Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.2012
Peer-review started: June 30, 2023
First decision: July 18, 2023
Revised: July 24, 2023
Accepted: August 4, 2023
Article in press: August 4, 2023
Published online: September 27, 2023
Processing time: 84 Days and 3.8 Hours
Computed tomography (CT) technology has been gradually used in the differentiation of small mesenchymal tumors of the stomach and intestines from smooth muscle tumours.
To explore the value of enhanced CT in the differentiation of small mesenchymal tumors of the stomach and intestines from smooth muscle tumours.
Clinical data of patients with gastric mesenchymal or gastric smooth muscle tu
CEA levels varied among the three groups in the following order: The gastric mesenchymal tumour group > the control group > the gastric smooth muscle tumour group. CA19-9 levels varied among the three groups in the following order: The gastric mesenchymal group > the gastric smooth muscle group > the control group, the difference was statistically significant (P < 0.05). ROC analysis showed that the area under the curve of CEA and CA19-9 was 0. 879 and 0. 782, respectively.
Enhanced CT has shown value in differentiating small mesenchymal tumors of the stomach and intestines from smooth muscle tumors.
Core Tip: Endoscopic ultrasound can accurately localise the lesion characteristics, and there are significant differences in the echogenic characteristics of intragastric mesenchymal tumours and smooth muscle tumours. In view of the fact that early metastases can occur in gastrointestinal mesenchymal tumours of less than 2 cm in diameter, endoscopic resection is recommended for the definitive diagnosis and simultaneous treatment or closer follow-up of intrinsic mesenchymal tumours with a clear echogenic border and less than 2 cm in diameter.
- Citation: Nie WJ, Jing Z, Hua M. Value of enhanced computed tomography in differentiating small mesenchymal tumours of the gastrointestinal from smooth muscle tumours. World J Gastrointest Surg 2023; 15(9): 2012-2020
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/2012.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.2012
Gastric cancer is one of the most common malignancies and is among the top three leading causes of cancer-related deaths worldwide[1-11]. According to the Japanese Classification Criteria for Gastric Cancer, early gastric cancer is defined as a lesion in which tumour infiltration is limited to the mucosa or submucosa without consideration of lymph node metastasis (LNM)[12]. In recent years, with the development of endoscopic techniques, endoscopic submucosal dissection (ESD) has been widely used for the treatment of early gastric cancer without LNM, and the indications for ESD in early gastric cancer published by the Japan Gastric Cancer Association classify gastric cancer into differentiated and undifferentiated types[13]. Gastrointestinal mesenchymal tumours (GIMTs), as a mesenchymal-derived tumour with specific histological features, are mainly located in the gastrointestinal tract and abdominal cavity and have a certain chance of malignant transformation and are therefore often diagnosed and treated differently from gastric smooth muscle tumours in clinical practice[14-20].
Although previous clinical reports have shown that GIMTs are rare[21], recent epidemiological studies have shown that 10%-30% of patients with GIMTs have no obvious clinical symptoms, but 15%-50% of patients may have metastases to the liver and abdominal cavity once detected, missing the best time for treatment. Currently, the clinical diagnosis of GIMTs mainly relies on imaging and pathology; however, imaging methods such as ultrasound endoscopy and computed tomography (CT) are influenced by the operator’s experience and image quality, and cannot accurately determine the nature of the lesion. Besides, the pathological examination requires endoscopy or surgery to obtain the pathological tissue, which is invasive and painful for patients, and pathological examination is not real-time[22-27]. As a convenient and common clinical test, CT has been widely used in the diagnosis, efficacy and prognosis of clinical tumors, and can be used for the identification of GIMTs[28]. Herein, we retrospectively analysed the clinical data of patients (volunteers) who received treatment or health check-ups in our hospital in recent years and investigated the value of CT in the differential diagnosis of patients with gastric mesenchymal tumours and gastric smooth muscle tumours.
