Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.700
Peer-review started: January 2, 2024
First decision: January 16, 2024
Revised: January 17, 2024
Accepted: February 20, 2024
Article in press: February 20, 2024
Published online: March 27, 2024
Processing time: 79 Days and 21 Hours
Gastric cancer (GC) is the fifth most common type of cancer and has the fourth highest death rate among all cancers. There is a lack of studies examining the impact of liver metastases on the effectiveness of immunotherapy in individuals diagnosed with GC.
To investigate the influence of liver metastases on the effectiveness and safety of immunotherapy in patients with advanced GC.
This retrospective investigation collected clinical data of patients with advanced stomach cancer who had immunotherapy at our hospital from February 2021 to January 2023. The baseline attributes were compared using either the Chi-square test or the Fisher exact probability method. The chi-square test and Kaplan-Meier survival analysis were employed to assess the therapeutic efficacy and survival duration in GC patients with and without liver metastases.
The analysis comprised 48 patients diagnosed with advanced GC, who were categorized into two groups: A liver metastasis cohort (n = 20) and a non-liver metastatic cohort (n = 28). Patients with liver metastasis exhibited a more deteriorated physical condition compared to those without liver metastasis. The objective response rates in the cohort with metastasis and the cohort without metastasis were 15.0% and 35.7% (P > 0.05), respectively. Similarly, the disease control rates in these two cohorts were 65.0% and 82.1% (P > 0.05), respectively. The median progression-free survival was 5.0 months in one group and 11.2 months in the other group, with a hazard ratio of 0.40 and a significance level (P) less than 0.05. The median overall survival was 12.0 months in one group and 19.0 months in the other group, with a significance level (P) greater than 0.05.
Immunotherapy is less effective in GC patients with liver metastases compared to those without liver metastasis.
Core Tip: To investigate the influence of liver metastases on the effectiveness and safety of immunotherapy in patients with advanced gastric cancer (GC). This retrospective investigation collected clinical data of patients with advanced stomach cancer who had immunotherapy at our hospital from February 2021 to January 2023. The baseline attributes were compared using either the Chi-square test or the Fisher exact probability method. The chi-square test and Kaplan-Meier survival analysis were employed to assess the therapeutic efficacy and survival duration in GC patients with and without liver metastases.
- Citation: Liu K, Wu CX, Liang H, Wang T, Zhang JY, Wang XT. Analysis of the impact of immunotherapy efficacy and safety in patients with gastric cancer and liver metastasis. World J Gastrointest Surg 2024; 16(3): 700-709
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/700.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.700
Gastric cancer (GC) is the fifth most common type of cancer and has the fourth highest death rate among all cancers[1-3]. The combination of fluorouracil and platinum is the predominant first-line chemotherapy treatment for HER2-negative advanced GC that is unresectable[4]. Nevertheless, its efficacy is limited, and the overall survival (OS) rate is notably poor (median OS < 1 year). Several phase III clinical trials[5-8] have demonstrated that the combination of chemotherapy and immunotherapy can enhance treatment efficacy and raise the OS rate in individuals diagnosed with advanced GC.
Despite this, the liver is an immune organ, and liver metastases not only stop the liver from responding to immunotherapy, but they also weaken the immune system as a whole, which means that systemic immunotherapy doesn’t work very well[9]. Backward studies[10-13] have shown that having liver metastases in people with non-small cell lung cancer (NSCLC) and melanoma can lower the response rate, progression-free survival (PFS), and OS rates of immunotherapy patients. This effect is observed regardless of other parameters, such as tumor mutation load and programmed cell death ligand 1 (PD-L1) expression[14]. Nevertheless, there is a lack of studies examining the impact of liver metastases on the effectiveness of immunotherapy in individuals diagnosed with GC.
This study retrospectively examined patients with advanced GC who received immunotherapy in the undergraduate department. The objective was to determine the impact of liver metastases on the efficacy of immunotherapy in individuals diagnosed with GC.
Data pertaining to GC patients undergoing immunotherapy at our hospital was gathered between February 2021 and January 2023.
(1) Histological or cytological diagnosis of GC has been confirmed; (2) GC is at stage IV according to the eighth edition of the TNM staging system of the International Union against Cancer; (3) The cancer is HER2 negative; (4) The patient has undergone immunotherapy; (5) There are no brain metastases; and (6) At least one measurable lesion is present.
(1) Individuals with other malignancies; and (2) Patients who have not received imaging assessment. The 48 patients were categorized into two groups, namely the non-liver metastasis cohort and the liver metastatic cohort, based on the presence or absence of liver metastases. Demographic information, ECOG score, disease stage, PD-L1 expression level, number of treatment lines, and treatment regimen were documented as baseline parameters. This project has been approved by the Ethics Committee of Hunan Provincial People’s Hospital.
The electronic imaging data of the patients were gathered and the effectiveness was assessed through a re-examination of the film. The effectiveness was assessed based on the evaluation criteria for solid tumor efficacy (RECIST1.1 criteria). The effectiveness was assessed based on complete response (CR), partial response (PR), stable disease (SD), and progressing disease (PD).
The desired outcome or result of a medical treatment or intervention, which aims to alleviate symptoms, improve health, or cure a disease.
In this study, a personalized immunotherapy regimen was provided for each patient with GC and liver metastasis. Differentiated treatment strategies were developed according to their pathological status, PD-L1 expression, and other characteristics in order to maximize the therapeutic effect and reduce the occurrence of adverse reactions. Immunotherapy regimen: albumin-paclitaxel chemotherapy (260 mg/m2, 1/3 wk) + Tirellizumab therapy (200 mg, 1/3 wk).
The objective response rate (ORR) was determined as the percentage of patients whose tumor volume decrease met the predetermined criteria and was sustained for the stipulated duration, calculated by adding the CR and PR ratios. The disease control rate (DCR) is calculated as the proportion of cases that achieved remission and SD after therapy, relative to the total number of cases that were evaluated. PFS was defined as the duration between the start of initial immunotherapy and either disease progression (PD) or death, while OS was defined as the duration between the start of initial immunotherapy and death.
Refers to the process of analyzing data using statistical methods. The statistical analysis was conducted using GraphPad Prism 8.0.1 software, and survival curves for PFS and OS were generated. The SPSS 25.0 software conducted supplementary statistical analysis. The baseline attributes of the two groups were compared using the Chi-square test or Fisher exact probability method. The comparison of mean age was done using a t-test.
The disparities in ORR and DCR between the two groups were examined using the chi-square test. The Kaplan-Meier estimator was employed for survival analysis, generating survival curves for PFS and OS. A log-rank test was utilized to examine the disparities in PFS and OS between the two cohorts. The Chi-square test was used to examine the counting data, while the t-test was used to investigate the continuous measurement data. A statistically significant difference was shown when the bilateral P value was less than 0.05 or 0.01.
This research encompassed 48 patients diagnosed with advanced stomach cancer, providing a comprehensive insight into the impact of immunotherapy on patients with this condition. The study cohort had an average age of 66.3 years, with a diverse age range spanning from 28 to 85 years. Of the participants, 64.6% were male, highlighting a balanced representation across genders. Additionally, 95.8% of the patients presented with adenocarcinoma, emphasizing the predominant histological subtype observed in this cohort.
Furthermore, the patients exhibited a range of physical conditions, with 77.1% having an ECOG PS score of 1 or higher, indicating varying levels of performance status. It is noteworthy that the distribution of gender, age, pathological status, PD-L1 expression, number of treatment lines, and treatment regimen did not reveal statistically significant differences between the two cohorts (all P > 0.05). This homogeneity in baseline characteristics enhances the robustness of the study, allowing for more reliable conclusions regarding the specific impact of immunotherapy. A crucial finding emerged when comparing patients with and without liver metastasis. Those with liver metastasis demonstrated significantly poorer physical conditions (P < 0.05), underscoring the challenges associated with this particular subset of advanced stomach cancer patients. This noteworthy difference is elucidated in detail in Table 1, providing a comprehensive breakdown of the relevant parameters.
| Clinical features | Hepatic metastases (n = 20) | No hepatic metastases (n = 28) | χ2 | P value |
| Age in yr | ||||
| < 65 | 4 (20.0) | 11 (39.3) | 2.020 | 0.212 |
| ≥ 65 | 16 (80.0) | 17 (60.7) | ||
| Gender | ||||
| Male | 13 (65.0) | 18 (64.3) | 0.003 | 0.999 |
| Female | 7 (35.0) | 10 (35.7) | ||
| ECOG score | ||||
| 0 | 6 (30.0) | 5 (17.8) | 9.116 | 0.011 |
| 1 | 8 (40.0) | 22 (78.6) | ||
| 2 | 6 (30.0) | 1 (3.6) | ||
| Histological type | ||||
| Adenocarcinoma | 19 (95.0) | 27 (96.4) | 2.117 | 0.347 |
| Signet-ring cell carcinoma | 0 (0.0) | 1 (3.6) | ||
| Unknown | 1 (5.0) | 0 (0.0) | ||
| PD-L1 expression | ||||
| ≥ 1% | 9 (45.0) | 11 (39.3) | 0.206 | 0.902 |
| < 1% | 10 (50.0) | 15 (53.6) | ||
| Unknown | 1 (5.0) | 2 (7.1) | ||
| Number of treatment lines | ||||
| 1 | 11 (55.0) | 14 (50.0) | 2.672 | 0.263 |
| 2 | 8 (40.0) | 8 (28.6) | ||
| ≥ 3 | 1 (5.0) | 6 (21.4) | ||
| Treatment plan | ||||
| Chemotherapy + immunotherapy | 19 (95.0) | 26 (92.8) | 0.777 | 0.658 |
| Antiangiogenic therapy + immunotherapy | 1 (5.0) | 1 (3.6) | ||
| Immunotherapy | 0 (0.0) | 1 (3.6) |
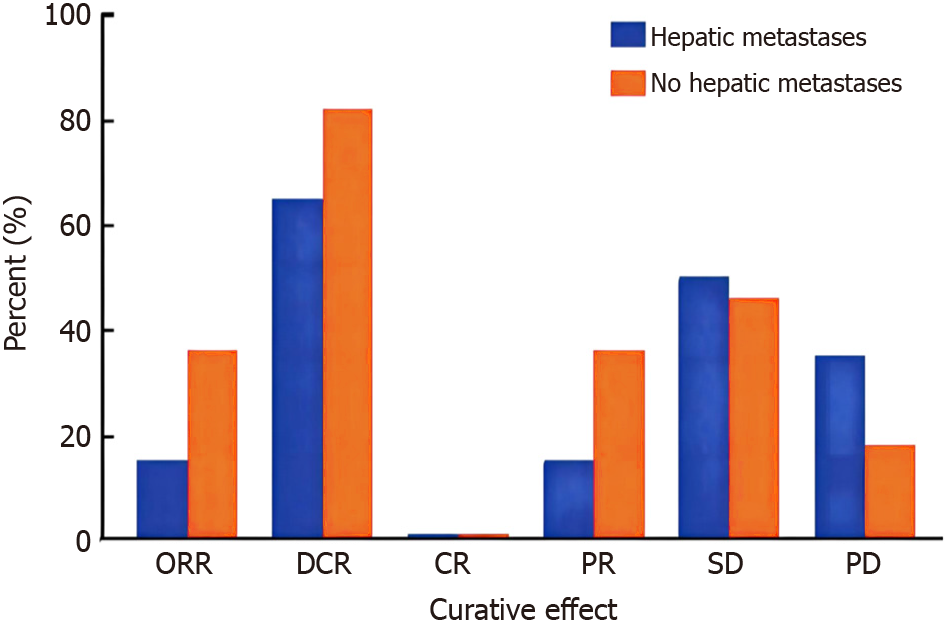
In the cohort of patients with liver metastases, 3 out of 20 patients (15.0%) obtained a PR and 10 out of 20 patients (50.0%) attained SD based on the RECIST1.1 criteria. Among the group of patients without liver metastases, 10 out of 28 individuals (35.7%) experienced a PR, while 13 out of 28 individuals (46.4%) achieved SD. In the liver metastatic cohort, the ORR and DCR were 15.0% and 35.7% (P > 0.05), respectively. In the non-liver metastasis cohort, the ORR and DCR were 65.0% and 82.1% (P > 0.05), respectively.
In the subset of patients with liver metastases, our study revealed a nuanced response to immunotherapy. Notably, 15.0% of these patients achieved a PR, and 50.0% experienced SD based on RECIST1.1 criteria. While these outcomes suggest a modest overall response, the ORR and DCR in this cohort were 15.0% and 35.7%, respectively, with no statistically significant difference (P > 0.05). This underscores the challenging nature of treating advanced stomach cancer with liver metastasis. Conversely, among patients without liver metastases, a more favorable response was observed. A higher percentage, 35.7%, achieved a PR, and 46.4% attained SD. The ORR and DCR in this non-liver metastasis cohort were 65.0% and 82.1%, respectively, with no significant difference (P > 0.05). These findings emphasize a more robust and clinically significant response to immunotherapy in patients without liver metastasis.
According to the study results, the rate of response to immunotherapy in GC patients with liver metastasis was lower compared to those without liver metastasis. However, this difference did not reach statistical significance (Figure 1).
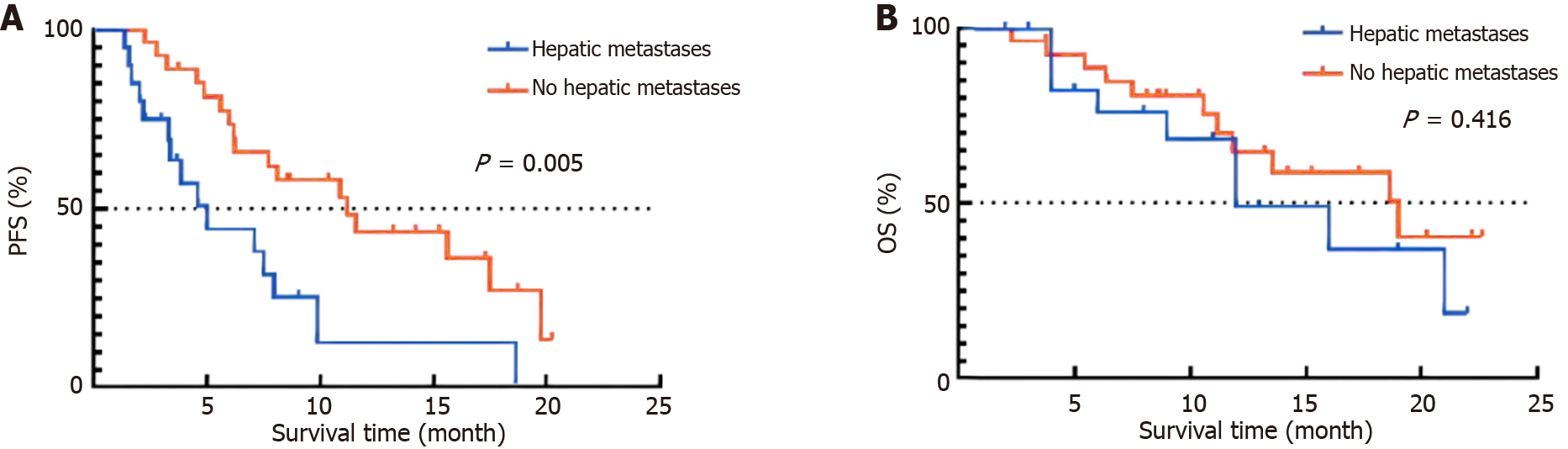
The median duration of follow-up was 18.9 months, with no patients experiencing a loss of follow-up until the most recent assessment. The Kaplan-Meier survival analysis revealed that the median PFS for GC patients in the liver metastasis group was 5.0 months, while it was 11.2 months for those in the non-liver metastasis group (hazard ratio = 0.40, P < 0.01). Additionally, the median OS was 12.0 months for the liver metastasis group and 19.0 months for the non-liver metastasis group (P > 0.05), as depicted in Figure 2. The findings indicated that the prognosis of GC patients who had immunotherapy and had liver metastasis was comparatively poorer than that of individuals without liver metastasis.
Out of the 48 patients diagnosed with GC, 15 patients who had liver metastasis and 20 patients who did not have liver metastases experienced adverse effects due to immunotherapy. Five patients with liver metastases and seven patients without liver metastasis experienced Grade 3 or higher treatment-related side events. There were no instances of treatment-related adverse events leading to withdrawal or death in either group of patients.
Among the 48 patients diagnosed with GC, 15 with liver metastasis and 20 without liver metastases encountered adverse effects from immunotherapy. Notably, five patients with liver metastases and seven without experienced Grade 3 or higher treatment-related side events. Importantly, no treatment-related adverse events led to withdrawal or mortality in either group. The predominant adverse events encompassed vomiting, nausea, and exhaustion in both cohorts. These findings underscore the tolerability of immunotherapy in advanced GC, with a manageable incidence of adverse effects. The absence of treatment-related withdrawals or fatalities suggests a favorable safety profile, providing reassurance for the clinical application of immunotherapy in this patient population. The predominant adverse events observed in both cohorts were vomiting, nausea, and exhaustion (Tables 2 and 3).
| Adverse reaction | Liver metastasis (n = 20) | No liver metastasis grade 1-2 (n = 28) | χ2 | P value |
| All events related to treatment | 10 (50.0) | 13 (46.4) | 0.060 | 0.999 |
| Nausea | 7 (35.0) | 7 (25.0) | 0.565 | 0.528 |
| Diarrhea | 6 (30.0) | 8 (29.0) | 0.012 | 0.999 |
| Fever | 5 (25.0) | 8 (29.0) | 0.075 | 0.999 |
| Peripheral neuropathy | 4 (20.0) | 6 (21.0) | 0.014 | 0.999 |
| Vomit | 7 (35.0) | 8 (29.0) | 0.224 | 0.755 |
| Fatigue | 5 (25.0) | 8 (29.0) | 0.075 | 0.999 |
| Anaemia | 6 (30.0) | 6 (21.0) | 0.457 | 0.520 |
| Anorexia | 5 (25.0) | 5 (18.0) | 0.361 | 0.721 |
| Rash | 3 (15.0) | 4 (14.0) | 0.005 | 0.999 |
| Thrombopenia | 4 (20.0) | 4 (14.0) | 0.274 | 0.703 |
| Abnormal liver function | 5 (25.0) | 4 (14.0) | 0.879 | 0.460 |
| Leukopenia | 4 (20.0) | 5 (18.0) | 0.035 | 0.999 |
| Adverse reaction | Liver metastasis (n = 20) | No liver metastasis grade ≥ 3 (n = 28) | χ2 | P value |
| All events related to treatment | 5 (25.0) | 7 (25.0) | 0.001 | 0.999 |
| Nausea | 4 (20.0) | 4 (14.0) | 0.274 | 0.073 |
| Diarrhea | 2 (10.0) | 3 (11.0) | 0.006 | 0.999 |
| Fever | 2 (10.0) | 4 (14.0) | 0.196 | 0.999 |
| Peripheral neuropathy | 2 (10.0) | 4 (14.0) | 0.196 | 0.999 |
| Vomit | 5 (25.0) | 5 (18.0) | 0.361 | 0.721 |
| Fatigue | 4 (20.0) | 5 (18.0) | 0.035 | 0.999 |
| Anaemia | 1 (5.0) | 4 (14.0) | 1.078 | 0.385 |
| Anorexia | 2 (10.0) | 4 (14.0) | 0.196 | 0.999 |
| Rash | 1 (5.0) | 2 (7.0) | 0.091 | 0.999 |
| Thrombopenia | 2 (10.0) | 1 (4.0) | 0.823 | 0.563 |
| Abnormal liver function | 2 (10.0) | 2 (7.0) | 0.125 | 0.999 |
| Leukopenia | 3 (15.0) | 4 (14.0) | 0.005 | 0.999 |
The outlook for patients with GC who have distant organ metastases is typically unfavorable[15]. The liver is the primary organ that GC spreads to, with a liver metastasis rate ranging from 36% to 40%[16-20]. Immune checkpoint inhibitors have emerged as a novel therapeutic choice for individuals with advanced malignancies. Several studies[21-24] have demonstrated that the existence of liver metastases prior to immunotherapy treatment in patients with melanoma and NSCLC leads to systemic immunosuppression, which subsequently leads to reduced effectiveness of immunotherapy[25]. Thus, may liver metastases serve as a constraint on the duration of immunotherapy’s advantages for patients with GC?
Currently, there is no substantial clinical investigation that has verified the correlation between liver metastases of GC and reduced effectiveness of immunotherapy in patients[26-28]. The study revealed that individuals with advanced GC who received immunotherapy had poorer health at the beginning of the study if they had liver metastases, in contrast to those without liver metastases[29]. This was because to the decreased treatment response rate and shorter PFS. What is the cause of these disparities? Hepatic immunological tolerance is a widely acknowledged notion that encompasses the following mechanisms: (1) Liver endothelial cells or immature DC cells present non-specific antigens to CD4+ and CD8+ T cells, causing the latter to differentiate into Treg cells and partially activated T cells, respectively, which will undergo passive cell death; (2) Liver metastases can decrease the density of CD8+ T cells at the periphery of invasive tumors; and (3) Preclinical model studies revealed that following immunotherapy, mouse primary tumors were heavily infiltrated by CD8+ T cells, and the level of immune cell infiltration decreased in the presence of liver metastasis. However, the initiation and activation of naive T cells were unaffected until they reached the liver, indicating that liver metastasis induces alterations in the systemic distribution of antigen-specific T cells[30-32]. Nevertheless, when liver metastases are present, there is a significant decrease in the quantity of antigen-specific CD8+ T cells in the primary tumor, tumor-draining lymph nodes, and peripheral blood[33]. Additionally, there is a notable decrease in the expression of labeled activated cytokines in T cells, as well as a significant reduction in the number and activation level of distal effector T cells.
This study has verified that the aforementioned findings are applicable to human diseases, specifically indicating that individuals with NSCLC and liver metastases have decreased absolute lymphocyte numbers compared to those without liver metastasis[34]. Primary tumor sequencing of metastatic patients, such as those with melanoma or NSCLC, revealed a reduction in T cell clonality and diversity, as well as a drop in T cell effector capacity, in patients with liver metastases. Studies have demonstrated that liver CD11b+F4/80+ bone marrow cells employ the Fas-FasL cell pathway to trigger the death of T cells in the liver[35]. This leads to a decrease in the distribution of T cells and induces systemic immunosuppression, ultimately resulting in the limited effectiveness of immunotherapy.
People with liver metastasis have a more deteriorated physical condition compared to people without liver metastasis. Research has demonstrated that liver metastasis leads to an escalation in the overall tumor burden, which subsequently results in a decline in the physical condition of patients. Studies[36-38] have demonstrated a negative correlation between the physical condition of patients with NSCLC and the effectiveness of immunotherapy. This could be attributed to the delayed response time of immunotherapy, which may not provide significant benefits to fragile patients who are at a heightened risk of early mortality. In addition, individuals experiencing poor health may require a combination of palliative and non-palliative corticosteroid treatments more frequently. The utilization of steroids is associated with diminished efficacy of immune checkpoint inhibitors. Further prospective trials are required to determine whether liver metastases or poor physical state in patients are associated with reduced efficacy of immunotherapy.
The primary constraints of this investigation, which involved a retrospective analysis conducted at a single location, are the inadequate duration of follow-up and the limited size of the sample, which hindered the acquisition of comprehensive OS data. Out of all the patients in this trial who had stomach cancer that had progressed to the liver, only two of them underwent liver mega lysis radiation in addition to immunotherapy.
Consequently, it is indeterminable whether the combo therapy enhances the liver’s immunological tolerance. Nevertheless, the findings of this study affirm that liver metastasis might cause a decline in the effectiveness of immunotherapy. Additionally, liver metastasis can serve as an unfavorable indicator of immunotherapy efficacy in individuals diagnosed with GC. Given these findings, it is imperative to conduct prospective investigations on individuals with liver metastases from GC to identify the optimal combination therapy that can overcome the liver’s immune tolerance, address the therapeutic challenges associated with liver metastases, and enhance the efficacy of immunotherapy in patients with liver metastases from GC.
Immunotherapy is less effective in GC patients with liver metastases compared to those without liver metastasis.
Gastric cancer (GC) is the fifth most common type of cancer and has the fourth highest death rate among all cancers. There is a lack of studies examining the impact of liver metastases on the effectiveness of immunotherapy in individuals diagnosed with GC.
This study retrospectively examined patients with advanced GC who received immunotherapy in the undergraduate department to investigate the influence of liver metastases.
To investigate the influence of liver metastases on the effectiveness and safety of immunotherapy in patients with advanced GC.
This retrospective investigation collected clinical data of patients with advanced stomach cancer who had immunotherapy at our hospital from February 2021 to January 2023. The baseline attributes were compared using either the Chi-square test or the Fisher exact probability method. The chi-square test and Kaplan-Meier survival analysis were employed to assess the therapeutic efficacy and survival duration in GC patients with and without liver metastases.
The analysis comprised 48 patients diagnosed with advanced GC, who were categorized into two groups: A liver metastasis cohort (n = 20) and a non-liver metastatic cohort (n = 28). Patients with liver metastasis exhibited a more deteriorated physical condition compared to those without liver metastasis. The objective response rates in the cohort with metastasis and the cohort without metastasis were 15.0% and 35.7% (P > 0.05), respectively. Similarly, the disease control rates (DCR) in these two cohorts were 65.0% and 82.1% (P > 0.05), respectively. The median progression-free survival was 5.0 months in one group and 11.2 months in the other group, with a hazard ratio of 0.40 and a significance level (P) less than 0.05. The median overall survival was 12.0 months in one group and 19.0 months in the other group, with a significance level (P) greater than 0.05.
Immunotherapy is less effective in GC patients with liver metastases compared to those without liver metastasis.
This study provides valuable insights into the efficacy and safety of immunotherapy in patients with GC and liver metastases. In the future, we will look at more detailed molecular level studies to explore the possibility of personalized therapy. In addition, we plan to strengthen the analysis of the mechanisms of immune response after treatment to reveal potential molecular markers of treatment success or failure. In clinical practice, we will strive to promote the translation of research results to provide patients with more personalized and precise treatment options. This series of future work will further promote the application of immunotherapy in GC and liver metastases, and bring more effective and safe treatment options to patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shang L, China S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Li S, Guo D, Sun Q, Zhang L, Cui Y, Liu M, Ma X, Liu Y, Cui W, Sun L, Teng L, Wang L, Lin A, Liu W, Zhuo W, Zhou T. MAPK4 silencing in gastric cancer drives liver metastasis by positive feedback between cancer cells and macrophages. Exp Mol Med. 2023;55:457-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 2. | Xia X, Zhang Z, Zhu C, Ni B, Wang S, Yang S, Yu F, Zhao E, Li Q, Zhao G. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13:1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 3. | Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, Li Z, Wang W, Xu H, Li B, Xu Z. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. 2022;41:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 128] [Reference Citation Analysis (0)] |
| 4. | Xie L, Qiu S, Lu C, Gu C, Wang J, Lv J, Fang L, Chen Z, Li Y, Jiang T, Xia Y, Wang W, Li B, Xu Z. Gastric cancer-derived LBP promotes liver metastasis by driving intrahepatic fibrotic pre-metastatic niche formation. J Exp Clin Cancer Res. 2023;42:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 5. | Jiang H, Yu D, Yang P, Guo R, Kong M, Gao Y, Yu X, Lu X, Fan X. Revealing the transcriptional heterogeneity of organ-specific metastasis in human gastric cancer using single-cell RNA Sequencing. Clin Transl Med. 2022;12:e730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 130] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 6. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Li D, Wang Y, Dong C, Chen T, Dong A, Ren J, Li W, Shu G, Yang J, Shen W, Qin L, Hu L, Zhou J. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023;42:83-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 158] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 8. | Dong Z, Zhang Y, Geng H, Ni B, Xia X, Zhu C, Liu J, Zhang Z. Development and validation of two nomograms for predicting overall survival and cancer-specific survival in gastric cancer patients with liver metastases: A retrospective cohort study from SEER database. Transl Oncol. 2022;24:101480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Li D, Zhang X, Jiang L. Molecular mechanism and potential therapeutic targets of liver metastasis from gastric cancer. Front Oncol. 2022;12:1000807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Lee J, Pang K, Kim J, Hong E, Lee J, Cho HJ, Park J, Son M, Park S, Lee M, Ooshima A, Park KS, Yang HK, Yang KM, Kim SJ. ESRP1-regulated isoform switching of LRRFIP2 determines metastasis of gastric cancer. Nat Commun. 2022;13:6274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 12. | Yang H, Hu Y, Weng M, Liu X, Wan P, Ma M, Zhang Y, Xia H, Lv K. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J Adv Res. 2022;37:91-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 168] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 13. | Chen J, Dang Y, Feng W, Qiao C, Liu D, Zhang T, Wang Y, Tian D, Fan D, Nie Y, Wu K, Xia L. SOX18 promotes gastric cancer metastasis through transactivating MCAM and CCL7. Oncogene. 2020;39:5536-5552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Zhang A, Zou X, Yang S, Yang H, Ma Z, Li J. Effect of NETs/COX-2 pathway on immune microenvironment and metastasis in gastric cancer. Front Immunol. 2023;14:1177604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Zhou YQ, Bao TS, Xie JX, Yao LL, Yu ST, Li Q, Huang PQ, Zhou WZ, Wang YY, Chen SY, Wang XQ, Zhang XL, Jiang SH, Yi SQ, Zhang ZG, Ma MZ, Hu LP, Xu J, Li J. The SLITRK4-CNPY3 axis promotes liver metastasis of gastric cancer by enhancing the endocytosis and recycling of TrkB in tumour cells. Cell Oncol (Dordr). 2023;46:1049-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | He Y, He P, Lu S, Dong W. KIFC3 Regulates the progression and metastasis of gastric cancer via Notch1 pathway. Dig Liver Dis. 2023;55:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Conde Monroy D, Ibañez-Pinilla M, Sabogal JC, Rey Chaves C, Isaza-Restrepo A, Girón F, Vanegas M, Ibañez-Villalba R, Mirow L, Siepmann T. Survival Outcomes of Hepatectomy in Gastric Cancer Liver Metastasis: A Systematic Review and Meta-Analysis. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Zhou P, Zheng ZH, Wan T, Wu J, Liao CW, Sun XJ. Vitexin Inhibits Gastric Cancer Growth and Metastasis through HMGB1-mediated Inactivation of the PI3K/AKT/mTOR/HIF-1α Signaling Pathway. J Gastric Cancer. 2021;21:439-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 20. | Ni B, He X, Zhang Y, Wang Z, Dong Z, Xia X, Zhao G, Cao H, Zhu C, Li Q, Liu J, Chen H, Zhang Z. Tumor-associated macrophage-derived GDNF promotes gastric cancer liver metastasis via a GFRA1-modulated autophagy flux. Cell Oncol (Dordr). 2023;46:315-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Yu D, Yang J, Jin M, Zhou B, Shi L, Zhao L, Zhang J, Lin Z, Ren J, Liu L, Zhang T, Liu H. Fecal Streptococcus Alteration Is Associated with Gastric Cancer Occurrence and Liver Metastasis. mBio. 2021;12:e0299421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Granieri S, Altomare M, Bruno F, Paleino S, Bonomi A, Germini A, Facciorusso A, Fagnani D, Bovo G, Cotsoglou C. Surgical treatment of gastric cancer liver metastases: Systematic review and meta-analysis of long-term outcomes and prognostic factors. Crit Rev Oncol Hematol. 2021;163:103313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Baba H, Kanda M, Sawaki K, Umeda S, Miwa T, Shimizu D, Tanaka C, Kobayashi D, Fujiwara M, Kodera Y, Fujii T. PRAME as a Potential Biomarker for Liver Metastasis of Gastric Cancer. Ann Surg Oncol. 2020;27:2071-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Alsina M, Arrazubi V, Diez M, Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. 2023;20:155-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 173] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 26. | Xie J, Fu L, Jin L. Immunotherapy of gastric cancer: Past, future perspective and challenges. Pathol Res Pract. 2021;218:153322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 442] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 28. | Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin Cancer Biol. 2022;86:566-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (1)] |
| 29. | Pous A, Notario L, Hierro C, Layos L, Bugés C. HER2-Positive Gastric Cancer: The Role of Immunotherapy and Novel Therapeutic Strategies. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Zeng Y, Zhang X, Li F, Wang Y, Wei M. AFF3 is a novel prognostic biomarker and a potential target for immunotherapy in gastric cancer. J Clin Lab Anal. 2022;36:e24437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Wang B, Zhang Z, Liu W, Tan B. Targeting regulatory T cells in gastric cancer: Pathogenesis, immunotherapy, and prognosis. Biomed Pharmacother. 2023;158:114180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Wang M, Yang G, Tian Y, Zhang Q, Liu Z, Xin Y. The role of the gut microbiota in gastric cancer: the immunoregulation and immunotherapy. Front Immunol. 2023;14:1183331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 34. | Mak TK, Li X, Huang H, Wu K, Huang Z, He Y, Zhang C. The cancer-associated fibroblast-related signature predicts prognosis and indicates immune microenvironment infiltration in gastric cancer. Front Immunol. 2022;13:951214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Entezam M, Sanaei MJ, Mirzaei Y, Mer AH, Abdollahpour-Alitappeh M, Azadegan-Dehkordi F, Bagheri N. Current progress and challenges of immunotherapy in gastric cancer: A focus on CAR-T cells therapeutic approach. Life Sci. 2023;318:121459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 455] [Article Influence: 151.7] [Reference Citation Analysis (0)] |
| 37. | Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 838] [Article Influence: 167.6] [Reference Citation Analysis (1)] |
| 38. | Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, You J, Wong BCY, Chen J, Ye W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology. 2022;163:154-162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |