INTRODUCTION
Over 500000 individuals undergo bariatric surgery annually worldwide[1]. Although bariatric surgery reduces the development of obesity-related diseases and improves all-cause mortality[2], it may also increase the risk of harmful drinking[3] and alter the liver’s susceptibility to injury in the setting of alcohol use[4]. In recent years, the practice of simultaneous bariatric surgery and liver transplantation has gained favor in patients with metabolic dysfunction-associated steatotic liver disease (MASLD) due to the potential improved peri-operative outcomes[5]. Thus, understanding alcohol-related disorders after bariatric surgery will be increasingly important in the clinical setting. In this article, we provide a scoping review of epidemiology, pathophysiology, and clinical outcomes of alcohol use disorder (AUD) and alcohol associated liver disease (ALD) after bariatric surgery and discuss opportunities to enhance post-operative care for these patients.
EPIDEMIOLOGY OF ALCOHOL USE AFTER BARIATRIC SURGERY
AUD is characterized by a pattern of alcohol consumption that persists despite negative personal and health consequences[6]. The prevalence of alcohol use has been steadily increasing with some data suggesting over 40% of adults engage in potentially harmful drinking[6]. An expanding body of literature suggests that bariatric surgery increases the risk of alcohol misuse. Estimates suggest that between 2%-33% of adult bariatric surgery patients develop AUD, with the peak incidence occurring in the second post-operative year[3,4,7,8]. This risk may be higher in adolescent bariatric surgery recipients with a recent multi-center study reporting an AUD prevalence of approximately 45% eight years post-surgery[9].
Multiple studies have identified the following as risk factors for harmful alcohol use after bariatric surgery: younger age, tobacco use, pre-surgical alcohol use, and reduced social support[8,10,11]. Additionally, one large prospective study identified higher household income and lower-level education as risk factors for AUD[7]. Patient sex influences risk as well: female sex is a risk factor for AUD in adolescent bariatric surgery recipients[12], while male sex is a risk factor in adult bariatric surgery recipients[10]. Although adult males have a higher risk of AUD, most bariatric surgery recipients with AUD are female, reflecting the overall higher proportion of females in the bariatric surgery population[3]. The risk of post-surgical AUD may also depend on the type of surgery performed. Some studies indicate a higher risk following gastric bypass procedures as compared to restrictive procedures[11,13], although this relationship is not yet fully elucidated[7,11,13].
Although pre-surgical alcohol use is associated with AUD following bariatric surgery, new onset alcohol use is also common[7,14-16]. In a recently published study, Kim et al[14] utilized a large commercial insurance database to assess alcohol use in patients who underwent bariatric surgery compared to patients who underwent cholecystectomy. They found that 2.8% of the post-bariatric surgery group developed de novo alcohol use or dependence compared to 1.7% of the post-cholecystectomy group. On subgroup analyses, Roux-en-Y gastric bypass (RYGB) was associated with a 150% higher risk of acquiring an alcohol-associated diagnosis (e.g., alcohol use, alcohol use, and alcohol-associated hepatitis) compared to post-cholecystectomy controls (95%CI 1.40-1.62, P < 0.001). In contrast, vertical sleeve gastrectomy (VSG) was associated with a slightly reduced risk of acquiring an alcohol-associated diagnosis. Notably, since this study was conducted using International Classification of Diseases, Tenth Revision (ICD-10) codes, the context of these diagnoses was not available. A multi-center retrospective study within the United States Veterans Affairs Healthcare System used the Alcohol Use Disorder Identification Test (AUDIT-C) to assess for harmful alcohol use among bariatric surgery patients (n = 1539 VSG, n = 854 RYGB) compared to non-surgical controls[15]. Among patients without pre-surgical alcohol use, new unhealthy alcohol use was significantly more common in the surgical groups at post operative years 3, 5, and 8. At the end of the study period, the risk of unhealthy alcohol use was 7.9% in VSG patients, 9.2% in RYGB patients, and 4.4%-4.5% in non-surgical control patients. Similarly, a recent prospective study observed a quadratic relationship between alcohol use and time after bariatric surgery, with annual increases in both quantity and frequency of alcohol consumption from years 1-7 after bariatric surgery[16]. This discrepancy in findings may be due to differing follow-up periods, as Kim et al[14] followed patients for a mean of 2.7 years whereas the latter two studies had an 8 year follow up and noted a significant increase in harmful alcohol use between 3-8 years post-surgery.
PATHOPHYSIOLOGY OF ALCOHOL USE DISORDER AFTER BARIATRIC SURGERY
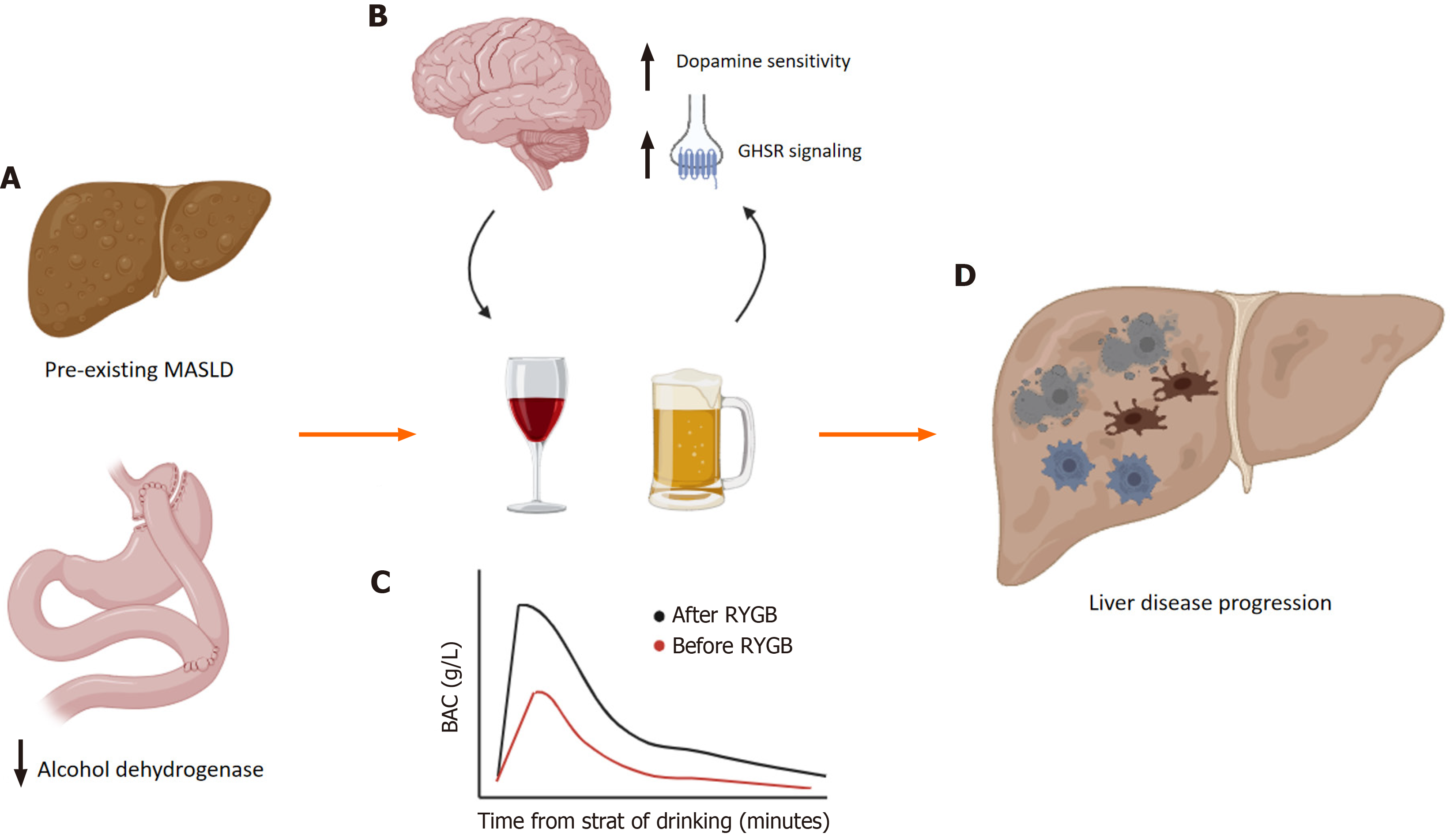
The mechanisms by which bariatric surgery increases the risk of AUD have been studied but are not fully understood. Several factors are likely to contribute, including the metabolic, anatomic, and downstream neurologic changes resulting from the surgery[3]. A basic understanding of each surgical procedure is important when considering post-operative alcohol use. Briefly, in RYGB, the stomach is divided into a small gastric pouch (using the proximal stomach) and a larger distal remnant. The gastric pouch is then connected directly to the mid-jejunum via the Roux - or alimentary - limb, bypassing the distal stomach and duodenum[17]. As a result, nutrients quickly pass from the stomach to the distal small intestine. Vertical sleeve gastrectomy involves stapling the stomach along a vertical line from the pylorus to the angle of His and removing the occluded portion which includes the fundus, body, and part of the antrum[18]. This results in a narrow tube-like stomach that is about 70%-80% smaller in size (Figure 1).
Figure 1 Mechanisms of alcohol related liver disease after bariatric surgery.
Several anatomic, metabolic, and neurohumoral changes occur after bariatric surgery. A: Changes in the gastric anatomy affect the metabolism and pharmacokinetics properties of alcohol and lead to enhanced alcohol sensitivity and reduced tolerance; B: These alterations are linked to changes in brain reward pathways that can increase the risk of developing alcohol use disorders; C: Further, reduced first pass metabolism results in increased delivery of alcohol to the liver; D: Together, these may result in earlier onset alcohol associated liver injury and chronic alcohol associated liver disease. MASLD: Metabolic dysfunction-associated steatotic liver disease; BAC: Blood alcohol concentration; GHSR, or ghrelin receptor: Growth hormone secretagogue receptor; RYGB: Roux-en-Y gastric bypass. Image created using Biorender.com.
Pharmacokinetics
The anatomic changes from both RYGB and VSG result in faster gastric emptying and reduced first-past metabolism[3,19,20]. As a result, blood alcohol concentration peaks faster and at higher concentrations after any given ingestion of alcohol. For instance, the oral bioavailability of alcohol has been shown to increase by more than 30% in women who undergo VSG[21]. Likewise, a study found that women with a history of RYGB experience more brisk and larger amplitude changes in blood alcohol concentration. Specifically, after consuming 26 g of alcohol (equivalent to about two shots of hard liquor), blood alcohol concentration increased to a level greater than the United States legal driving limit in less than 10 min[22].
In addition to objective measurements, patients report subjectively increased response to alcohol. A qualitative study evaluating the addictive properties of alcohol following bariatric surgery reported that patients experienced the feeling of intoxication faster than they did prior to surgery[23]. Other studies have used patient-facing questionnaires including the Addiction Research Center Inventory and the Alcohol Sensitivity Questionnaire to quantify subjective response to alcohol. In these studies, patients reported increased feelings of drunkenness and higher sensitivity to alcohol after bariatric surgery[24,25]. Because fast onset drugs are known to have higher addictive potential[26], this brisk increase in response to alcohol has been thought to play a major role in the development of post-bariatric surgery AUD. It was thought that reduced first-pass metabolism after bariatric surgery was due a relative reduction in gastric alcohol dehydrogenase activity. However, studies evaluating alcohol dehydrogenase have been inconsistent, and currently there is insufficient evidence to support that reduced alcohol dehydrogenase activity is the primary factor behind the rapid increases in blood alcohol concentration observed after bariatric surgery[22,27,28].
Hormonal and reward pathways
Metabolic changes influencing alcohol use after bariatric surgery arise from alterations in the secretion of multiple gut hormones that occur after both RYGB and VSG[3,19,29]. Two key hormones linking alcohol use and bariatric surgery are ghrelin and glucagon-like peptide-1 (GLP-1).
Ghrelin: The role of ghrelin in the development of AUD after bariatric surgery has been explored extensively. Early theories proposed that changes in ghrelin levels directly influence alcohol intake after RYGB. However, it is now thought that hormonal changes affect reward-seeking pathways in the brain[30-32]. Pre-clinical models have demonstrated that RYGB surgery is associated with a heightened response to alcohol in central reward centers[30,31] and that this heightened response can be attenuated by antagonizing the ghrelin receptor: growth hormone secretagogue receptor (GHSR) 1a[31]. In fact, a recent study found that GHSR activity regulates the tonic rate of neuronal firing in a key reward center in the brain, the ventral tegmental area (VTA). In this study, researchers treated mice with a GHSR-antagonist, and observed a reduction in the action potential firing rate of VTA dopaminergic neurons in non-RYGB mice but observed no effect in mice with RYGB[32]. They concluded that alcohol misuse after RYGB may be related to reduced GHSR control of the VTA dopaminergic system. Similar animal models have been developed for VSG[33,34]. In contrast to RYGB models, mice with VSG demonstrated reduced alcohol seeking behavior compared to control mice. This finding was initially attributed to the removal of the ghrelin producing part of the stomach, but this was refuted when administration of ghrelin at different concentrations failed to impact ethanol consumption. Interestingly, administration of low dose GHSR antagonist induced a significant reduction in alcohol consumption in the VSG mice but not in control mice[35]. Collectively these data demonstrate that GHSR signaling is linked to changes in brain reward pathways including enhanced dopamine sensitivity and increased reward response to alcohol after bariatric surgery. Differences observed between pre-clinical models of VSG and RYGB may help explain differences in the prevalence of AUD in these groups.
GLP-1: GLP-1 is a gut-brain peptide that regulates appetite and food intake, and it has been increasingly recognized for its role in alcohol-mediated behaviors. For instance, animal studies have shown that administration of liraglutide, a GLP-1 agonist, attenuates the rewarding properties of alcohol[36,37]. Specifically, liraglutide has been shown to impair the release of dopamine in the nucleus accumbens of mice after acute injections of ethanol. After treatment with liraglutide, mice were less likely to demonstrate preference for areas where ethanol was in their environment. Data from these preclinical models suggest that GLP-1 receptor agonists may attenuate the ability of alcohol to activate the mesolimbic dopamine system and may reduce alcohol intake. Interestingly, a nation-wide cohort study in Denmark found that GLP-1 agonists were associated with a lower incidence of alcohol-related health events, though this protective effect was limited to the first 3 months of therapy[38]. While these findings highlight the potential clinical relevance of GLP-1 pathways in treating AUD, the use of GLP-1 receptor agonists in reducing alcohol intake following weight reduction surgery has not been studied.
Psychology and addiction
When discussing potential mechanisms of increased alcohol use after bariatric surgery, it is worth mentioning the “addiction transfer” hypothesis. This theory assumes that patients pursuing bariatric surgery have addictive eating habits that are transferred to alcohol consumption post-operatively when food intake is limited by appetite[39]. However, there are several lines of evidence which demonstrate that addiction transfer alone is insufficient to explain observed trends in post-bariatric surgery alcohol use.
First, studies have shown that pre-operative addictive eating behaviors and binge eating do not increase the likelihood of developing AUD[40,41] which directly opposes the idea that post-surgical AUD originates from food-related addictive behaviors. Furthermore, increased alcohol use has been observed in patients following prophylactic total gastrectomy, even in the absence of obesity or history of addictive eating behaviors[42,43]. Second, the tendency for alcohol use to increase one to two years after bariatric surgery contradicts the addiction transfer theory as the reduction in food intake occurs almost immediately post-operatively[44]. Third, the incidence of AUD after bariatric surgery varies depending on the type of surgery, suggesting that changes inherent to each surgical procedure play a role in the development of AUD[7].
Additionally, the anatomic changes resulting from these surgeries alter the metabolism and pharmacokinetics of alcohol[45]. Post-surgery weight loss combined with reduced gastric volume and accelerated gastric emptying results in higher blood alcohol concentrations and leads to increased alcohol sensitivity and lower tolerance[46]. These changes are likely critical in the development of AUD after weight loss surgeries. While the addiction transfer hypothesis may explain a behavioral component in a minority of patients, its general application can increase stigma and discourage care seeking amongst the majority[45]. Therefore, healthcare providers should be cautious when referencing this hypothesis to avoid perpetuating stigma in a disorder that has a clear biological basis.
ALCOHOL-RELATED OUTCOMES AFTER BARIATRIC SURGERY
Beyond AUD, bariatric surgery is associated with an increased risk of developing ALD. There are likely several reasons for this relationship. Patients undergoing bariatric surgery likely have some baseline hepatic disease, commonly in the form of steatotic liver disease[47]. Obesity and alcohol consumption have a synergistic effect on the liver; patients with a BMI over 35 are more likely to develop liver damage from any given amount of alcohol compared to patients with a BMI under 35[48,49]. While bariatric surgery can reduce hepatic fibrosis and/or eliminate steatohepatitis in 70%-80% of patients achieving a minimum 5-10-kilogram weight loss[50], it remains unclear if the liver continues to be predisposed to alcohol-associated damage, particularly when exposed to relatively higher concentrations of alcohol after surgery.
The exact prevalence of ALD after bariatric surgery is unknown. Two recent studies estimate the incidence of ALD to be 0.7% in bariatric surgery recipients[4] compared to 0.3% in the general population[51,52]. Mellinger et al[4] compared the risk of alcohol-associated cirrhosis (AC) in obese patients who underwent bariatric surgery and those who did not in two time periods: before 2008 and from 2008 to 2016. While initial unadjusted analyses suggested no increased risk of AC in the bariatric surgery group, further analyses revealed a significant interaction between the time period and patient gender. Adjusted analyses demonstrated that women who underwent bariatric surgery had a 2.1-times greater risk of AC (95%CI 1.8-2.4) and men had a 1.3-times greater risk of AC (95%CI 1.1-1.6) compared to their counterparts who did not undergo the surgery. In another study, Alvarado et al[52] assessed the risk of ALD following bariatric surgery compared to other abdominal surgeries in a large patient cohort (n = 1,075,514). They found that bariatric surgery recipients were more likely to develop both ALD (95%CI 1.22-1.37) and AC (95%CI 1.37-1.42). Additionally, they observed a significant increase in ALD and AC cases among bariatric surgery patients from 2005 to 2015, which aligns with Mellinger et al’s findings about the long-term risk of AC[4].
Bariatric surgery status has also been linked to poorer outcomes in patients with ALD. In a study of 2,634 patients with alcohol-associated hepatitis, those with prior RYGB had a higher 30-day readmission rate (P < 0.01) and increased overall mortality (P = 0.03) compared to non-surgical patients[53]. Conversely, an analysis of the National Inpatient Sample found that prior RYGB was associated with an increased risk of hepatic encephalopathy and infection but was not associated with higher mortality among patients hospitalized for ALD[54]. It is possible that the higher rate of adverse outcomes, despite a relatively younger patient population, is due to the delivery of higher concentrations of alcohol to the liver for any given ingestion of alcohol. Ultimately, the mortality benefits of bariatric surgery are attenuated by the development of alcohol-associated co-morbidities in patients who develop AUD[55].
BARIATRIC SURGERY AND LIVER TRANSPLANTATION
In line with the trends for ALD, there has been an increase in liver transplants for AC among post-bariatric surgery patients. Patients with a history of bariatric surgery that progress to needing a liver transplant in the setting of ALD are typically younger, female, and are more likely to be diagnosed with mood disorders compared to those without a history of bariatric surgery[56]. This cohort tends to present with more severe hepatic decompensation and progresses to liver transplant significantly faster than those who have not undergone bariatric surgery[57]. Post-liver transplant outcomes in this cohort have not been extensively studied, though a small study found that post-transplant biliary complications and rejection rates were similar in patients with and without a history of RYGB.
Over the last decade, there has been a trend towards performing simultaneous liver transplant and bariatric surgery (usually VSG) in eligible patients. Although limited, a few small studies reporting patient outcomes have emerged in recent years[58-62]. Generally, simultaneous bariatric surgery has not shown to increase liver transplant related mortality. Two prospective studies showed that patients with simultaneous surgeries had lower degrees of hepatic steatosis and fewer weight-related co-morbidities three years post-surgery compared to patients who had liver transplant alone[60,61]. It is important to note that post-surgical alcohol use is not addressed in these reports. Therefore, it remains unclear whether the risk of AUD in this cohort is similar to that in the general population. For those who do develop AUD, the risk of recurrent ALD is likely very different. While the pre-surgical fibrosis is obviated by transplantation, post-transplant patients are at risk of complications like strictures, chronic cholestasis, and rejection. These complications may interact with alcohol and result in liver dysfunction. To-date, no studies have addressed these clinical questions or assessed the risk of AUD or ALD in patients who have undergone simultaneous liver transplant-bariatric surgery.
FUTURE DIRECTIONS: CLINICAL CARE FOR AUD AFTER BARIATRIC SURGERY
Currently, there are no screening guidelines for AUD following bariatric surgery. Providers should counsel patients that there is no known “safe” level of alcohol intake and that standard recommendations for alcohol consumption (less than 7 drinks per week for women, less than 14 drinks per week for men) generally do not apply after the procedure. When concerns arise, screening questionnaires used in the general population, such as the AUDIT-C, can be used to identify potentially harmful alcohol use. The AUDIT-C is a three-question survey that can help categorize alcohol use behaviors over the past 12 months[63]. The AUDIT-C has proven effective in detecting AUD and predicting long-term outcomes, including hospitalizations for alcohol-related diagnoses. A 2019 study by White et al[16] assessed the use of the AUDIT-C in women with a history of bariatric surgery and found that a score ≥ 3 had the highest combined sensitivity (76.4%) and specificity (81.6%) for identifying alcohol-related health problems. Using validated questionnaires may be more helpful than serum markers of alcohol consumption (e.g., phosphatidylethanol), which may vary unpredictably in patients with a history of bariatric surgery[64].
While early screening is important for early diagnosis, bariatric surgery centers may also consider more intensive evaluation later, particularly in the second post-operative year[7]. Notably, patients undergoing simultaneous liver transplant and bariatric surgery may experience compound risk for AUD when considering the potential for psychological distress after liver transplantation. Therefore, these patients should receive counseling on risk reduction strategies and undergo similar, if not more stringent, post-operative monitoring for alcohol use compared to individuals with other liver disease etiologies. In patients who develop liver disease, identifying the primary etiology may be challenging for clinicians. While non-invasive markers exist to identify non-alcohol associated liver diseases, they often lack specificity. In patients with suspected alcohol use, liver biopsy may be required to assess the relative contribution of alcohol to liver damage[65-67].
CONCLUSION
Patients who have undergone bariatric surgery represent a high-risk cohort for alcohol-related health conditions including alcohol-associated liver disease. More information is needed to develop clinical recommendations for AUD and ALD in this cohort, though it is essential to advise patients that there is no “safe” level of alcohol consumption after bariatric surgery. Future studies should focus on identifying risk factors for hepatic fibrosis and ALD development, evaluating the effectiveness of medication-associated therapies for AUD in bariatric surgery recipients, and assessing alcohol-related outcomes in patients who undergo simultaneous liver transplant-bariatric surgery.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Skok P, Slovenia S-Editor: Gong ZM L-Editor: A P-Editor: Xu ZH