Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.85
- This article has been retracted.
- Retraction in: World J Gastrointest Surg. Jan 27, 2025; 17(1): 101330 See also: Errata, Retraction, Duplicate Publication and Comment Policy
Peer-review started: September 14, 2023
First decision: November 17, 2023
Revised: November 24, 2023
Accepted: December 21, 2023
Article in press: December 21, 2023
Published online: January 27, 2024
Processing time: 132 Days and 18.6 Hours
Gastric cancer is one of the most common malignant tumors in the digestive system, ranking sixth in incidence and fourth in mortality worldwide. Since 42.5% of metastatic lymph nodes in gastric cancer belong to nodule type and peripheral type, the application of imaging diagnosis is restricted.
To establish models for predicting the risk of lymph node metastasis in gastric cancer patients using machine learning (ML) algorithms and to evaluate their pre
Data of a total of 369 patients who underwent radical gastrectomy at the Depart
Among the seven ML models, except for SVM, the other ones exhibited higher accuracy and reliability, and the influences of various risk factors on the models are intuitive.
The ML models developed exhibit strong predictive capabilities for lymph node metastasis in gastric cancer, which can aid in personalized clinical diagnosis and treatment.
Core Tip: The purpose of this study was to explore the performance of machine learning based models for the risk assessment of lymph node metastasis in patients with gastric cancer. We used seven different methods to analyze our data. After training, the algorithm with the highest average area under the receiver operating characteristic curve was selected as the optimal algorithm.
- Citation: Lu T, Lu M, Wu D, Ding YY, Liu HN, Li TT, Song DQ. Predictive value of machine learning models for lymph node metastasis in gastric cancer: A two-center study. World J Gastrointest Surg 2024; 16(1): 85-94
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/85.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.85
Gastric cancer is one of the most common malignant tumors in the digestive system, ranking sixth in the world in incidence and fourth in mortality[1]. At present, gastric cancer typically is managed with comprehensive treatment that includes surgery. However, the overall 5-year survival rate remains below 50%[2]. In the Tumor-Node-Metastasis staging system of the American Joint Committee on Cancer, N represents the number of lymph node metastases, which is itself an independent factor in predicting the overall survival rate of gastric cancer patients[3]. However, there are some di
Artificial intelligence refers to the ability of machines to independently replicate typical human intellectual processes[6]. Artificial intelligence has various applications in the medical field, encompassing image processing, computer vision, machine learning (ML), artificial neural networks (ANNs), and convolutional neural networks (CNNs). ML can assist physicians in interpreting clinical data through the computer-aided diagnostic (CAD) system. The CAD can be cate
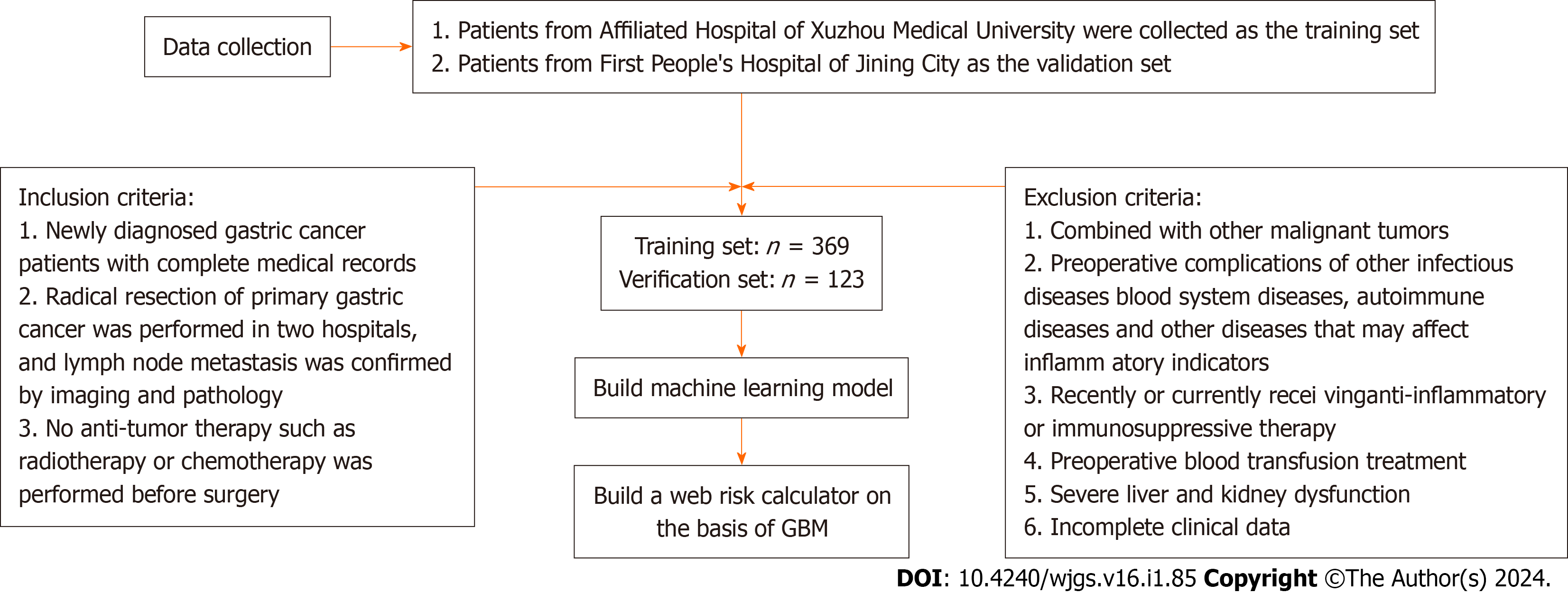
A total of 369 patients who underwent radical gastrectomy at the Department of General Surgery of the Affiliated Hospital of Xuzhou Medical University (Xuzhou, China) from March 2016 to November 2019 were enrolled as the train
Clinical data, such as patient name, age, gender, and other clinicopathological data, including routine blood parameters, tumor location, maximum tumor diameter, depth of invasion, and the presence or absence of lymph node metastasis, were collected from all patients. Blood samples were collected in the morning on an empty stomach on the day after admission to determine neutrophil count, platelet count, monocyte count, and lymphocyte count using the Sysmex XE-2100 Automatic Blood Analyzer. Carcinoembryonic antigen (CEA) level in the blood was also measured. The pan-immune-inflammation value (PIV) and CEA level were utilized to establish clinical prediction models. PIV was calculated as (neutrophil count × platelet count × monocyte count)/lymphocyte count.
Continuous variables are expressed as the mean ± SD, and categorical variables are presented as percentages. LR was employed to identify the independent risk factors associated with lymph node metastasis in gastric cancer patients. This analysis allowed for the calculation of odds ratios (ORs) and their corresponding 95% confidence intervals. An OR greater than 1 indicated that the variable was a positive risk factor affecting the outcome, while an OR less than 1 suggested that the variable was a negative risk factor influencing the outcome. Statistical significance was defined as a P value of less than 0.05. The statistical analyses and modeling procedures were carried out using SPSS 20.0 software (IBM, Armonk, NY, United States) and R-Studio 25.0 software (R Foundation for Statistical Computing, Vienna, Austria). Several packages were utilized to train models and draw relevant graphs, with the caret package applied for training and vali
The training dataset was combined with the validation dataset, and seven ML algorithms were employed to establish prediction models. LR is a classification algorithm designed to establish a relationship between a feature and the proba
Performance evaluation of the models involved various metrics, including accuracy, recall, and other indicators. The primary indicator for predicting binary classification results was the area under the receiver operating characteristic curve (AUC). This metric varies from 0 to 1, with higher values signifying a superior performance. Additionally, for models with two outcomes, the area under the accuracy-recall curve was utilized, illustrating the trade-off between true accuracy and positive predictive value, and the F1 score, defined as the harmonic mean of recall and accuracy. The mo
The comparison of clinical data between the two groups is presented in Table 1. Gender, age, tumor location, and surgical method exhibited no significant differences between the two groups (P > 0.05). In the training dataset, the proportion of patients with total gastrectomy, neurovascular invasion, and maximum tumor diameter > 5 cm was significantly higher in patients with lymph node metastasis than in those without (P < 0.05). In the verification dataset, the number of patients who were aged > 60 years old and had neurovascular invasion and maximum tumor diameter > 5 cm was significantly greater in patients with lymph node metastasis than in those without (P < 0.05).
| Clinical data | Training set | P value | Validation set | P value | ||||
| No lymph node metastasis (n = 141) | Lymph node metastasis (n = 228) | No lymph node metastasis (n = 51) | Lymph node metastasis (n = 72) | |||||
| Gender | 1.017 | 0.313 | 1.126 | 0.289 | ||||
| Male | 99 (70.2) | 171 (75.0) | 33 (64.7) | 53 (73.6) | ||||
| Female | 42 (29.8) | 57 (25.0) | 0 | 18 (35.3) | 19 (26.4) | |||
| Age (yr) | 0.015 | 0.901 | 4.729 | 0.030 | ||||
| ≤ 60 | 64 (45.4) | 105 (46.1) | 27 (52.9) | 24 (33.3) | ||||
| > 60 | 77 (54.6) | 123 (53.9) | 24 (47.1) | 48 (66.7) | ||||
| Mode of operation | 7.816 | 0.005 | 3.578 | 0.059 | ||||
| Partial gastrectomy | 113 (80.1) | 152 (66.7) | 43 (84.3) | 50 (69.4) | ||||
| Total gastrectomy | 28 (19.9) | 76 (33.3) | 8 (15.7) | 22 (30.6) | ||||
| Tumor invasion depth | -11.022 | < 0.001 | -7.114 | < 0.001 | ||||
| T1 | 61 (43.3) | 13 (5.7) | 30 (58.8) | 4 (5.6) | ||||
| T2 | 42 (29.8) | 22 (9.6) | 13 (25.5) | 13 (18.1) | ||||
| T3 | 21 (14.9) | 64 (28.1) | 6 (11.8) | 27 (37.5) | ||||
| T4 | 17 (12.1) | 129 (56.6) | 2 (3.9) | 28 (38.9) | ||||
| Tumor site | 0.716 | 0.699 | 0.392 | 0.822 | ||||
| Gastric body | 24 (17.0) | 32 (14.0) | 18 (35.3) | 22 (30.6) | ||||
| Gastric antrum | 73 (51.8) | 126 (55.3) | 26 (51.0) | 38 (52.8) | ||||
| Gastric cardia | 44 (31.2) | 70 (30.7) | 7 (13.7) | 12 (16.7) | ||||
| Nerve or vascular invasion | 128.649 | < 0.001 | 54.772 | < 0.001 | ||||
| No | 108 (76.6) | 39 (17.1) | 42 (82.4) | 11 (15.3) | ||||
| Yes | 33 (23.4) | 189 (82.9) | 9 (17.6) | 61 (84.7) | ||||
| Maximum tumor diameter | 38.634 | < 0.001 | 8.323 | 0.004 | ||||
| ≤ 5 cm | 122 (86.5) | 126 (55.3) | 46 (90.2) | 49 (68.1) | ||||
| > 5 cm | 19 (13.5) | 102 (44.7) | 5 (9.8) | 23 (31.9) | ||||
| PIV | 132.00 (80.73, 226.80) | 190.72 (106.49, 311.44) | -3.606 | < 0.001 | 149.43 (91.73, 217.49) | 173.59 (102.20, 274.73) | -1.586 | 0.113 |
| CEA | 2.47 (1.53, 3.58) | 2.90 (1.82, 6.87) | -3.189 | 0.001 | 2.65 (1.47, 3.95) | 4.91 (1.97, 9.02) | -2.331 | 0.020 |
The results of Mann-Whitney U test revealed that there were no statistically significant differences in the depth of infiltration, PIV, or CEA level between the two groups (P > 0.05). It was found that the depth of infiltration and CEA level in patients with lymph node metastasis were significantly higher than those in patients without (P < 0.05). In the training dataset, the infiltration depth, PIV, and CEA level in patients with lymph node metastasis were significantly greater than those in patients without (P < 0.05).
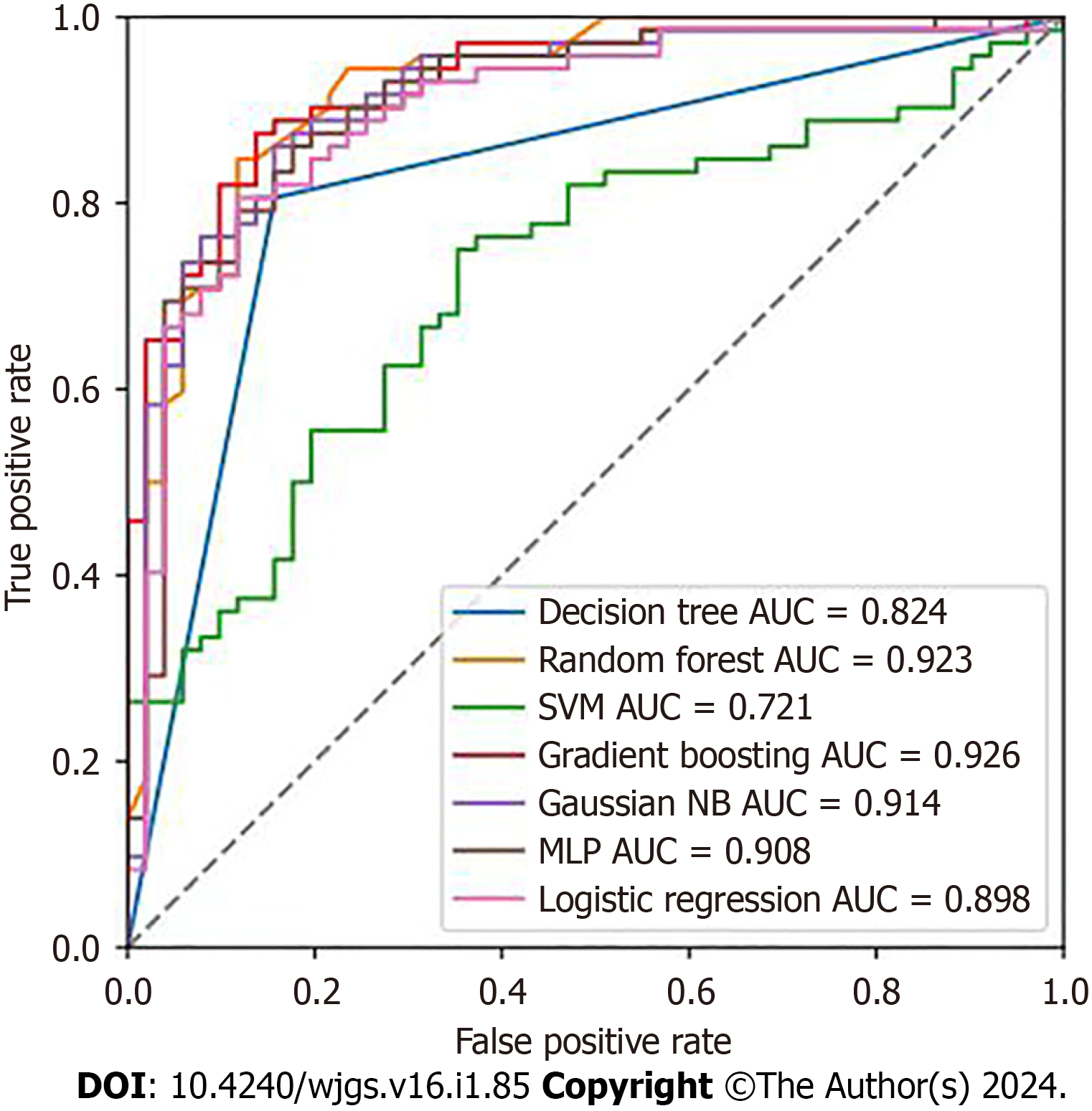
In order to compare the predictive performance of the seven ML-based models, this study employed ten-fold cross-validation and utilized the AUC value, validated on the test dataset, as the primary metric for assessing their perfor
| Model | AUC | Accuracy | Kappa | Sensitivity (recall rates) | Specificity |
| DT | 0.824 | 0.821 | 0.638 | 0.806 | 0.843 |
| RF | 0.923 | 0.854 | 0.702 | 0.847 | 0.882 |
| SVM | 0.721 | 0.585 | 0.000 | 0.750 | 0.547 |
| GBM | 0.927 | 0.870 | 0.734 | 0.875 | 0.863 |
| NB | 0.914 | 0.821 | 0.640 | 0.861 | 0.843 |
| MLP | 0.907 | 0.837 | 0.665 | 0.882 | 0.824 |
| LR | 0.898 | 0.821 | 0.636 | 0.806 | 0.882 |
As a result of the limited early detection of gastric cancer, over 50% of patients are diagnosed at advanced stages or with metastasis. At present, surgery is the main method for the treatment of gastric cancer, and lymph node metastasis is regarded as the main factor affecting the stage, grade, and survival rate of gastric cancer[26,27]. Therefore, early predi
ML represents an evolving frontier in the field of medicine, drawing substantial resources to connect computer science and statistical analysis with medical challenges. ML has the capacity to effectively handle extensive, diverse, and intricate medical data. Consequently, the implementation of ML techniques in medicine is widely regarded as the cornerstone of future endeavors in biomedical research, personalized medicine, and computer-aided diagnosis[28,29]. Specifically, the operational framework of ML involves development of algorithms to execute numerous tasks, refining the algorithms iteratively to optimize performance. Ultimately, this process yields a model that establishes connections between multiple variables and target outcomes. In the present study, clinical data were collected, and ML algorithms were employed to develop a model for assessing the risk of lymph node metastasis in gastric cancer. By leveraging multiple variables, clini
In this study, in addition to some clinicopathological data, hematological indicators, namely, immunoinflammatory factors (PIV and CEA), were utilized to develop the prediction models. PIV is a novel blood-based biomarker that inte
Using ML, seven models were established for comparative analysis, utilizing the AUC as the benchmark for assess
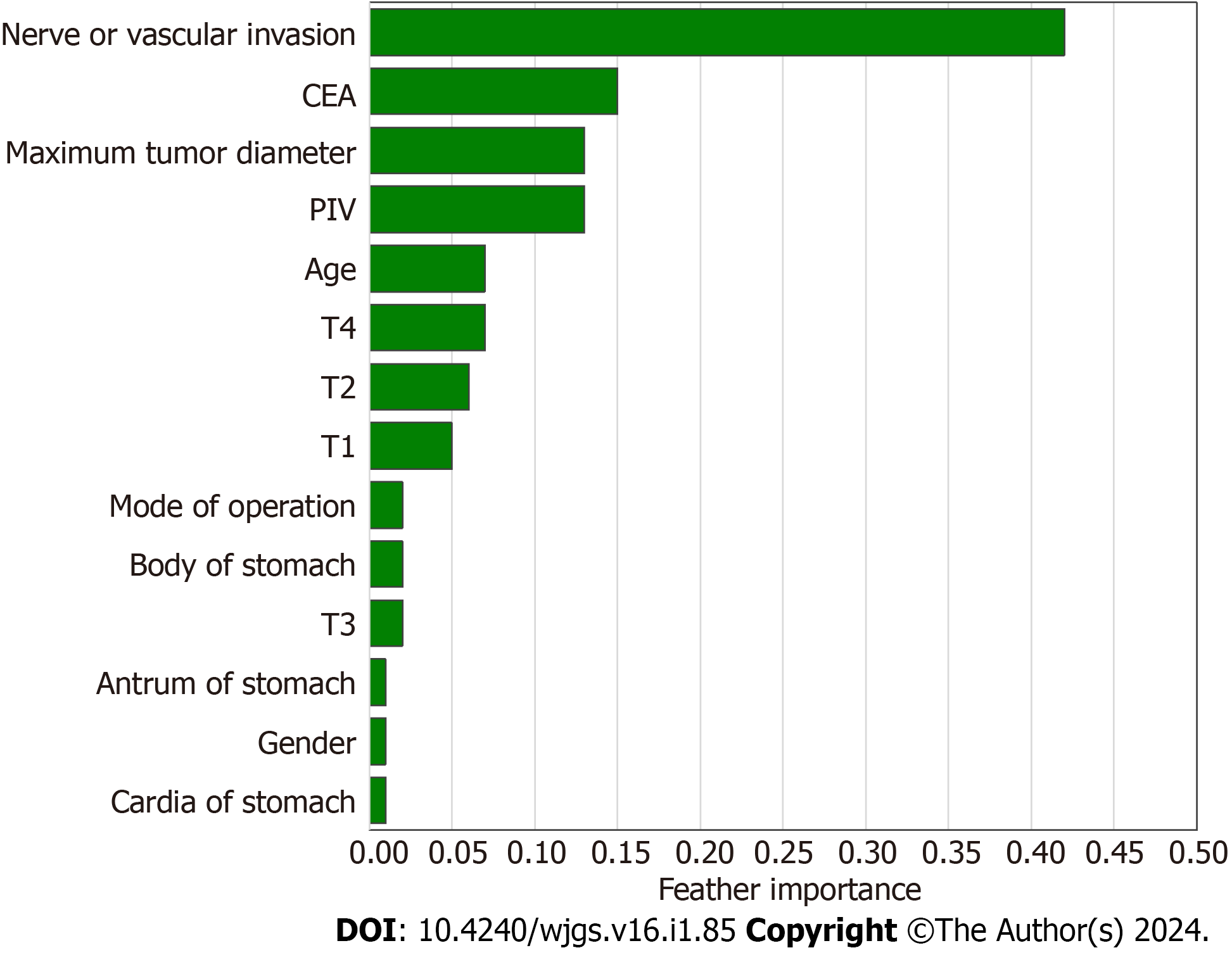
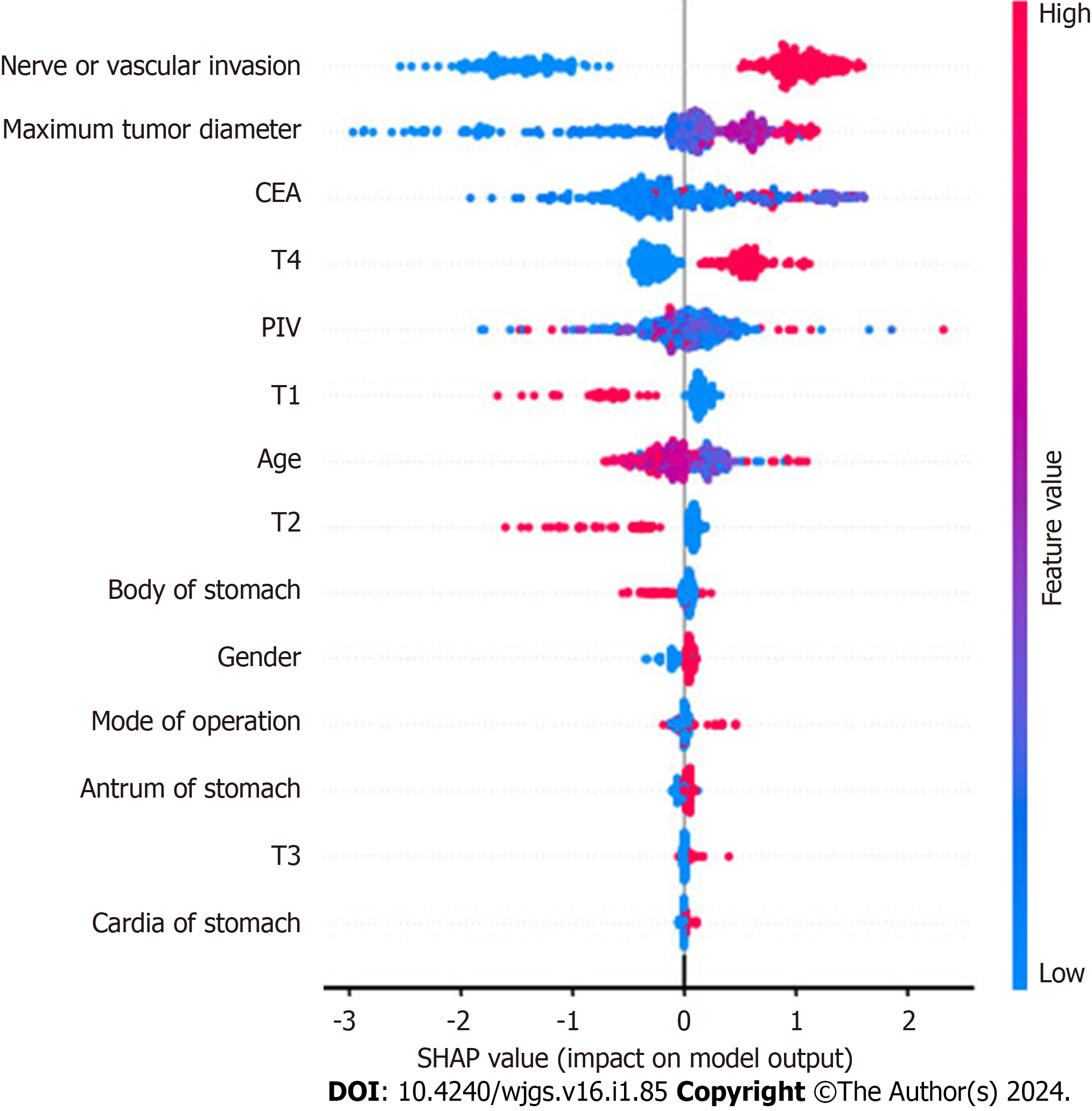
Using the best-performing GBM model, feature importance assessment was conducted. This analysis highlighted the significance of specific indicators within the model, providing new insights into the model’s structure. To understand the relationship between the direction of lymph node metastasis in gastric cancer and the importance of its main predictors, a SHAP summary plot was drawn. This method was utilized to explain the predictions of ML models. SHAP-Beeswarm diagrams, a common visualization tool in SHAP method, display the effect of each feature on the predicted results. The horizontal axis of the plot represents the SHAP value, indicating the contribution of each feature to the predicted result, while the vertical axis represents the feature name. Each data point in the diagram represents a sample, with its hori
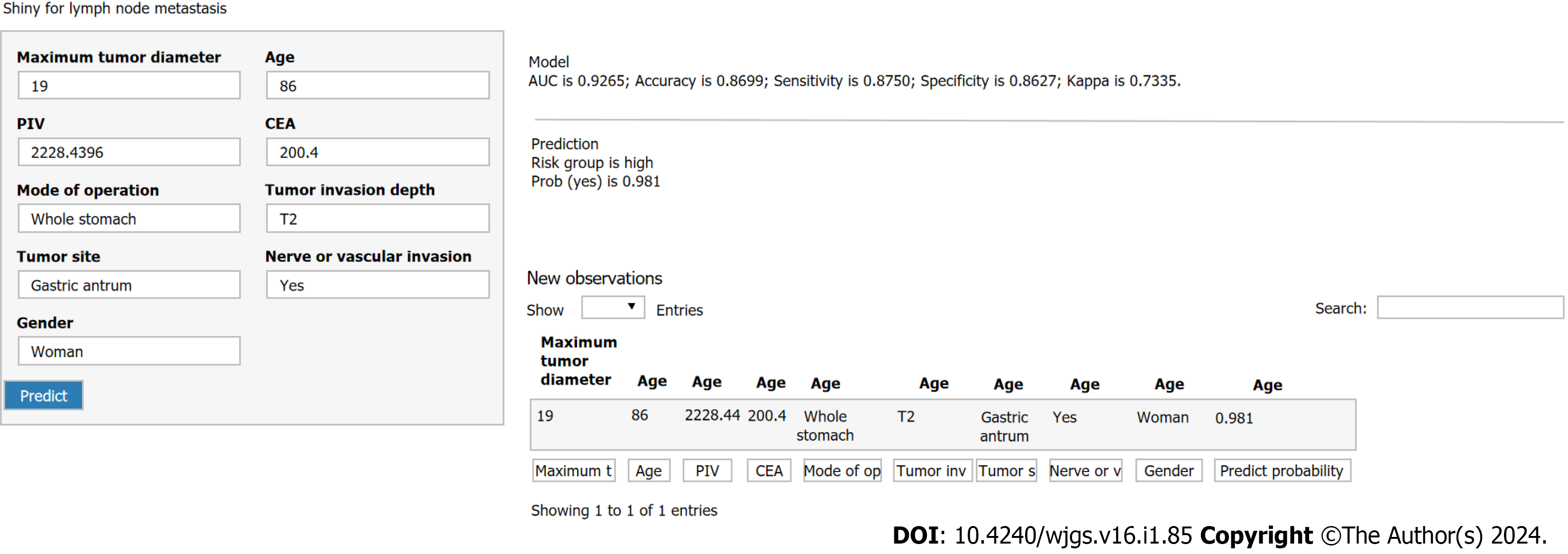
According to the optimal GBM model, a web-based risk calculator was developed. By inputting patients’ clinical characteristics, it can directly predict the probability of lymph node metastasis in patients with gastric cancer. This tool is user-friendly and straightforward, making it accessible for healthcare practitioners. It serves as a valuable resource in diagnosis and treatment, providing significant support for clinicians.
In summary, based on the clinicopathological data of 492 gastric cancer patients in two centers, ML algorithms were utilized to establish clinical models and conduct cross-validation, and AUC values were finally compared to draw conclusions. In addition to SVM, other ML models have exhibited promising accuracy and reliability, as well as better predictive value for gastric cancer lymph node metastasis. Among them, GBM outperformed the others, with the highest predictive value and accuracy. This study demonstrated that ML could reveal the potential of clinical data to reflect di
Gastric cancer is one of the most common malignant tumors of the digestive system, ranking sixth in incidence and fourth in mortality worldwide. Machine learning (ML) represents an evolving frontier in the field of medicine, drawing sub
Using machine learning-based models to predict lymph node metastasis of gastric cancer is helpful to individualized diagnosis and treatment of gastric cancer patients.
Based on the clinicopathological data of 492 gastric cancer patients in two centers, we used ML algorithms to establish clinical models and conduct cross-validation, and finally compared the area under the receiver operating characteristic curve to draw conclusions. In addition to support vector machine, other ML models have good accuracy and reliability, and have better predictive value for gastric cancer lymph node metastasis. Among them, gradient boosting machine (GBM) has the best performance and the highest predictive value and accuracy. Through this study, ML can dig out the ability of clinical data to reflect disease, which can help clinicians evaluate patients' conditions and make better treatment decisions.
Seven machine algorithm models were built with data from two centers, and then their performance was evaluated. Based on GBM model, a web-based online estimator and Shapley Additive Explanations summary plot were established.
ML can tap into the ability of clinical data to reflect disease, which can help clinicians assess patients' conditions and make better treatment decisions.
ML algorithms have been used to establish an optimal prediction model for lymph node metastasis in gastric cancer, which is helpful for clinical risk stratification and individualized diagnosis and treatment of gastric cancer patients.
In the future, multi-center data are needed to verify the external applicability of our model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng C, China; Lee KS, South Korea S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Xu ZH
| 1. | Salvatori S, Marafini I, Laudisi F, Monteleone G, Stolfi C. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 119] [Reference Citation Analysis (1)] |
| 2. | National Health Commission Of The People's Republic Of China. Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version). Chin J Cancer Res. 2019;31:707-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Jin C, Jiang Y, Yu H, Wang W, Li B, Chen C, Yuan Q, Hu Y, Xu Y, Zhou Z, Li G, Li R. Deep learning analysis of the primary tumour and the prediction of lymph node metastases in gastric cancer. Br J Surg. 2021;108:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Wang H, Gong H, Tang A, Cui Y. Neutrophil/lymphocyte ratio predicts lymph node metastasis in patients with gastric cancer. Am J Transl Res. 2023;15:1412-1420. [PubMed] |
| 5. | Li C, Tian XJ, Qu GT, Teng YX, Li ZF, Nie XY, Liu DJ, Liu T, Li WD. Clinical value of regional lymph node sorting in gastric cancer. World J Gastrointest Oncol. 2022;14:2393-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2829] [Article Influence: 471.5] [Reference Citation Analysis (0)] |
| 7. | Li Y, Xie F, Xiong Q, Lei H, Feng P. Machine learning for lymph node metastasis prediction of in patients with gastric cancer: A systematic review and meta-analysis. Front Oncol. 2022;12:946038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Bhinder B, Gilvary C, Madhukar NS, Elemento O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021;11:900-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 319] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 9. | Mainali G. Artificial Intelligence in Medical Science: Perspective from a Medical Student. JNMA J Nepal Med Assoc. 2020;58:709-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Seifert R, Weber M, Kocakavuk E, Rischpler C, Kersting D. Artificial Intelligence and Machine Learning in Nuclear Medicine: Future Perspectives. Semin Nucl Med. 2021;51:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Luo R, Gao J, Gan W, Xie WB. Clinical-radiomics nomogram for predicting esophagogastric variceal bleeding risk noninvasively in patients with cirrhosis. World J Gastroenterol. 2023;29:1076-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (5)] |
| 12. | Ma Y, Lu Q, Yuan F, Chen H. Comparison of the effectiveness of different machine learning algorithms in predicting new fractures after PKP for osteoporotic vertebral compression fractures. J Orthop Surg Res. 2023;18:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 2303] [Article Influence: 230.3] [Reference Citation Analysis (0)] |
| 14. | Zhou CM, Wang Y, Yang JJ, Zhu Y. Predicting postoperative gastric cancer prognosis based on inflammatory factors and machine learning technology. BMC Med Inform Decis Mak. 2023;23:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Song X, Liu X, Liu F, Wang C. Comparison of machine learning and logistic regression models in predicting acute kidney injury: A systematic review and meta-analysis. Int J Med Inform. 2021;151:104484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 16. | Koga S, Zhou X, Dickson DW. Machine learning-based decision tree classifier for the diagnosis of progressive supranuclear palsy and corticobasal degeneration. Neuropathol Appl Neurobiol. 2021;47:931-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Collin FD, Durif G, Raynal L, Lombaert E, Gautier M, Vitalis R, Marin JM, Estoup A. Extending approximate Bayesian computation with supervised machine learning to infer demographic history from genetic polymorphisms using DIYABC Random Forest. Mol Ecol Resour. 2021;21:2598-2613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl Vis Sci Technol. 2020;9:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 257] [Reference Citation Analysis (2)] |
| 19. | Citko W, Sienko W. Inpainted Image Reconstruction Using an Extended Hopfield Neural Network Based Machine Learning System. Sensors (Basel). 2022;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Dinh A, Miertschin S, Young A, Mohanty SD. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inform Decis Mak. 2019;19:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 21. | Wu Y, Fang Y. Stroke Prediction with Machine Learning Methods among Older Chinese. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Cha GW, Moon HJ, Kim YC. Comparison of Random Forest and Gradient Boosting Machine Models for Predicting Demolition Waste Based on Small Datasets and Categorical Variables. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Senders JT, Staples P, Mehrtash A, Cote DJ, Taphoorn MJB, Reardon DA, Gormley WB, Smith TR, Broekman ML, Arnaout O. An Online Calculator for the Prediction of Survival in Glioblastoma Patients Using Classical Statistics and Machine Learning. Neurosurgery. 2020;86:E184-E192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Chang CH, Lin CH, Lane HY. Machine Learning and Novel Biomarkers for the Diagnosis of Alzheimer's Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 25. | Peiffer-Smadja N, Rawson TM, Ahmad R, Buchard A, Georgiou P, Lescure FX, Birgand G, Holmes AH. Machine learning for clinical decision support in infectious diseases: a narrative review of current applications. Clin Microbiol Infect. 2020;26:584-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 258] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 26. | Ma D, Zhang Y, Shao X, Wu C, Wu J. PET/CT for Predicting Occult Lymph Node Metastasis in Gastric Cancer. Curr Oncol. 2022;29:6523-6539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 27. | Li X, Zhou H, Zhao X, Peng H, Luo S, Feng J, Heng J, Liu H, Ge J. Establishment and Validation for Predicting the Lymph Node Metastasis in Early Gastric Adenocarcinoma. J Healthc Eng. 2022;2022:8399822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Obermeyer Z, Emanuel EJ. Predicting the Future - Big Data, Machine Learning, and Clinical Medicine. N Engl J Med. 2016;375:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1627] [Article Influence: 180.8] [Reference Citation Analysis (0)] |
| 29. | Bayliss L, Jones LD. The role of artificial intelligence and machine learning in predicting orthopaedic outcomes. Bone Joint J. 2019;101-B:1476-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | DeVries Z, Hoda M, Rivers CS, Maher A, Wai E, Moravek D, Stratton A, Kingwell S, Fallah N, Paquet J, Phan P; RHSCIR Network. Development of an unsupervised machine learning algorithm for the prognostication of walking ability in spinal cord injury patients. Spine J. 2020;20:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | Bien N, Rajpurkar P, Ball RL, Irvin J, Park A, Jones E, Bereket M, Patel BN, Yeom KW, Shpanskaya K, Halabi S, Zucker E, Fanton G, Amanatullah DF, Beaulieu CF, Riley GM, Stewart RJ, Blankenberg FG, Larson DB, Jones RH, Langlotz CP, Ng AY, Lungren MP. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: Development and retrospective validation of MRNet. PLoS Med. 2018;15:e1002699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 32. | Craik A, He Y, Contreras-Vidal JL. Deep learning for electroencephalogram (EEG) classification tasks: a review. J Neural Eng. 2019;16:031001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 536] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 33. | MacEachern SJ, Forkert ND. Machine learning for precision medicine. Genome. 2021;64:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (79)] |
| 34. | Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284:603-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 497] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 35. | Seligman B, Tuljapurkar S, Rehkopf D. Machine learning approaches to the social determinants of health in the health and retirement study. SSM Popul Health. 2018;4:95-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 267] [Article Influence: 33.4] [Reference Citation Analysis (0)] |