was not associated with early ePOR. The discrepancies may be due to sample sizes and analytical methodologies used in the prior studies. Additionally, VAT volume can be influenced by several factors such as age, BMI, and sex, so differences in study settings amongst the studies (China vs Australia vs United States) could have contributed to the discrepancies. Similarly, severe vasa recta engorgement and mesenteric lymphadenopathy were not associated with ePOR. Clinically, these parameters are often reported to describe severe disease activity and are associated with deep ulcers on endoscopy, which has been associated with medically recalcitrant CD[18]. Thus, these parameters may have prognostic value for treatment response rather than POR.
This study has several notable strengths. First, we included a large cohort of surgical CD patients with available pre-operative imaging and early post-operative endoscopic evaluation in a US population. Prior published studies included subjects from China and Australia, and anthropomorphic composition can vary among different racial groups and countries. Moreover, practice patterns can differ among regions of the world, so findings from prior studies may not be applicable to the US patient population. Second, we used three-dimensional measures to calculate VAT metrics. This approach is more accurate compared to two-dimensional measurements because it accounts for variations in fat distribution among individuals. Additionally, the level where the inflamed mesentery is located varies between individuals, so a single slice two-dimensional measurement at a pre-specified vertebral level, which was commonly performed in prior studies, can miss the affected region. We also recognize limitations of this study. First, this was a retrospective, single center study. Second, the study only included subjects with available pre-operative CT scans, so there is a risk for selection bias. However, MRI has very limited imaging parameters that can be extracted retrospectively and measuring radiodensity is currently not technologically possible. CT has the advantage of more extractable parameters to provide more prognostic information about POR. Nonetheless, future studies with advanced imaging processing techniques with MRI are needed as CD patients often undergo MRI too. Finally, our cohort was predominantly European ancestry, so our findings must be interpreted with caution in surgical CD patients with non-European ancestry. Future studies in non-European CD patients are also needed to validate our findings.
In conclusion, in a cohort of surgical CD patients with available pre-operative CT imaging, we described the prognostic value of several pre-operative radiographic mesenteric parameters for early ePOR and found decreased VAT radiodensity is associated with early ePOR and increased ePOR severity. If confirmed by larger, prospective studies, VAT radiodensity can potentially improve risk stratification for POR in CD. Additionally, further investigation is needed to evaluate if MAT histomorphology and dysregulated inflammatory pathways in MAT are associated with POR to better define the biologic link between MAT and POR.
Strategies for mitigating post-operative recurrence (POR) of Crohn’s disease (CD) require accurate risk stratification of patients for POR, but aside from smoking, internal penetrating disease, and prior surgeries, many risk factors are variably supported by the literature. Inflammatory mesenteric adipose tissue has been implicated in the pathogenesis of POR in CD, but its prognostic value is uncertain, in-part, due to difficulties studying it non-invasively.
Accurate risk stratification for POR of CD is important for informing post-operative management strategies to mitigate POR. Many prognostic markers have modest predictive accuracy. Thus, identifying new and accurate prognostic markers for POR is important.
The objective of this study was to establish the prognostic value of pre-operative radiographic mesenteric parameters for early endoscopic POR.
We conducted a retrospective cohort study if CD patients undergoing ileocecal and/or small bowel resection with available CT abdomen/pelvis within 6 months prior to surgery and underwent endoscopic evaluation for POR within 15 months after surgery. The primary outcome was early endoscopic POR defined as Rutgeerts ≥ i2 on post-operative endoscopy within 15 months of after surgery. Multivariable regression analyses were performed to determine the independent association between pre-operative radiographic mesenteric parameters and POR.
Analysis of 139 subjects found lower visceral adipose tissue (VAT) radiodensity was independently associated with early endoscopic POR (adjusted odds ratio: 0.59, 95% confidence interval: 0.38-0.90).
VAT radiodensity is a potentially novel imaging prognostic marker for POR in CD.
While larger studies are needed to validate our findings, VAT radiodensity is potentially a novel prognostic marker for POR that can be easily extracted from available CT imaging and help inform risk of POR. Lower VAT radiodensity has been suggested to reflect poor fat quality, so translational studies are required to understand possible underlying mechanisms of poor mesenteric fat quality that may contribute to POR and are reflected in VAT radiodensity.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology, No. 44884; American Gastroenterological Association, No. C-1362610; Crohn's and Colitis Foundation, No. 8-13469898.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang XL, China S-Editor: Gong ZM L-Editor: A P-Editor: Zhao S
| 1. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4105] [Article Influence: 513.1] [Reference Citation Analysis (110)] |
| 2. | Tsai L, Ma C, Dulai PS, Prokop LJ, Eisenstein S, Ramamoorthy SL, Feagan BG, Jairath V, Sandborn WJ, Singh S. Contemporary Risk of Surgery in Patients With Ulcerative Colitis and Crohn's Disease: A Meta-Analysis of Population-Based Cohorts. Clin Gastroenterol Hepatol. 2021;19:2031-2045.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 3. | Stevens TW, Haasnoot ML, D'Haens GR, Buskens CJ, de Groof EJ, Eshuis EJ, Gardenbroek TJ, Mol B, Stokkers PCF, Bemelman WA, Ponsioen CY; LIR!C study group. Laparoscopic ileocaecal resection vs infliximab for terminal ileitis in Crohn's disease: retrospective long-term follow-up of the LIR!C trial. Lancet Gastroenterol Hepatol. 2020;5:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 4. | Lichtenstein L, Ron Y, Kivity S, Ben-Horin S, Israeli E, Fraser GM, Dotan I, Chowers Y, Confino-Cohen R, Weiss B. Infliximab-Related Infusion Reactions: Systematic Review. J Crohns Colitis. 2015;9:806-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 5. | Jani M, Dixon WG, Chinoy H. Drug safety and immunogenicity of tumour necrosis factor inhibitors: the story so far. Rheumatology (Oxford). 2018;57:1896-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Regueiro M, Velayos F, Greer JB, Bougatsos C, Chou R, Sultan S, Singh S. American Gastroenterological Association Institute Technical Review on the Management of Crohn's Disease After Surgical Resection. Gastroenterology. 2017;152:277-295.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 930] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 8. | Nguyen GC, Loftus EV Jr, Hirano I, Falck-Ytter Y, Singh S, Sultan S; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the Management of Crohn's Disease After Surgical Resection. Gastroenterology. 2017;152:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | Moss AC. Prevention of postoperative recurrence of Crohn's disease: what does the evidence support? Inflamm Bowel Dis. 2013;19:856-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Arkenbosch JHC, Beelen EMJ, Dijkstra G, Romberg-Camps M, Duijvestein M, Hoentjen F, van der Marel S, Maljaars PWJ, Jansen S, de Boer NKH, West RL, Horjus CS, Stassen LPS, van Schaik FDM, van Ruler O, Jharap BJH, Visschedijk M, Janssen A, Erler NS, Doukas M, Ooms AHAG, Kats-Ugurlu G, van der Woude CJ, de Vries AC. Prophylactic Medication for the Prevention of Endoscopic Recurrence in Crohn's Disease: a Prospective Study Based on Clinical Risk Stratification. J Crohns Colitis. 2023;17:221-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | Li Y, Zhu W, Zuo L, Shen B. The Role of the Mesentery in Crohn's Disease: The Contributions of Nerves, Vessels, Lymphatics, and Fat to the Pathogenesis and Disease Course. Inflamm Bowel Dis. 2016;22:1483-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Gu P, Dube S, McGovern DPB. Medical and Surgical Implications of Mesenteric Adipose Tissue in Crohn's Disease: A Review of the Literature. Inflamm Bowel Dis. 2023;29:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Coffey CJ, Kiernan MG, Sahebally SM, Jarrar A, Burke JP, Kiely PA, Shen B, Waldron D, Peirce C, Moloney M, Skelly M, Tibbitts P, Hidayat H, Faul PN, Healy V, O'Leary PD, Walsh LG, Dockery P, O'Connell RP, Martin ST, Shanahan F, Fiocchi C, Dunne CP. Inclusion of the Mesentery in Ileocolic Resection for Crohn's Disease is Associated With Reduced Surgical Recurrence. J Crohns Colitis. 2018;12:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 14. | Zhu Y, Qian W, Huang L, Xu Y, Guo Z, Cao L, Gong J, Coffey JC, Shen B, Li Y, Zhu W. Role of Extended Mesenteric Excision in Postoperative Recurrence of Crohn's Colitis: A Single-Center Study. Clin Transl Gastroenterol. 2021;12:e00407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Li Y, Stocchi L, Liu X, Rui Y, Liu G, Remzi FH, Shen B. Presence of Granulomas in Mesenteric Lymph Nodes Is Associated with Postoperative Recurrence in Crohn's Disease. Inflamm Bowel Dis. 2015;21:2613-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Rahier JF, Dubuquoy L, Colombel JF, Jouret-Mourin A, Delos M, Ferrante M, Sokol H, Hertogh GD, Salleron J, Geboes K, Desreumaux P. Decreased lymphatic vessel density is associated with postoperative endoscopic recurrence in Crohn's disease. Inflamm Bowel Dis. 2013;19:2084-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Sakurai T, Katsuno T, Saito K, Yoshihama S, Nakagawa T, Koseki H, Taida T, Ishigami H, Okimoto KI, Maruoka D, Matsumura T, Arai M, Yokosuka O. Mesenteric findings of CT enterography are well correlated with the endoscopic severity of Crohn's disease. Eur J Radiol. 2017;89:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn's disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Gu P, Chhabra A, Chittajallu P, Chang C, Mendez D, Gilman A, Fudman DI, Xi Y, Feagins LA. Visceral Adipose Tissue Volumetrics Inform Odds of Treatment Response and Risk of Subsequent Surgery in IBD Patients Starting Antitumor Necrosis Factor Therapy. Inflamm Bowel Dis. 2022;28:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Yarur AJ, Abreu MT, Deepak P, Beniwal-Patel P, Papamichael K, Vaughn B, Bruss A, Sekhri S, Moosreiner A, Gu P, Kennedy W, Dubinsky M, Cheifetz A, Melmed GY. Patients With Inflammatory Bowel Diseases and Higher Visceral Adipose Tissue Burden May Benefit From Higher Infliximab Concentrations to Achieve Remission. Am J Gastroenterol. 2023;118:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Yarur AJ, Bruss A, Moosreiner A, Beniwal-Patel P, Nunez L, Berens B, Colombel JF, Targan SR, Fox C, Melmed GY, Abreu MT, Deepak P. Higher Intra-Abdominal Visceral Adipose Tissue Mass Is Associated With Lower Rates of Clinical and Endoscopic Remission in Patients With Inflammatory Bowel Diseases Initiating Biologic Therapy: Results of the Constellation Study. Gastroenterology. 2023;165:963-975.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Holt DQ, Moore GT, Strauss BJ, Hamilton AL, De Cruz P, Kamm MA. Visceral adiposity predicts post-operative Crohn's disease recurrence. Aliment Pharmacol Ther. 2017;45:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Li Y, Zhu W, Gong J, Zhang W, Gu L, Guo Z, Cao L, Shen B, Li N, Li J. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn's disease. Colorectal Dis. 2015;17:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1221] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 25. | Chen BSE, Jiang WY, Tu DS. A hierarchical Bayes model for biomarker subset effects in clinical trials. Comput Stat Data An. 2014;71:324-334. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Fang T, Mackillop W, Jiang WY, Hildesheim A, Wacholder S, Chen BSE. A Bayesian method for risk window estimation with application to HPV vaccine trial. Comput Stat Data An. 2017;112:53-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Zhang YN, Fowler KJ, Hamilton G, Cui JY, Sy EZ, Balanay M, Hooker JC, Szeverenyi N, Sirlin CB. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br J Radiol. 2018;91:20170959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 28. | Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | Shah RV, Allison MA, Lima JA, Abbasi SA, Eisman A, Lai C, Jerosch-Herold M, Budoff M, Murthy VL. Abdominal fat radiodensity, quantity and cardiometabolic risk: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2016;26:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2430] [Cited by in RCA: 2482] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 31. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 3626] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 32. | Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 2505] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 33. | Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 605] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 34. | Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I, Hamaguchi M, Nishimura S, Manabe I, Matsuda T, Kimura K, Inoue H, Inagaki Y, Aoe S, Yamasaki S, Ogawa Y. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun. 2014;5:4982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 35. | Vila IK, Badin PM, Marques MA, Monbrun L, Lefort C, Mir L, Louche K, Bourlier V, Roussel B, Gui P, Grober J, Štich V, Rossmeislová L, Zakaroff-Girard A, Bouloumié A, Viguerie N, Moro C, Tavernier G, Langin D. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7:1116-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 36. | Chun TH. Peri-adipocyte ECM remodeling in obesity and adipose tissue fibrosis. Adipocyte. 2012;1:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, Mente A, Rao S, Yusuf S, Gerstein HC, Sharma AM. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One. 2011;6:e22112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Shiramizu D, Gu P, Mujukian A, Lee Y, Nurzynska K, Chang E, Fleshner P, McGovern D, Gertych A. Computational Pathology Approach To Identify Micro And Macroscopic Features of Uninvolved Ileum Predictive Of Postoperative Recurrence In Crohn’s Disease. Digestive Diseases Week. Chicago, IL, USA. 2023. |