The clinical data of patients with gastric mesenchymal or gastric smooth muscle tumours who were treated in our hospital from May 2018 to April 2023 were retrospectively analyzed. Patients were divided into the gastric mesenchymal tumor group and the gastric smooth muscle tumor group respectively (n = 50 cases per group). Clinical data of 50 healthy volunteers who underwent physical examination in the same hospital during the same period were selected and included in the control group. The gastric mesenchymal tumor group included 24 males and 26 females, aged 38 to 72 years (mean age: 56.73 ± 7.46 years) and with a body mass index (BMI) of 18-24 kg/m2 (mean BMI: 21.76 ± 2.21 kg/m2). The gastric smooth muscle tumor group included 22 males and 28 females, aged 36 to 71 years (mean age: 57.11 ± 7.18 years) and with a BMI of 18-24 kg/m2 (mean BMI: 21.89 ± 2.14 kg/m2). The control group included 21 males and 29 females, aged 38-70 years (mean age: 55.82 ± 7.39 years) and with a BMI of 18-24 kg/m2 (mean BMI: 21.76 ± 2.21 kg/m2). The differences between the three groups were not statistically significant (P > 0. 05) and were comparable. Confidentiality of all patient information was maintained in this study.
Inclusion criteria including: (1) Patients with gastric mesenchymal tumour or gastric smooth muscle tumour, all con
Exclusion criteria including: (1) Organic heart, liver, or kidney dysfunction; (2) Patients with combined cancer of other tissues or a history of radiotherapy; (3) Unable to participate in this study due to psychiatric illness or other reasons; (4) Combined coagulation disorders or autoimmune diseases; and (5) A history of gastrectomy.
Approximately 5 mL of fasting venous blood was collected from all patients (or volunteers) during the preoperative examination, centrifuged and stored at -80 °C, and the serum levels of carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), CA-125 and cytokeratin 19 fragment antigen 21-1 (CYFRA21-1) were mea
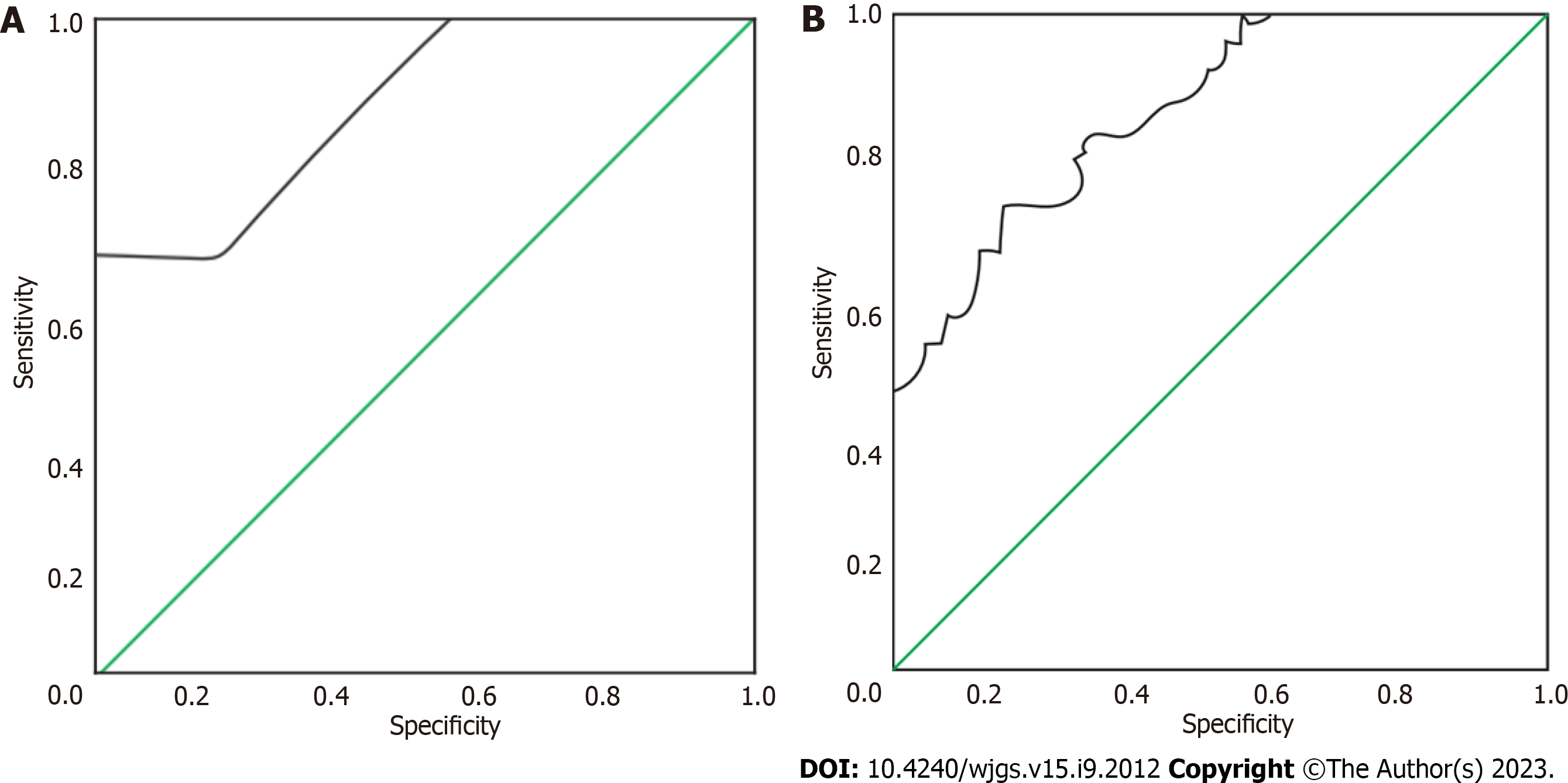
Serum levels of CEA, AFP, CA19-9, CA-125 and CYFRA21-1 levels were compared among the three groups. The value of CEA and CA19-9 in identifying gastric mesenchymal tumours was analysed using the receiver operating characteristic (ROC) curve. The ROC curves of CEA and CA19-9 in identifying gastric mesenchymal tumours were plotted separately based on the pathological results of the patients. The area of the lower curve for each measure was calculated, and the area of the lower curve > 0.5 indicated that the measure had diagnostic efficacy, and the closer it was to 1, the higher its diagnostic efficacy.
All data were processed using SPSS 22. 0 statistical software and were expressed as mean ± standard deviation (mean ± SD) or percentages (%). The χ2 test was used to analyze categorical variables. One-way ANOVA was used to compare multiple groups. The ROC curve was used to analyze the value of CEA and CA19-9 in the diagnosis of gastric mesen
Both intragastric mesenchymal tumours and smooth muscle tumours were found in the fundus and body of the stomach, with no statistically significant difference in the distribution of lesions (P = 0.32). The diameter of mesenchymal tumours was larger than that of smooth muscle tumours, and the difference was statistically significant (P < 0.05). Both mesenchymal and smooth muscle tumours were smooth, erosive, or ulcerated in surface morphology, with no statistically significant difference (P = 0.61). The intrinsic muscular layer and the mucosal muscular layer were the most common sites of origin for both mesenchymal tumours and smooth muscle tumours. The difference was not statistically significant (P = 1.0). At endoscopic ultrasound (EUS), mesenchymal tumours appeared as hypoechoic lesions and smooth muscle tumours appeared as hypoechoic and isoechoic lesions. In terms of echogenicity, the echogenic non-uniformity of mesenchymal tumours was more pronounced than that of smooth muscle tumours (P < 0.05) see Table 1.
| Clinical parameters | Intragastric mesenchymal tumour | Smooth muscle tumour |
| Male | 24 (48.0) | 22 (44.0) |
| Female | 26 (52.0) | 28 (56.0) |
| Age, yr | 50.5 ± 14.0a | 57.9 ± 9.5 |
| Location, % | ||
| Cardia | 0 | 1 (2.0) |
| Gastric base | 26 (52.0) | 12 (24.0) |
| Gastric body | 18 (36.0) | 11 (22.0) |
| Gastric sinus | 9 (18.0) | 2 (4.0) |
| Diameter, mm | 16.2 ± 9.9a | 9.7 ± 5.0 |
| Level of origin, % | ||
| Mucosal muscle layer | 2 (3.8) | 1 (2.0) |
| Inherent muscle layer | 48 (96.0) | 25 (50.0) |
| Indicated ulcers, % | ||
| Yes | 6 (12.0) | 2 (4.0) |
| None | 47 (94.0) | 24 (48.0) |
| Echo characteristics, % | ||
| Low echo | 50 (100) | 47 (94.0) |
| Waiting for an echo | 0 | 1 (2.0) |
| High echo | 0 | |
| Echo uniformity, % | ||
| Uniformity | 25 (49.1)a | 50 (100) |
| Non-homogeneous | 27 (50.0)a | 0 |
CEA levels varied among the three groups in the following order: The gastric mesenchymal tumour group > the control group > the gastric smooth muscle tumour group. CA19-9 levels varied among the three groups in the following order: The gastric mesenchymal group > the gastric smooth muscle group > the control group, the differences were statistically significant (P < 0. 05) see Table 2.
| Group | n | CEA (ng/mL) | CA19-9 (ng/mL) | CA-125 (kU/L) | AFP (ng/mL) | CYFRA21-1 (U/mL) |
| Gastric mesenchymal tumour group | 50 | 1.53 ± 0.24 | 9.32 ± 2.18 | 44.34 ± 10.67 | 5.46 ± 1.18 | 8.17 ± 1.57 |
| Gastric smooth muscle tumour group | 50 | 0.67 ± 0.15a | 8.37 ± 1.81a | 41.56 ± 8.74 | 5.03 ± 0.86 | 7.44 ± 1.69 |
| Control group | 50 | 1.04 ± 0.18a,b | 6.45 ± 1.39a,b | 39.67 ± 7.98 | 5.29 ± 0.74 | 7.67 ± 1.88 |
| F value | 198.507 | 25.753 | 2.608 | 2.100 | 1.888 | |
| P value | 0.000 | 0.000 | 0.078 | 0.127 | 0.156 |
The area under the curve (AUC) for the identification of gastric mesenchymal tumours by CEA and CA19-9 was 0. 879 and 0. 782, respectively (Table 3). The ROC curves for the identification of gastric mesenchymal tumours by CEA and CA19-9 are shown in Figure 1.
| Indicators | Area under the curve | Standard error | P value | 95%CI | Optimal cut-off value | Sensitivity | Specificity |
| CEA | 0.879 | 0.036 | 0.000 | 0.808-0.950 | 1.145 | 0.850 | 0.675 |
| CA19-9 | 0.782 | 0.050 | 0.000 | 0.684-0.880 | 8.880 | 0.600 | 0.800 |
The treatment methods for GIMTs have been rapidly changing with the development of medical treatment technology in recent years, and there are various methods commonly used for differential diagnosis in clinical practice[29-35]. However, the sensitivity of single tumour markers is low, and there is a certain degree of underdiagnosis; thus, combined detection of tumour markers is necessary for the diagnosis of GIMTs[36]. The present study analysed the expression of CEA, AFP, CA19-9, CA-125 and CYFRA21-1 in patients with gastric mesenchymal and smooth muscle tumours to provide a reference for clinical diagnosis. The results showed that CEA levels varied among the three groups in the following order: The gastric mesenchymal tumour group > control group > gastric smooth muscle tumour group, and CA19-9 levels varied in the following order: The gastric mesenchymal tumour group > gastric smooth muscle group > control group, suggesting that CEA and CA19-9 were differentially expressed in patients (or volunteers) with different gastric lesions. Tumour markers are chemical substances that reflect the presence of tumours and are synthesised and released by tumour cells during tumourigenesis and proliferation or are important for the host’s responsiveness to cancer[37]. Their formation or change in expression in the blood can indicate the nature of the tumour and thus help the clinician to understand their role in tumour histogenesis, cell differentiation and cell function. Common tumour markers can be classified into embryonic antigens, glycoproteins, kinins, enzymes and oncogene products according to their composition, with CEA being a protein and CA19-9 and CA-125 being glycoantigens[38].
Mesenchymal and smooth muscle tumours are the predominant mesenchymal-derived tumours of the gastrointestinal tract and the most common cause of submucosal lesions. Mesenchymal tumours originate from the interstitial cells of Cajal or mesenchymal stem cells in the gastrointestinal tract. It is currently thought that mutations in the C-kit or platelet-derived growth factor receptor A gene activation are important causes of mesenchymal tumours. Mesenchymal tumours are characterised by dynamic non-directional differentiation and potential malignancy, and even mesenchymal tumours with very low malignant potential may metastasise[39].
Pathological examination and immunohistochemistry are the gold standard for differentiating mesenchymal tumours from smooth muscle tumours. EUS is a non-invasive method that can assist in the diagnosis of the nature of the lesion and the choice of treatment by observing the level of origin, size and echogenicity of the lesion. The identification of the differences by comparing EUS features of intragastric mesenchymal tumours with those of smooth muscle tumours may spare patients with smooth muscle tumours from undergoing resection, while smaller diameter mesenchymal tumours may be diagnosed early, and intervention may be possible.
The data from this study show that mesenchymal tumours are more common than smooth leiomyosarcomas in the augmentation of the stomach, which is consistent with the finding in national studies[40-43]. Both appear as round or oval submucosal masses on plain endoscopy, with some visible surface erosions or ulcers, making differential diagnosis difficult. On EUS, mesenchymal tumours are usually of intramucosal origin, with a few originating in the mucosal layer, and appear as round or oval masses, which may be homogeneously hypoechoic, heterogeneously echogenic or hype
Therefore, the number of cells, their tight arrangement, the presence of liquefied necrosis, calcification and the amount of fibrous cell content are the factors that make up the ultrasound interface and the pathological basis of the ultrasound image in submucosal tumours. As previously discussed, mesenchymal tumours are richer in cells, more variable in their morphology and arrangement and more likely to undergo secondary changes than smooth muscle tumours, leading to differences in their echogenic characteristics.
In summary, EUS can accurately localise lesion characteristics, and there are significant differences in the echogenic characteristics of intragastric mesenchymal and smooth muscle tumours. Since early metastases can occur in GIMTs of less than 2 cm in diameter, endoscopic resection is recommended for the definitive diagnosis and simultaneous treatment or closer follow-up of intrinsic mesenchymal tumours with a clear echogenic border and less than 2 cm in diameter. The diagnosis of smooth muscle tumour is more likely for lesions with homogeneous echogenicity, well-defined borders and an intrinsic muscle layer of less than 2 cm in diameter, and patients can be advised to follow up. Overall, EUS can provide a strong basis for differentiating mesenchymal and smooth muscle tumours of less than 2 cm in diameter in the stomach and for clinical decision-making.
With the development of computed tomography technology, the differentiation of small mesenchymal tumors of the stomach and intestines from for smooth muscle tumors has been gradually used in this method.
To retrospectively analyze the clinical data of patients with gastric mesenchymal tumor and gastric smooth muscle tumor treated in our hospital from May 2018 to April 2023, and include them into the gastric mesenchymal tumor group and the gastric smooth muscle tumor group respectively, both groups consisted of 50 cases, and the clinical data of 50 healthy volunteers who received physical examination in our hospital during the same period were selected and included into the control group; to compare the serum carcinoembryonic antigen (CEA), alpha-fetoprotein, carbohydrate antigen 19-9 (CA19-9).
The Kappa test was used to analyse the consistency of the combined CEA and CA19-9 test in identifying gastric mesen
Clinical data of patients with gastric mesenchymal or gastric smooth muscle tumours who were treated in our hospital from May 2018 to April 2023 were retrospectively analysed. The value of CEA and CA19-9 in the identification of gastric mesenchymal tumours was analysed using the receiver operating characteristic curve.
When comparing the CEA levels of the three groups, the gastric mesenchymal tumour group > the control group > the gastric smooth muscle tumour group; when comparing the CA19-9 levels of the three groups, the gastric mesenchymal group > the gastric smooth muscle group > the control group, the difference was statistically significant (P < 0.05). The area under the curve of CEA and CA19-9 was 0. 879 and 0. 782, respectively, by receiver operating characteristic analysis.
We proposed the theory of nodular and vesicle types domestically through observation, and made breakthroughs in overcoming the issue of inaccurate diagnosis based on a few independent early-stage case reports.
According to the different partings under endoscopy, the clinical symptoms, therapeutic efficacy, and prognosis of patients with primary enteric lymphangiectasis were observed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alorro MG, Australia; Sherf-Dagan S, Israel S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 918] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 2. | Xing JJ, Huang WP, Wang F, Chai YR, Gao JB. Computed tomography features and clinicopathological characteristics of gastric glomus tumor. BMC Gastroenterol. 2022;22:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Papanikolaou IS, Triantafyllou K, Kourikou A, Rösch T. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc. 2011;3:86-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Jo VY, Doyle LA. Refinements in Sarcoma Classification in the Current 2013 World Health Organization Classification of Tumours of Soft Tissue and Bone. Surg Oncol Clin N Am. 2016;25:621-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Tanaka J, Oshima T, Hori K, Tomita T, Kim Y, Watari J, Oh K, Hirota S, Matsumoto T, Miwa H. Small gastrointestinal stromal tumor of the stomach showing rapid growth and early metastasis to the liver. Dig Endosc. 2010;22:354-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Virani N, Pang J, Lew M. Cytologic and Immunohistochemical Evaluation of Low-Grade Spindle Cell Lesions of the Gastrointestinal Tract. Arch Pathol Lab Med. 2016;140:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kudo M. Immuno-Oncology Therapy for Hepatocellular Carcinoma: Current Status and Ongoing Trials. Liver Cancer. 2019;8:221-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Vij M, Agrawal V, Kumar A, Pandey R. Gastrointestinal stromal tumors: a clinicopathological and immunohistochemical study of 121 cases. Indian J Gastroenterol. 2010;29:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Miura H, Tanaka K, Umeda Y, Ikenoyama Y, Yukimoto H, Hamada Y, Yamada R, Tsuboi J, Nakamura M, Katsurahara M, Horiki N, Nakagawa H. Usefulness of magnifying endoscopy with acetic acid and narrow-band imaging for the diagnosis of duodenal neoplasms: proposal of a diagnostic algorithm. Surg Endosc. 2022;36:8086-8095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Hirano K, Nagata A, Akahane K, Yonekura H, Tomioka I, Kobayashi T, Kawa S, Shimakura K, Oguchi H, Furuta S. [Clinical significance of the fecal chymotrypsin test in chronic pancreatitis--comparative study of its value with the pancreozymin-secretin test, PFD test, and endoscopic retrograde pancreatography]. Nihon Shokakibyo Gakkai Zasshi. 1985;82:2964-2972. [PubMed] |
| 11. | Sakamoto H, Kitano M, Kudo M. Diagnosis of subepithelial tumors in the upper gastrointestinal tract by endoscopic ultrasonography. World J Radiol. 2010;2:289-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 12. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (9)] |
| 13. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Xiu H, Zhao CY, Liu FG, Sun XG, Sun H, Liu XS. Comparing about three types of endoscopic therapy methods for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Scand J Gastroenterol. 2019;54:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Gheorghe G, Bacalbasa N, Ceobanu G, Ilie M, Enache V, Constantinescu G, Bungau S, Diaconu CC. Gastrointestinal Stromal Tumors-A Mini Review. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Fernandes MR, Ghezzi CLA, Grezzana-Filho TJ, Feier FH, Leipnitz I, Chedid AD, Cerski CTS, Chedid MF, Kruel CRP. Giant hepatic extra-gastrointestinal stromal tumor treated with cytoreductive surgery and adjuvant systemic therapy: A case report and review of literature. World J Gastrointest Surg. 2021;13:315-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kim HJ, Park JY, Kim BJ, Kim JG, Kim HS, Park JM. Gastric Gastrointestinal Stromal Tumor with Repeated Recurrence at the Anastomosis Site in a Very Elderly Patient. Korean J Gastroenterol. 2020;76:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Martínez-Camblor P, Pardo-Fernández JC. Smooth time-dependent receiver operating characteristic curve estimators. Stat Methods Med Res. 2018;27:651-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Chiu PWY, Yip HC, Teoh AYB, Wong VWY, Chan SM, Wong SKH, Ng EKW. Per oral endoscopic tumor (POET) resection for treatment of upper gastrointestinal subepithelial tumors. Surg Endosc. 2019;33:1326-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Mekras A, Krenn V, Perrakis A, Croner RS, Kalles V, Atamer C, Grützmann R, Vassos N. Gastrointestinal schwannomas: a rare but important differential diagnosis of mesenchymal tumors of gastrointestinal tract. BMC Surg. 2018;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 22. | Roberto GA, Rodrigues CMB, Peixoto RD, Younes RN. Gastric neuroendocrine tumor: A practical literature review. World J Gastrointest Oncol. 2020;12:850-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (8)] |
| 23. | Balakrishnan M, George R, Sharma A, Graham DY. Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep. 2017;19:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 24. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 25. | Rausei S, Dionigi G, Boni L. Evaluation of the Seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2011;117:2823-4; author reply 2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Seo HS, Lee GE, Kang MG, Han KH, Jung ES, Song KY. Mixed Histology Is a Risk Factor for Lymph Node Metastasis in Early Gastric Cancer. J Surg Res. 2019;236:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K, Nakajima T. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Russel SM, Geraghty JR, Renaldy H, Thompson TM, Hirshfield LE. Training for Professional Uncertainty: Socialization of Medical Students Through the Residency Application Process. Acad Med. 2021;96:S144-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Santiago JM, Sasako M, Osorio J. [TNM-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric Cancer. Towards simplicity and standardisation in the management of gastric cancer]. Cir Esp. 2011;89:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1335] [Article Influence: 333.8] [Reference Citation Analysis (2)] |
| 31. | Ikeguchi M, Murakami D, Kanaji S, Ohro S, Maeta Y, Yamaguchi K, Tatebe S, Kondo A, Tsujitani S, Kaibara N. Lymph node metastasis of gastric cancer: comparison of Union International Contra Cancer and Japanese systems. ANZ J Surg. 2004;74:852-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1327] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 33. | Park HK, Lee KY, Yoo MW, Hwang TS, Han HS. Mixed Carcinoma as an Independent Prognostic Factor in Submucosal Invasive Gastric Carcinoma. J Korean Med Sci. 2016;31:866-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Yang S, Gu X, Tao R, Huo J, Hu Z, Sun F, Ni J, Wang X. Relationship between histological mixed-type early gastric cancer and lymph node metastasis: A systematic review and meta-analysis. PLoS One. 2022;17:e0266952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Yang P, Zheng XD, Wang JM, Geng WB, Wang X. Undifferentiated-predominant mixed-type early gastric cancer is more aggressive than pure undifferentiated type: a systematic review and meta-analysis. BMJ Open. 2022;12:e054473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 36. | Song KY, Hyung WJ, Kim HH, Han SU, Cho GS, Ryu SW, Lee HJ, Kim MC; Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Is gastrectomy mandatory for all residual or recurrent gastric cancer following endoscopic resection? A large-scale Korean multi-center study. J Surg Oncol. 2008;98:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Ryu KW, Choi IJ, Doh YW, Kook MC, Kim CG, Park HJ, Lee JH, Lee JS, Lee JY, Kim YW, Bae JM. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol. 2007;14:3428-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakaya N, Nakamura T, Shimosegawa T. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: "eCura system". Am J Gastroenterol. 2017;112:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 39. | Takizawa K, Ono H, Muto M. Current indications of endoscopic submucosal dissection for early gastric cancer in Japan. Jpn J Clin Oncol. 2019;49:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Mikami K, Hirano Y, Futami K, Maekawa T. Expansion of lymph node metastasis in mixed-type submucosal invasive gastric cancer. Asian J Surg. 2018;41:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Sun F, Zhang S, Wang X, Yao M, Zhang C, Liu Z, Ai S, Guan W, Wang M. Mixed Histologic Type is a Risk Factor for Lymph Node Metastasis in Submucosal Invasive Early Gastric Cancer. J Surg Res. 2023;282:160-167. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Komatsu S, Ichikawa D, Miyamae M, Shimizu H, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Kishimoto M, Otsuji E. Histological mixed-type as an independent prognostic factor in stage I gastric carcinoma. World J Gastroenterol. 2015;21:549-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Nie RC, Yuan SQ, Li YF, Chen YM, Chen XJ, Zhu BY, Xu LP, Zhou ZW, Chen S, Chen YB. Clinicopathological Characteristics and Prognostic Value of Signet Ring Cells in Gastric Carcinoma: A Meta-Analysis. J Cancer. 2017;8:3396-3404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Chen JN, Wang QW, Zhang QW, Tang ZR, Li XB. Poorly differentiated is more significant than signet ring cell component for lymph node metastasis in mixed-type early gastric cancer: a retrospective study from a large-volume hospital. Surg Endosc. 2021;35:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |