Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3675
Revised: September 18, 2024
Accepted: October 18, 2024
Published online: December 27, 2024
Processing time: 230 Days and 5.3 Hours
Previous studies suggest that appendectomy has a protective effect against ulcera
To explore the correlation between appendectomy and the onset of UC.
A total of 313 patients with newly diagnosed UC and 313 healthy individuals were selected for this study. According to whether their appendix was removed before the diagnosis of UC, patients were divided into appendectomized and non-appendectomized groups. Their general clinical data, appendectomy history, disease severity, extent of involvement, and blood routine test results were collec
The study revealed that the average time interval for the diagnosis of UC after appendectomy was 14.72 ± 13.87 years. 55.81% patients were diagnosed with UC five years after appendectomy. Among them, eight patients underwent appendec
Appendectomy may delay the onset of UC, reduce disease severity, and lessen the scope of involvement.
Core Tip: Our study found that appendectomy may delay the onset of ulcerative colitis (UC), reduce disease severity, and lessen the scope of involvement. This study provided evidence that appendectomy plays an important role in the occurrence and development of UC. A further prospective randomized trial evaluating the improvement of appendectomy on the disease course of UC may be feasible.
- Citation: Cui M, Shi C, Yao P. Protective effect of appendectomy against the onset of ulcerative colitis: A case-control study. World J Gastrointest Surg 2024; 16(12): 3675-3684
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3675.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3675
Ulcerative colitis (UC) is a chronic intestinal inflammatory disease with an unclear etiology and pathogenesis, mainly involving the sigmoid colon and rectum. The lesions are mostly limited to the mucosa and submucosa, and rarely involve the entire thickness of the colon[1]. The main clinical manifestations are abdominal pain, recurrent diarrhea, and the presence of mucus and blood in stools. In severe cases, UC can lead to toxic megacolon and may even progress to cancer. More than 25% of patients experience extraintestinal manifestations. The overall incidence of UC in China is about 0.0116% and shows a significant upward trend[2]. Owing to the unclear cause and the lack of a complete cure, the disease course of patients with UC is prolonged, with alternate periods of acute attacks and remission, significantly reducing their quality of life[3].
Current studies suggest that the pathogenesis of UC is a result of the combined effects of immune, environmental, and other factors[4]. Among these, abnormal immune function plays a major role[5]. The appendix is an immune organ and an important component of gut-associated lymphoid tissue. Compared with the colon, the appendix is rich in T lym
In 1958, Lumb and Protheroe[7] first found that periappendicular inflammatory lesions could act as skip lesions of UC, occurring not only in the UC of the right side or total colon, but also in patients with mild UC with lesions limited to the left colon or rectum. This finding suggests that appendicitis or periappendicular inflammatory lesions might play a certain role in the occurrence of UC. Later, Rutgeerts et al[8] found that compared to patients who had not undergone appendectomy, those who had undergone appendectomy in the past had a 29.4 times lower risk of developing UC. Cosnes et al[9] found that appendectomy can reduce disease activity in patients with UC and reduce the risk of colectomy. Mayer and Eisenhardt[10] suggested that the protective effect of appendectomy on UC may be closely related to T lymphocytes. In different mouse models of colitis, including those with T-cell receptor alpha mutation and models induced by dextran sulphate sodium salt, removal of inflamed cecum patches (akin to the human appendix) could prevent the occurrence of colitis[11]. Further research on cellular changes during appendicitis found that Paneth cells increased, goblet cells decreased, and crypt abscesses increased significantly, similar to the cellular changes observed during colonic inflammation[12]. However, the exact relationship between the appendix and the pathogenesis of UC remains unclear. Recent studies suggest that CD4+ and CD8+ regulatory T cells, natural killer T cells, and immunoglobulin A in the appendix may play a major role in the pathogenesis of UC[12,13].
Many international studies suggest that undergoing an appendectomy before being diagnosed with UC can afford a protective effect against the occurrence and development of the disease. However, there is a scarcity of research on the relationship between appendectomy and UC in China. This study aims to study the correlation between a history of appendectomy and the clinical characteristics of newly diagnosed UC.
Following a review and approval by the ethics committee (approval No. K202303-20), this study enrolled 626 participants, comprising 313 patients newly diagnosed with UC and 313 healthy individuals without any underlying diseases, from the First Affiliated Hospital of Xinjiang Medical University from January 2011 to December 2021. Participants were categorized into two groups based on their UC diagnosis: The UC group (313 cases) and the non-UC group (313 cases). Within the UC group, participants were further divided based on whether they had undergone an appendectomy before being diagnosed with UC, resulting in 43 cases (13.74%) in the appendectomized group and 270 cases (86.26%) in the non-appendectomized group.
The study included patients with UC who were newly diagnosed according to the “Consensus Opinion on the Diagnosis and Treatment of Inflammatory Bowel Disease (2018, Beijing)”[14] along with healthy individuals without any under
Individuals previously diagnosed with Crohn’s disease or those with an unclear diagnosis; patients with UC who also had infectious colitis, ischemic colitis, or other severe intestinal diseases, as well as those who had previously undergone a colectomy; those with a history of tonsillectomy; individuals diagnosed with hypertension, diabetes, or coronary heart disease; and cases with incomplete data were excluded from the study.
The baseline data of patients collected in this retrospective case–control study included age, sex, nationality (Han vs ethnic minorities), area of residence (urban vs rural), education level (categorized into low and middle education equivalent to high school and below, and higher education, which is beyond high school), smoking history (smokers vs non-smokers), drinking history (with and without a history of alcohol consumption), history of appendectomy, disease severity, extent of disease involvement, and blood routine indicators. The blood routine indicators included white blood cell (WBC) count, neutrophil count, lymphocyte count, monocyte count, red blood cell (RBC) count, hemoglobin (Hb), platelet (PLT) count, neutrophil-to-lymphocyte ratio (NLR), and PLT-to-lymphocyte ratio (PLR). The study then aimed to analyze the correlation between appendectomy and the clinical features of the onset of UC.
The modified Mayo score system[14] was used to evaluate the activity of the disease, by considering factors such as the frequency of defecation, the degree of blood in the stool, endoscopic findings, and the overall evaluation of the physician. The extent of lesions was classified according to the Montreal classification[15] into three categories: E1, E2, and E3, E1 represents involvement limited to the rectum, E2 represents involvement limited to a proportion of the colorectum distal to the splenic flexure, and E3 represents involvement extends proximal to the splenic flexure.
Data were analyzed using SPSS version 26.0 (IBM, Armonk, NY, United States). The measurement data were tested for normal distribution. Data that conformed to a normal distribution were expressed as the mean ± SD, and comparisons between groups were conducted using the two-independent sample t-test. Data not conforming to a normal distribution were represented by the median (lower quartile, upper quartile), with the Mann–Whitney U test used for between-group comparisons. Categorical data were expressed as numbers and percentages (%), and the Chi-square test was used for comparisons between groups. Blood routine factors influencing the onset of UC were analyzed using binary logistic regression analysis. The dependent variable in this analysis was the diagnosis of UC, with a score of 1 indicating UC and 0 indicating non-UC. A P-value of < 0.05 was considered statistically significant.
A total of 626 patients were included in this study, which were divided into the UC group (313 cases) and the non-UC group (313 cases), and their clinical data were compared and analyzed. According to the baseline clinical data, no statistically significant differences were observed between the two groups in terms of age, sex, and education level. However, statistically significant differences were noted in nationality, area of residence, smoking habits, and alcohol consumption, as shown in Table 1.
| UC (n = 313) | Non-UC (n = 313) | t/χ2 | P value | |
| Age | 42.50 ± 13.94 | 41.84 ± 13.71 | 0.590 | 0.556 |
| Sex | 0.058 | 0.810 | ||
| Male | 176 (56.23) | 173 (55.27) | ||
| Female | 137 (43.77) | 140 (44.73) | ||
| Nationality | 29.344 | < 0.001 | ||
| Han | 159 (50.8) | 225 (71.88) | ||
| Ethnic minorities | 154 (49.2) | 88 (28.12) | ||
| Education level | 2.334 | 0.127 | ||
| Low and middle education | 183 (58.47) | 164 (52.40) | ||
| Higher education | 130 (41.53) | 149 (47.60) | ||
| Region of residence | 8.395 | 0.004 | ||
| Urban | 214 (68.37) | 246 (78.59) | ||
| Rural | 99 (31.63) | 67 (21.41) | ||
| Smoking history | 6.157 | 0.013 | ||
| Smoking | 58 (18.53) | 84 (26.84) | ||
| No smoking | 255 (81.47) | 229 (73.16) | ||
| Drinking history | 10.102 | 0.001 | ||
| Drinking | 43 (13.74) | 74 (23.64) | ||
| No drinking | 270 (86.26) | 239 (76.36) | ||
| History of appendectomy | 37.541 | < 0.001 | ||
| Appendectomized | 43 (13.74) | 3 (0.96) | ||
| Nonappendectomized | 270 (86.26) | 310 (99.04) |
Blood routine test results showed statistically significant differences in WBC count, neutrophil count, lymphocyte count, monocyte count, RBC count, Hb, PLT count, NLR, and PLR between the groups, as shown in Table 2.
| UC (n = 313) | Non-UC (n = 313) | Z | P value | |
| WBC (× 109/L) | 6.96 (5.41-9.08) | 5.81 (4.91-7.19) | -5.980 | < 0.001 |
| Neutrophile granulocyte (× 109/L) | 4.15 (3.04-5.97) | 3.16 (2.51-4.12) | -7.319 | < 0.001 |
| Lymphocyte (× 109/L) | 1.77 (1.37-5.97) | 2.01 (1.66-2.45) | 4.778 | < 0.001 |
| Monocytes (× 109/L) | 0.52 (0.38-0.69) | 0.40 (0.32-0.52) | -7.385 | < 0.001 |
| RBC (× 1012/L) | 4.42 (3.93-4.80) | 4.53 (4.16-4.95) | 3.449 | < 0.001 |
| Hb (g/L) | 128.00 (108.75-143.00) | 138.00 (125.00-152.00) | 6.348 | < 0.001 |
| PLT (× 109/L) | 277.50 (211.75-350.25) | 230.00 (193.00-264.00) | -6.733 | < 0.001 |
| NLR | 2.38 (1.62-3.68) | 1.55 (1.20-2.07) | -9.520 | < 0.001 |
| PLR | 154.00 (120.64-219.67) | 111.91 (91.11-143.25) | -9.251 | < 0.001 |
The variables from the blood routine tests mentioned above were incorporated into a binary logistic regression analysis, and the fit of the regression equation was evaluated (P = 0.651), indicating that the equation fitted well. The results showed that monocyte count and Hb levels were the factors influencing the onset of UC (P < 0.05). Specifically, monocyte count was a risk factor for the development of UC [odds ratio (OR) = 16.039, 95%CI: 4.312–59.653, P < 0.001], whereas Hb level served as a protective factor (OR = 0.975, 95%CI: 0.961–1.005, P < 0.001), as shown in Table 3.
| β | SE | Wald | P value | OR | 95%CI | ||
| Lower limit | Upper limit | ||||||
| WBC | 0.121 | 0.104 | 1.361 | 0.243 | 1.129 | 0.921 | 1.383 |
| Neutrophile Granulocyte | -0.043 | 0.112 | 0.148 | 0.701 | 0.958 | 0.769 | 1.193 |
| Lymphocyte | -0.452 | 0.317 | 2.026 | 0.155 | 0.637 | 0.342 | 1.186 |
| Monocytes | 2.775 | 0.670 | 17.146 | 0.000 | 16.039 | 4.312 | 59.653 |
| RBC | 0.296 | 0.252 | 1.384 | 0.239 | 1.345 | 0.821 | 2.204 |
| Hb | -0.026 | 0.007 | 12.238 | 0.001 | 0.975 | 0.961 | 0.989 |
| PLT | 0.001 | 0.002 | 0.056 | 0.812 | 1.001 | 0.996 | 1.005 |
| NLR | 0.260 | 0.238 | 1.196 | 0.274 | 1.297 | 0.814 | 2.066 |
| PLR | 0.003 | 0.003 | 0.764 | 0.382 | 1.003 | 0.996 | 1.010 |
| Constant | -0.154 | 0.879 | 0.031 | 0.861 | 0.858 | ||
Among 313 patients with UC, 176 (56.23%) were male and 137 (43.77%) were female. There was no significant difference in age of onset, scope of lesions and degree of disease activity between male and female patients, as shown in Table 4.
| Male (n = 176) | Female (n = 137) | t/χ2 | P value | |
| Onset age | 42.46 ± 13.75 | 42.54 ± 14.24 | -0.50 | 0.96 |
| Scope of lesions | 4.90 | 0.09 | ||
| E1 | 17 (9.66) | 25 (18.25) | ||
| E2 | 66 (37.50) | 46 (33.57) | ||
| E3 | 93 (52.84) | 66 (48.18) | ||
| Degree of disease activity | 2.29 | 0.52 | ||
| Clinical remission | 6 (3.41) | 2 (1.46) | ||
| Mild | 29 (16.48) | 29 (21.17) | ||
| Moderate | 112 (63.64) | 82 (59.85) | ||
| Severe | 29 (16.48) | 24 (17.52) |
Patients with UC were divided into the appendectomized group (43 cases, 13.74%) and the non-appendectomized group (270 cases, 86.26%). The average age at onset of UC in the appendectomized group was 49.51 ± 14.16 years, which was higher than the average age of 41.38 ± 13.61 years observed in the non-appendectomized group (P < 0.05). Among men diagnosed with incipient UC, the average age in the appendectomized group was 50.52 ± 13.95 years, and that in the non-appendectomized group was 41.37 ± 13.40 years (P < 0.05). Among women diagnosed with incipient UC, the average age in the appendectomized group was 48.55 ± 14.62 years, and that in the non-appendectomized group was 41.39 ± 13.94 (P < 0.05; Table 5).
| Appendectomized | Non-appendectomized | t value | P value | |
| Onset age | 49.51 ± 14.16 | 41.38 ± 13.61 | -3.621 | <0.001 |
| Onset age of male | 50.52 ± 13.95 | 41.37 ± 13.40 | -2.903 | 0.004 |
| Onset age of female | 48.55 ± 14.62 | 41.39 ± 13.94 | -2.188 | 0.029 |
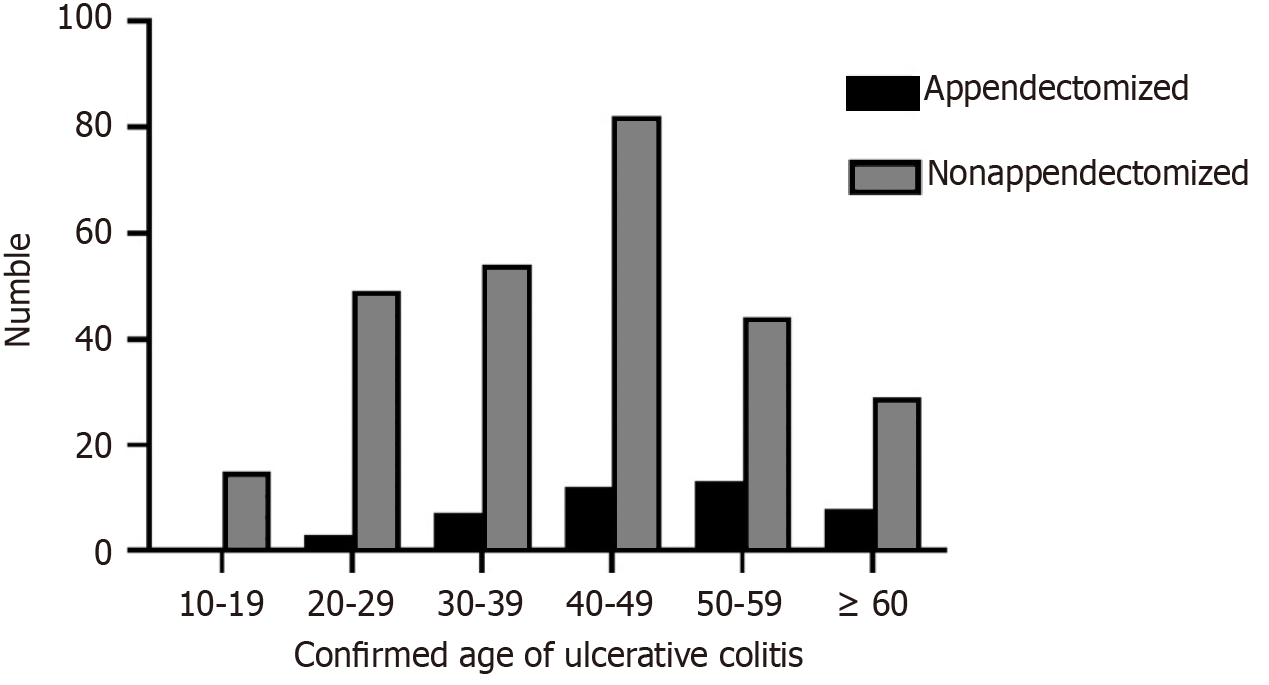
After stratification based on age, it was found that the most frequent age range for the diagnosis of UC in the appendectomized group was 50–59 years (30.23%), whereas in the non-appendectomized group, it was 40–49 years (30.37%), as shown in Figure 1. There was a significant difference in the incidence of onset of UC between the non-appendectomized and appendectomized groups in the age range of 50–59 years (χ2 = 4.837, P = 0.028), as shown in Table 6.
| Onset age | Appendectomized | Non-appendectomized | χ2 | P value |
| 10-19 | 0 (0.00) | 15 (5.56) | 2.509 | 0.113 |
| 20-29 | 3 (6.98) | 49 (18.15) | 3.342 | 0.068 |
| 30-39 | 7 (16.28) | 54 (20.00) | 0.327 | 0.567 |
| 40-49 | 12 (27.91) | 82 (30.37) | 0.107 | 0.743 |
| 50-59 | 13 (30.23) | 44 (16.30) | 4.837 | 0.028 |
| ≥ 60 | 8 (18.60) | 26 (9.63) | 3.086 | 0.079 |
The average interval between appendectomy and the onset of UC was 14.72 ± 13.87 years. Eight patients (18.60%) with UC had undergone appendectomy before the age of 20 years, and all were diagnosed with UC five years later. Among the patients who underwent appendectomy after the age of 20 years, 8 (18.60%) were diagnosed with UC within a year after surgery, 11 (25.58%) were diagnosed with UC two to five years after surgery, and 16 (37.21%) were diagnosed with UC more than five years after surgery. Overall, more than half of the patients (55.81%) were diagnosed with UC more than five years after undergoing appendectomy.
No significant differences were found in sex, education level, smoking habits, and alcohol consumption between the two groups. Compared with the non-appendectomized group, the onset age of UC in the appendectomized group was higher, with a greater proportion of Han patients and patients residing in cities for a long time. In addition, in the appendectomized group, the degree of disease activity of UC was significantly lower; the proportion of patients in clinical remission and mild disease was higher, and that of patients with severe disease was lower. The scope of UC lesions in the appendectomized group was limited, with a higher proportion of E1 and E2, whereas a lower proportion of E3 Lesions, as shown in Table 7.
| Appendectomized (n = 43) | Non-appendectomized (n = 270) | t/χ2 | P value | |
| Age | 49.51 ± 14.16 | 41.38 ± 13.61 | -3.621 | < 0.001 |
| Sex | 1.107 | 0.293 | ||
| Male | 21 (48.84) | 155 (57.41) | ||
| Female | 22 (51.16) | 115 (42.59) | ||
| Nationality | 7.716 | 0.007 | ||
| Han | 30 (69.77) | 129 (47.78) | ||
| Ethnic minorities | 13 (30.23) | 141 (52.22) | ||
| Region of residence | 7.202 | 0.007 | ||
| Urban | 37 (86.05) | 177 (65.56) | ||
| Rural | 6 (13.95) | 93 (34.44) | ||
| Education level | 0.509 | 0.476 | ||
| Low and middle education | 23 (53.49) | 160 (59.26%) | ||
| Higher education | 20 (46.51) | 110 (40.74%) | ||
| Smoking history | 0.167 | 0.682 | ||
| Smoking | 7 (16.28) | 51 (18.89) | ||
| No smoking | 36 (83.72) | 219 (81.11) | ||
| Drinking history | 1.923 | 0.166 | ||
| Drinking | 3 (6.98) | 40 (14.81) | ||
| No drinking | 40 (93.02) | 230 (85.19) | ||
| Degree of disease activity | 48.739 | < 0.001 | ||
| Clinical remission | 4 (9.3) | 4 (1.48) | 9.109 | 0.003 |
| Mild | 22 (51.16) | 36 (13.33) | 35.162 | < 0.001 |
| Moderate | 16 (37.21) | 178 (65.93) | 12.981 | < 0.001 |
| Severe | 1 (2.33) | 52 (19.26) | 7.562 | 0.006 |
| Scope of lesions | 21.631 | < 0.001 | ||
| E1 | 13 (30.23) | 29 (10.74) | 12.130 | < 0.001 |
| E2 | 21 (48.84) | 91 (33.70) | 3.697 | 0.055 |
| E3 | 9 (20.93) | 150 (55.56) | 17.793 | < 0.001 |
UC is a chronic intestinal inflammatory disease characterized by repeated attacks that seriously affect patients’ quality of life. According to previous studies, as an immune organ, the appendix contains abundant immune cells, which are activated during inflammation and can produce immunoglobulins, cytokines, and chemokines, participating in immune response and playing an important role in maintaining intestinal microecological stability and modulating the immune system[12]. Current studies suggest that the pathogenesis of UC is closely related to the interaction between immune factors and intestinal microbes. An appendectomy may affect the course of UC by preventing the recolonization of harmful bacteria and reducing the production of immunoglobulins and cytokines[16].
Among the 313 UC patients, the UC patients in urban areas were more than those in rural areas, which is consistent with the findings of most studies[17]. It may be related to the difference in lifestyle between urban and rural residents. Current research has shown that patients in cities experienced more psychological stress and fatigue[18]. Meanwhile, a western diet with high red meat, fatty foods and refined sugars in city can also closely related to UC.
In this study, compared with the non-appendectomized group, the appendectomized group of patients with newly diagnosed UC showed significantly lower disease activity. There was a higher proportion of patients in clinical remission or with mild disease and a lower proportion of patients with severe UC. In addition, the range of lesions was limited, with a higher proportion of E2 and E1, whereas a lower proportion of E3 lesions, which is consistent with the results of Radford–Smith and other studies conducted in China. The study conducted by Radford–Smith in Australia[19], which involved 307 UC patients, showed that among 21 patients with UC in the appendectomized group, 20 had mild disease and did not require immunosuppressants or colectomy, 3 of these did not receive maintenance treatment, and 17 were treated only with oral 5-aminosalicylic acid; while 71 patients in the non-appendectomized group required continuous immunotherapy. No patient in the appendectomized group underwent colectomy for severe colitis, while 60 (21.4%) patients in the non-appendectomized group underwent colectomy, indicating a negative association between appendectomy and UC and suggesting that the clinical condition of patients who have undergone an appendectomy tends to be milder.
A study conducted on 402 patients with UC in Shanghai found that compared with the non-appendectomized group, the disease severity in the appendectomized group was lower, with a higher proportion of patients with mild disease (45.45% vs 32.23%). In addition, more patients had lesions limited to the rectum (36.36% vs 9.21%), and fewer cases had extensive colon involvement (18.18% vs 48.59%). This study supports the hypothesis that a prior appendectomy can reduce the disease activity of UC and result in a relatively limited range of lesions[20].
Hb was found to be a protective factor against the onset of UC, whereas monocytes were found to be a risk factor. Some studies have reported that peripheral blood monocytes are related to the severity of UC[21,22], exhibiting increased expression in patients with severe disease and decreased expression in those with moderate and mild disease. However, in both instances, monocyte levels are higher compared to the normal population. Some studies have also shown that the capacity for bacterial phagocytosis by monocytes in patients with inflammatory bowel disease (IBD) significantly increases within 2 hours, suggesting that peripheral blood monocytes can be activated[23].
Anemia and fatigue are common in patients with IBD. In a retrospective analysis of 465 patients with IBD, 51.6% of patients had anemia, which was more common in women than in men (P < 0.001). Iron deficiency anemia was the most common, followed by anemia of chronic disease[24]. In addition, in patients with UC, Hb levels decreased as systemic inflammation increased[25]. Reinisch et al[26] suggested that low Hb levels were similar to high levels of inflammatory markers, which might indicate adverse outcomes in patients at an early stage of UC. These findings are consistent with the results of our study.
In this study, a total of eight patients with UC who underwent appendectomy before the age of 20 years were diagnosed with UC five years later, consistent with the findings of Lowenfels and Maisonneuve[27], Frisch et al[28]. Hansen et al[29] screened 2004 patients with UC in Denmark and found that undergoing appendectomy before the onset of UC during adolescence (age < 20 years) can reduce the incidence of UC. Kiasat et al[30] also confirmed that ap
Although an increasing number of studies and experimental evidence show that appendectomy (with or without appendicitis) plays an important role in the occurrence and development of UC, the exact mechanism underlying this relationship remains unclear. Current studies have proved that the intestinal microbiome of patients with UC is dis
In China, very few studies have reported on the relationship between UC and the role of appendectomy in its occurrence and development, indicating a need for more basic studies and long-term clinical follow-up studies to clarify this relationship and its mechanisms. At present, there is no consensus regarding the use of appendectomy in the treatment of UC. The limitation of this study is that our study is a small sample, single center study. Hence, multicenter, large-scale, prospective, randomized controlled studies are still needed to further clarify the indications for appen
In summary, we found that appendectomy may delay the onset of UC, reduce disease severity, and lessen the scope of involvement.
The authors thank the platform support of the First Affiliated Hospital of Xinjiang Medical University.
| 1. | Du L, Ha C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 359] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 2. | He Q, Li JD. [Epidemiological progress of inflammatory bowel disease]. Shiyong Yixue Zazhi. 2019;35:18. [DOI] [Full Text] |
| 3. | Zhong C, Cheng X, Jia B, Xiong P, Lu J, Zhang P, Liu X, Chen Y. Gancao Xiexin decoction combined with mesalazine in the treatment of ulcerative colitis: A protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e23038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Singh N, Bernstein CN. Environmental risk factors for inflammatory bowel disease. United European Gastroenterol J. 2022;10:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 5. | Roberts-Thomson IC, Bryant RV, Costello SP. Uncovering the cause of ulcerative colitis. JGH Open. 2019;3:274-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Sucic L, Galati-Fournier V, Kym U, Pfeifle VA, Gros SJ, Schäfer KH, Holland-Cunz S, Keck S. Increased regulatory T cells in pediatric acute appendicitis. Pediatr Allergy Immunol. 2018;29:104-108. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Lumb G, Protheroe RH. Ulcerative colitis; a pathologic study of 152 surgical specimens. Gastroenterology. 1958;34:381-407. [PubMed] |
| 8. | Rutgeerts P, D'Haens G, Hiele M, Geboes K, Vantrappen G. Appendectomy protects against ulcerative colitis. Gastroenterology. 1994;106:1251-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 149] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Cosnes J, Carbonnel F, Beaugerie L, Blain A, Reijasse D, Gendre JP. Effects of appendicectomy on the course of ulcerative colitis. Gut. 2002;51:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Mayer L, Eisenhardt D. Lack of induction of suppressor T cells by intestinal epithelial cells from patients with inflammatory bowel disease. J Clin Invest. 1990;86:1255-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 129] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Mizoguchi A, Mizoguchi E, Chiba C, Bhan AK. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med. 1996;184:707-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Kooij IA, Sahami S, Meijer SL, Buskens CJ, Te Velde AA. The immunology of the vermiform appendix: a review of the literature. Clin Exp Immunol. 2016;186:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Yazdani R, Azizi G, Abolhassani H, Aghamohammadi A. Selective IgA Deficiency: Epidemiology, Pathogenesis, Clinical Phenotype, Diagnosis, Prognosis and Management. Scand J Immunol. 2017;85:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Wu KC, Liang J, Ran ZH, Qian JM, Yang H. [Chinese consensus on diagnosis and treatment of inflammatory bowel disease]. Zhongguo Shiyong Neike Zazhi. 2018;38:9. [DOI] [Full Text] |
| 15. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2353] [Article Influence: 123.8] [Reference Citation Analysis (2)] |
| 16. | Sahami S, Kooij IA, Meijer SL, Van den Brink GR, Buskens CJ, Te Velde AA. The Link between the Appendix and Ulcerative Colitis: Clinical Relevance and Potential Immunological Mechanisms. Am J Gastroenterol. 2016;111:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Borowitz SM. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front Pediatr. 2022;10:1103713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 18. | Bai A, Guo Y, Shen Y, Xie Y, Lu N. Gender-related and city- and countryside-related differences in patients with ulcerative colitis in a Chinese population. Intern Med. 2008;47:2103-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Radford-Smith GL, Edwards JE, Purdie DM, Pandeya N, Watson M, Martin NG, Green A, Newman B, Florin TH. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn's disease. Gut. 2002;51:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Chen D, Ma J, Luo S, Lu L, Wan X, Ben Q. Effects of Appendectomy on the Onset and Course of Ulcerative Colitis in Chinese Patients. Gastroenterol Res Pract. 2018;2018:2927891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Génot N, Mewton N, Bresson D, Zouaghi O, Francois L, Delwarde B, Kirkorian G, Bonnefoy-Cudraz E. Bioelectrical impedance analysis for heart failure diagnosis in the ED. Am J Emerg Med. 2015;33:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Furukawa S, Ikeda Y, Yagi S, Miyake T, Shiraishi K, Tange K, Hashimoto Y, Mori K, Ninomiya T, Suzuki S, Shibata N, Murakami H, Ohashi K, Hasebe A, Tomida H, Yamamoto Y, Takeshita E, Hiasa Y. Association Between Peripheral Blood Monocyte Count and Mucosal Healing in Japanese Patients With Ulcerative Colitis. Clin Transl Gastroenterol. 2021;12:e00429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Mee AS, Szawatakowski M, Jewell DP. Monocytes in inflammatory bowel disease: phagocytosis and intracellular killing. J Clin Pathol. 1980;33:921-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Bengi G, Keyvan H, Durmaz SB, Akpınar H. Frequency, types, and treatment of anemia in Turkish patients with inflammatory bowel disease. World J Gastroenterol. 2018;24:4186-4196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425-4433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 673] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 26. | Reinisch W, Reinink AR, Higgins PD. Factors associated with poor outcomes in adults with newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Lowenfels AB, Maisonneuve P. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;345:223; author reply 223-223; author reply 224. [PubMed] |
| 28. | Frisch M, Pedersen BV, Andersson RE. Appendicitis, mesenteric lymphadenitis, and subsequent risk of ulcerative colitis: cohort studies in Sweden and Denmark. BMJ. 2009;338:b716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Hansen TS, Jess T, Vind I, Elkjaer M, Nielsen MF, Gamborg M, Munkholm P. Environmental factors in inflammatory bowel disease: a case-control study based on a Danish inception cohort. J Crohns Colitis. 2011;5:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Kiasat A, Ekström LD, Marsk R, Löf-Granström A, Gustafsson UO. Childhood appendicitis and future risk of inflammatory bowel disease - A nationwide cohort study in Sweden 1973-2017. Colorectal Dis. 2022;24:975-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 31. | Fantodji C, Jantchou P, Parent ME, Rousseau MC. Appendectomy and risk for inflammatory bowel disease: effect of age and time post appendectomy - a cohort study. BMJ Open Gastroenterol. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |