Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.1986
Peer-review started: May 24, 2023
First decision: June 13, 2023
Revised: July 4, 2023
Accepted: July 31, 2023
Article in press: July 31, 2023
Published online: September 27, 2023
Processing time: 121 Days and 11.7 Hours
Adenocarcinoma of the esophagogastric junction has a center of origin within 5 cm of the esophagogastric junction. Surgical resection remains the main treatment. A transthoracic approach is recommended for Siewert I adenocarcinoma of the esophagogastric junction and a transabdominal approach is recommended for Siewert III adenocarcinoma of the esophagogastric junction. However, there is a need to determine the optimal surgical approach for Siewert II adenocarcinoma of the esophagogastric junction to improve lung function and the prognosis of patients.
To investigate and compare the surgical effects, postoperative changes in pulmonary function, and prognoses of two approaches to treating combined esophagogastric cancer.
One hundred and thirty-eight patients with combined esophagogastric cancer treated by general and thoracic surgeries in our hospital were selected. They were divided into group A comprising 70 patients (transabdominal approach) and group B comprising 68 patients (transthoracic approach) based on the surgical approach. The indexes related to surgical trauma, number of removed lymph nodes, indexes of lung function before and after surgery, survival rate, and survival duration of the two groups were compared 3 years after surgery.
The duration of surgery, length of hospital stay, and postoperative drainage duration of the patients in group A were shorter than those of the patients in group B, and the volume of blood loss caused by surgery was lower for group A than for group B (P < 0.05). At the one-month postoperative review, the first second, maximum ventilation volume, forceful lung volume, and lung volume values were higher for group A than for group B (P < 0.05). Preoperatively, the QLQ-OES18 scale scores of the patients in group A were higher than those in group B on re-evaluation at 3 mo postoperatively (P < 0.05). The surgical complication rate of the patients in group A was 10.00%, which was lower than that of patients in group B, which was 23.53% (P < 0.05).
Transabdominal and transthoracic surgical approaches are comparable in treating combined esophagogastric cancer; however, the former results in lesser surgical trauma, milder changes in pulmonary function, and fewer complications.
Core Tip: Surgical resection remains the main treatment for adenocarcinoma of the esophagogastric junction. The transthoracic approach is recommended for Siewert I, and the transabdominal approach for Siewert III adenocarcinomas of the esophagogastric junction. However, the optimal surgical approach for Siewert II adenocarcinoma of the esophagogastric junction remains inconclusive. We found that the transabdominal approach has the advantage of lesser surgical trauma, lesser impact on patients' pulmonary function, and fewer complications.
- Citation: Sun CB, Han XQ, Wang H, Zhang YX, Wang MC, Liu YN. Effect of two surgical approaches on the lung function and prognosis of patients with combined esophagogastric cancer. World J Gastrointest Surg 2023; 15(9): 1986-1994
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/1986.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.1986
Adenocarcinoma of the esophagogastric junction has a center of origin within 5 cm of the esophagogastric junction. It is classified as a separate disease because of its anatomical location and biological characteristics, and its incidence is increasing significantly globally[1]. Surgical resection remains the main treatment. The transthoracic and transabdominal approaches are recommended for Siewert I and III adenocarcinomas of the esophagogastric junction, respectively, for complete resection[2]. However, the optimal surgical approach for Siewert II adenocarcinoma of the esophagogastric junction has not been established[3]. The three common approaches for the surgical treatment of Siewert II adenocarcinoma of the esophagogastric junction are transthoracic, transabdominal, and combined transthoracic and abdominal[4]. However, the transthoracic and transabdominal approaches are more commonly used. This study aimed to investigate and compare the surgical effects, changes in postoperative pulmonary function, and differences in prognosis associated with the transthoracic and transabdominal epigastric approaches for the treatment of adenocarcinoma of the esophagogastric junction.
One hundred and thirty-eight patients with combined esophagogastric cancer surgically treated by general and thoracic surgeries in our hospital were recruited between July 2015 and June 2017 for this study. Based on the surgical approach, they were divided into group A comprising 70 patients (transabdominal approach) and group B comprising 68 patients (transthoracic approach). The inclusion criteria were as follows: (1) Esophageal cancer diagnosed according to the criteria in the NCCN Esophageal Cancer Guidelines 2015 V3 edition[5]; (2) age of 19 to 75 years; (3) esophageal cancer confirmed by biopsies taken by fiberoptic esophagoscopy before surgery and Siewert type II adenocarcinoma confirmed by pathology; and (4) absence of distant metastasis during preoperative examination. The exclusion criteria were as follows: (1) Patients requiring surgery due to emergencies, such as obstruction, perforation, or bleeding; (2) coagulation disorders; (3) palliative tumor resection; (4) previous history of open-heart or open abdominal surgery; (5) preoperative history of radiotherapy; and (6) missing data. The study protocol did not violate the relevant medical ethics requirements.
The characteristics of group A were as follows: Age range of 42–75 years with an average of 58.3 ± 7.0 years; gender distribution: 40 males and 30 females; TNM tumor stages: 13 cases of stage I, 30 cases of stage II, and 27 cases of stage III; tumor lesion diameter of 5.27 ± 1.40 cm; and Lauren's staging: 50 cases of intestinal type, 6 cases of diffuse type, 14 cases of mixed type.
The characteristics of group B were as follows: Age range of 40-75 years with an average of 56.8 ± 6.9 years; gender distribution: 34 males and 34 females; tumor TNM stages: 16 cases of stage I, 30 cases of stage II, and 22 cases of stage III; tumor lesion diameter of 5.10 ± 1.35 cm; Lauren typing: 52 cases of intestinal type, 4 cases of diffuse type, and 12 cases of mixed type. The baseline information of the two patient groups was above that of the baseline comparison, and the difference was not statistically significant (P > 0.05).
All patients were diagnosed preoperatively using gastroscopy and underwent cardiac color Doppler ultrasonography, pulmonary function test, chest computed tomography (CT), abdominal CT, routine electrocardiogram, routine biochemical tests, and coagulation function tests (Figure 1).
Group A: Transabdominal approach to surgical treatment (Figure 2). After the successful induction of general anesthesia, the patient was placed on his back and disinfected routinely. An incision was made in the middle of the upper abdomen, the abdomen was opened in layers, and the transverse colon was elevated. The gastrocolic ligament was opened along its upper edge, on the left near the splenic flexure of the colon, and on the right at the hepatic flexure of the colon, exposing the greater omentum and the anterior lobe of the transverse mesocolon. There was an upward separation along the greater curvature of the stomach. The left vessel of the gastric omentum was ligated, and the lymph nodes were swept. The primordial band of the liver and stomach was cut along the lesser curvature of the stomach on the right side of the cardia, the stomach was turned upward, and the gastric artery and vein were severed. The right and left diaphragmatic angles were opened, the anterior and posterior vagus nerve trunks were separated, and the lower end of the esophagus was freed and cut 3-5 cm from the cardia. The anastomotic stapler was placed in the esophagus, and the anastomosis was fixed in a purse string. The closing apparatus was placed close to the gastric body and sutured, and the esophagogastric anastomosis was strengthened intermittently. After the abdominal cavity was irrigated to stop bleeding, an abdominal drain was placed, and the abdomen was closed layer-by-layer. The abdominal and lower mediastinal lymph nodes were dissected.
Group B: Transthoracic approach to surgical treatment. After the successful induction of general anesthesia for transthoracic surgical access, the patient was placed in the supine position and routinely disinfected. An incision was made in the middle of the upper abdomen, and the abdomen was opened in layers. The transverse colon was elevated. The gastrocolic ligament was opened along its upper edge, on the left near the splenic flexure of the colon, and on the right to the hepatic flexure of the colon, exposing the greater omentum and the anterior lobe of the transverse mesocolon. There was an upward separation along the greater curvature of the stomach, the left vessel of the gastric omentum was ligated, and the lymph nodes were swept. The primordial band of the liver and stomach was separated along the lesser curvature of the stomach on the right side of the cardia, the stomach was turned upward, and the gastric artery and vein were severed. The right and left diaphragmatic angles were opened, the anterior and posterior vagus nerve trunks were separated, and the lower end of the esophagus was freed and cut 3-5 cm from the cardia. The anastomotic stapler was placed in the esophagus, and the anastomosis was secured in a purse string. The closing apparatus was placed close to the gastric body and sutured, and the esophagogastric anastomosis was strengthened intermittently. After the abdominal cavity was irrigated to stop bleeding, an abdominal drain was placed, and the abdomen was closed layer-by-layer. The mediastinal and perigastric lymph nodes were dissected.
The tumor center, number of lymph nodes, and positive status were confirmed based on the postoperative pathological findings of the specimen. R staging of the residual disease was used to determine the rate of radical resection: r0 indicated no signs of a residual tumor to the naked eye and under the microscope; r1 indicated a residual tumor that was not visible to the naked eye but was visible at the margin under the microscope; and r2 indicated a tumor visible to the naked eye at the cut edge.
We compared the duration of surgery, bleeding volume, number of cleared lymph nodes, number of positive lymph nodes, positive margin rate, and length of hospital stay. We also compared the two groups based on the four indicators of forced expiratory volume within the first second (FEV1), maximum ventilation volume (MVV), forceful lung volume (FVC), and lung volume (VC) as percentages of their expected values; surgical complications; and 3-year survival rates and durations before and after surgery.
Pulmonary function was measured preoperatively and 1 mo postoperatively. A JAEGER Flowscreen pulmonary function tester (Jaeger, Germany) was used to examine the patients while fully awake and in the sitting position[6]. The main indices included VC, FVC, FEV, and MVV, and the data are expressed as percentages of the actual value to the desired value.
The quality of survival was assessed preoperatively and at 3 mo postoperatively. The QLQ-OES18 scale for esophageal cancer[7] was used to evaluate the quality of survival; it contains 19 questionnaire items, 18 of which have a score range of 0-3, and one which has a score range of 0-4, with a total score of 58. Higher scores indicated higher quality of survival for the patient.
Statistical analysis was performed using SPSS 21.0 software. Measurement data such as duration of surgery, length of hospital stay, and bleeding volume were expressed as mean and standard deviation (x ± s) for both groups. The t-test was used to compare the two groups. The χ2 test was used to compare the counting data of the groups. The Kaplan-Meier method was used for survival analysis. Statistical significance was set at P < 0.05.
The durations of surgery, lengths of hospital stay, and postoperative drainage durations of patients in group A were shorter than those in group B. Bleeding caused by surgery was lower in group A than in group B (P < 0.05), and there was no statistically significant difference in the positive incision margin rate between the patients in groups A and B (P > 0.05) (Table 1).
| Group | n | Operation time (min) | Bleeding volume (mL) | Positive rate of cutting edge (%) | Postoperative drainage time (d) | Postoperative landing time (h) | Length of stay (d) |
| A group | 70 | 168.1 ± 15.7 | 136.8 ± 36.1 | 1 (1.43) | 3.71 ± 0.84 | 27.81 ± 7.51 | 12.30 ± 2.13 |
| B group | 68 | 188.0 ± 19.3 | 188.2 ± 43.7 | 3 (4.41) | 4.40 ± 1.22 | 29.40 ± 7.82 | 13.54 ± 2.35 |
| t/χ2 | -6.653 | -7.542 | 1.091 | -3.879 | -1.218 | -3.250 | |
| P value | 0.000 | 0.000 | 0.296 | 0.000 | 0.225 | 0.001 |
There were no statistically significant differences in the number of cleared lymph nodes, positive lymph nodes, lower mediastinal lymph nodes, subdiaphragmatic lymph nodes, or abdominal lymph nodes between groups A and B (P > 0.05) (Table 2).
| Group | n | Number of lymph nodes cleaned | Number of positive lymph nodes | Inferior mediastinal lymph nodes | Subphrenic lymph node | Number of abdominal lymph nodes |
| A group | 70 | 33.87 ± 3.82 | 3.65 ± 1.20 | 3.81 ± 1.20 | 2.56 ± 0.72 | 27.50 ± 2.95 |
| B group | 68 | 34.33 ± 2.90 | 3.92 ± 1.53 | 4.03 ± 1.15 | 2.74 ± 0.75 | 27.56 ± 3.02 |
| t value | -0.795 | -1.155 | -1.099 | -1.438 | -0.118 | |
| P value | 0.428 | 0.250 | 0.274 | 0.153 | 0.906 |
The preoperative values of FEV1, MVV, FVC, and VC for the patients in groups A and B were not significantly different (P > 0.05). However, the values obtained during the review conducted one month after surgery showed higher percentages of FEV1, MVV, FVC, and VC relative to their expected values for group A than for group B (P < 0.05) (Table 3).
| Group | n | Preoperative | 1 mo after operation | t value | P value | Preoperative | 1 mo after operation | t value | P value |
| FEV1 (%) | MVV (%) | ||||||||
| A group | 70 | 95.66 ± 8.64 | 90.21 ± 8.50 | 3.734 | 0.000 | 98.16 ± 9.26 | 93.48 ± 9.11 | 2.992 | 0.003 |
| B group | 68 | 97.03 ± 8.11 | 86.30 ± 7.76 | 7.937 | 0.000 | 96.32 ± 8.58 | 88.75 ± 8.36 | 5.248 | 0.000 |
| t value | -0.960 | 2.820 | 1.210 | 3.175 | |||||
| P value | 0.339 | 0.006 | 0.228 | 0.002 | |||||
| FVC (%) | VC (%) | ||||||||
| A group | 70 | 97.34 ± 8.14 | 92.36 ± 6.06 | 4.067 | 0.000 | 93.06 ± 4.85 | 90.01 ± 4.43 | 3.854 | 0.000 |
| B group | 68 | 99.03 ± 7.93 | 89.51 ± 7.24 | 7.359 | 0.000 | 94.41 ± 5.00 | 88.26 ± 5.25 | 7.048 | 0.000 |
| t value | -1.235 | 2.510 | -1.610 | 2.119 | |||||
| P value | 0.219 | 0.013 | 0.11 | 0.036 | |||||
Before surgery, the QLQ-OES18 scores of patients in groups A and B were compared, and the difference was not statistically significant (P > 0.05). When re-evaluated 3 mo after surgery, the QLQ-OES18 scores for the patients in group A were higher than those for the patients in group B (P < 0.05) (Table 4).
| Group | n | Preoperative | 3 mo after operation | t value | P value |
| A group | 70 | 31.83 ± 6.60 | 43.09 ± 5.57 | -10.816 | 0.000 |
| B group | 68 | 30.50 ± 5.78 | 40.14 ± 5.42 | -10.100 | 0.000 |
| t value | 1.258 | 3.152 | |||
| P value | 0.211 | 0.002 |
The surgical complication rate of 10.00% for group A was lower than that of 23.53% for group B (P < 0.05; Table 5).
| Group | n | Pulmonary infection | Abdominal infection | Anastomotic fistula | Pyothorax | Incision infection | Pleural effusion | Complication rate (%) |
| A group | 70 | 1 | 2 | 1 | 0 | 1 | 2 | 7 (10.00) |
| B group | 68 | 7 | 0 | 3 | 2 | 1 | 3 | 16 (23.53) |
| χ2 | 4.546 | |||||||
| P value | 0.033 |
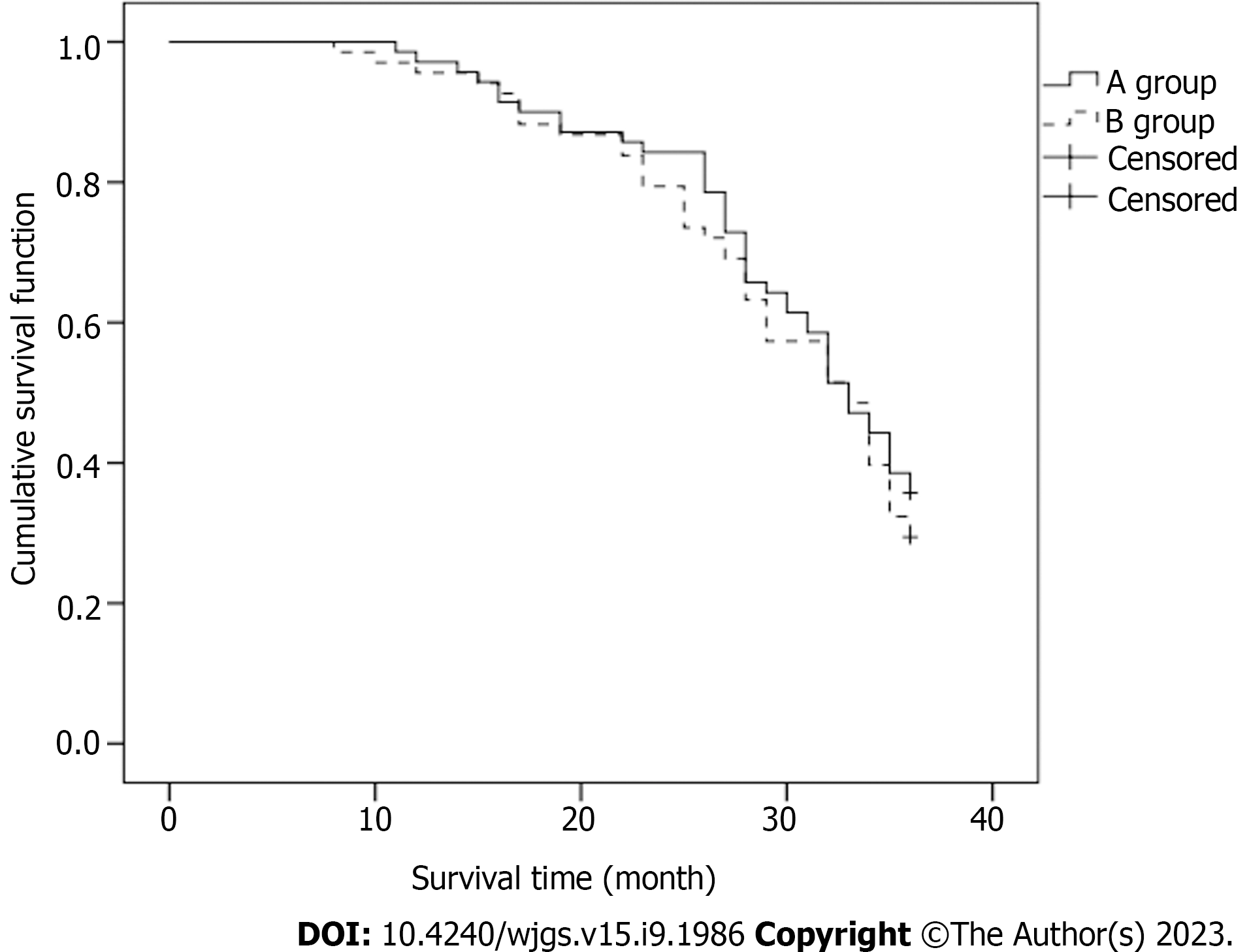
After 3 years of postoperative follow-up, there was no statistically significant difference in the survival rate of 35.71% for group A relative to 29.41% for group B (P > 0.05) (Table 6).
| Group | n | Subsist | Die |
| A group | 70 | 25 (35.71) | 45 (64.29) |
| B group | 68 | 20 (29.41) | 48 (70.59) |
| χ2 | 0.623 | ||
| P value | 0.430 | ||
The median duration of survival was 30.0 mo for the patients in group A and 29.0 mo for those in group B. The difference between the two groups was not statistically significant (P > 0.05) (Figure 2).
Adenocarcinoma of the esophagogastric junction is considered a special type of tumor because of its anatomical location and physiological function. It is independent of esophageal and gastric cancers and is more common in patients with Siewert II and III types[8]. Surgery for adenocarcinoma of the esophagogastric junction is usually performed by a gastrointestinal or thoracic surgeon or both. However, the choice of surgical approach for the Siewert II type has not been established. The advantage of combined thoracoabdominal therapy for adenocarcinoma of the esophagogastric junction is that it allows for complete dissection of the abdominal and mediastinal lymph nodes. However, this surgical approach is more invasive, increases the risk of surgery, and does not significantly improve the long-term survival of patients; therefore, most scholars recommend the transthoracic or transabdominal approach[9]. The advantage of the transthoracic approach is that it can completely expose the structures and tissues of the esophagus and cardia; however, exposure of the tissues near the distal stomach and spleen is poor. The transabdominal approach can fully expose the abdomen and facilitate the dissection of the abdominal lymph node; however, exposure to the distal esophagus is poor[9-11].
The duration of surgery and the severity of intraoperative blood loss are lateral reflections of surgical trauma and are interrelated[12]. Less intraoperative bleeding ensures less obstruction of the surgical field and a shorter duration of bleeding. The results of this study showed that the duration of surgery, length of hospital stay, and postoperative drainage of the patients in group A were shorter than those of the patients in group B. The blood loss during surgery was significantly lower for Group A than for Group B. This indicates that the transabdominal is less traumatic than the transthoracic approach. The pectoral muscle or the rib cage of the patient needs to be severed to expose the left side of the chest cavity, which is rich in intercostal vessels, and bleeding can easily occur when opening the chest. The transabdominal approach, which involves entering the abdominal cavity through the white line of the abdomen, results in less severe blood loss and a shorter duration of surgery. However, it is difficult to expose the organs and lymph nodes in the abdominal cavity, the scope of surgery is relatively small, the duration of surgery is prolonged, and intraoperative bleeding and duration of surgery may be further prolonged if adhesions and anatomical abnormalities are present in the abdominal cavity during exposure. The results of this study showed no statistically significant difference in the positive margin rate between the two groups, indicating that the outcomes of the two surgical approaches were comparable. Complete removal of regional lymph nodes is a key factor for the long-term survival of patients with Siewert type II adenocarcinoma of the esophagogastric junction and helps improve the long-term survival of patients during the progressive stage[13]. Studies have shown[14-18] that lymph node metastases are more likely to metastasize to the abdominal cavity than to the thoracic cavity in patients with type II esophagogastric junction cancer. Therefore, more attention should be paid to the removal of abdominal lymph nodes during surgery for type II esophagogastric junction cancer. In this study, there were no statistically significant differences in the number of removed lymph nodes, positive lymph nodes, lower mediastinal lymph nodes, subdiaphragmatic lymph nodes, or abdominal lymph nodes between the two groups. Increased attention of the surgeon combined with the use of laparoscopy and other techniques has resulted in no significant difference in the number of positive lymph node dissections associated with the current transabdominal and transthoracic approaches.
FEV1, MVV, FVC, and VC, expressed as percentages of the expected values, were higher for group A than for group B. Both groups showed different degrees of decline. Owing to the residual effects of general anesthesia, early postoperative pain, and the use of a chest strap, chest compliance decreased, and respiratory function decreased. The differences in postoperative pulmonary function associated with the two surgical approaches in this study were mainly attributed to the transabdominal approach through the right anterolateral incision, which maintained the integrity of the diaphragm during open thoracotomy with relatively little damage to the chest wall muscles. In addition, a shorter duration of surgery of the thoracic cavity resulted in less interference with the lung tissue. The transthoracic approach caused more severe damage to the chest wall muscles, impairment of the diaphragmatic integrity, greater interference with the lung tissue, and a greater deterioration of lung function. Group A had a significantly lower rate of surgical complications than group B. The transthoracic approach may have disrupted the normal muscles of the chest during the left thoracic incision, requiring incision and re-suturing of the diaphragm and damaging the respiratory muscles; this was detrimental to postoperative sputum expulsion and affected respiratory function. The intraoperative collapse of the left lung; ventilation of the right lung; intra-thoracic surgical involvement and compression of the lung tissue, heart, and blood vessels; and postoperative chest tube placement increase the inflammatory response in the thoracic cavity. Transthoracic surgery destroys the intercostal nerves of the patient, results in more severe postoperative pain than abdominal surgery, and is more likely to result in sputum accumulation. As a result, the incidence of pulmonary complications was higher for group B, which may have led to longer postoperative hospital stays. Patients in group A had higher QLQ-OES18 scores than those in group B when re-evaluated 3 mo postoperatively. Patients who underwent surgery with the transabdominal approach had a better prognosis. The 3-year postoperative follow-up evaluation revealed no statistically significant differences between the survival rates and median durations of survival of the two groups. The results of this study are consistent with those of previous studies[3,19-23], showing that the treatment of Siewert II esophagogastric junction cancer through the left thorax and abdomen is equally reliable.
Current clinical studies on the treatment of combined esophagogastric cancer mainly compare the efficacies and adverse effects of the different approaches[24-27]; they less frequently focus on the changes in the parameters of pulmonary function. In this study, the clinical outcomes, lymph node removal, and lung function recovery of patients with combined oesophagogastric cancer treated using transabdominal and transthoracic surgical approaches were studied comprehensively. This was more conducive to finding a more advantageous surgical approach. There were some limitations and shortcomings in the design of this study. It was a single-center retrospective study with a short duration of follow-up. Therefore, the results need to be confirmed by prospective, multicenter, randomized controlled clinical studies.
Transabdominal and transthoracic surgical approaches for the treatment of combined esophagogastric cancer are comparable. However, the former has the advantages of milder surgical trauma, less impact on pulmonary function, and fewer complications. Thus, it is suitable for older patients with frailty, cardiopulmonary insufficiency, or more complicated diseases.
Different types of esophagogastric junction adenocarcinoma have different operation methods.
We need to determine the optimal surgical approach for Siewert II adenocarcinoma of the esophagogastric junction to improve lung function and the prognosis of patients.
To investigate and compare the surgical effects, postoperative changes in pulmonary function, and prognoses of two approaches to treating combined esophagogastric cancer.
Patients with esophageal gastric cancer who received combined treatment in our hospital were selected, and the relevant indicators were compared after grouping.
The transabdominal approach has the advantages of less trauma, less impact on lung function and fewer complications.
Transabdominal surgical approaches is suitable for older patients with frailty, cardiopulmonary insufficiency, or more complicated diseases.
It was a single-center retrospective study with a short duration of follow-up. Therefore, the results need to be confirmed by prospective, multicenter, randomized controlled clinical studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Terashima M, Japan; Wei W, China S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Chevallay M, Bollschweiler E, Chandramohan SM, Schmidt T, Koch O, Demanzoni G, Mönig S, Allum W. Cancer of the gastroesophageal junction: a diagnosis, classification, and management review. Ann N Y Acad Sci. 2018;1434:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Türeci O, Sahin U, Schulze-Bergkamen H, Zvirbule Z, Lordick F, Koeberle D, Thuss-Patience P, Ettrich T, Arnold D, Bassermann F, Al-Batran SE, Wiechen K, Dhaene K, Maurus D, Gold M, Huber C, Krivoshik A, Arozullah A, Park JW, Schuler M. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 3. | Kumamoto T, Kurahashi Y, Niwa H, Nakanishi Y, Okumura K, Ozawa R, Ishida Y, Shinohara H. True esophagogastric junction adenocarcinoma: background of its definition and current surgical trends. Surg Today. 2020;50:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Urabe M, Matsusaka K, Ushiku T, Fukuyo M, Rahmutulla B, Yamashita H, Seto Y, Fukayama M, Kaneda A. Adenocarcinoma of the stomach and esophagogastric junction with low DNA methylation show poor prognoses. Gastric Cancer. 2023;26:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Manabe N, Matsueda K, Haruma K. Epidemiological Review of Gastroesophageal Junction Adenocarcinoma in Asian Countries. Digestion. 2022;103:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Treese C, Hartl K, Pötzsch M, Dahlmann M, von Winterfeld M, Berg E, Hummel M, Timm L, Rau B, Walther W, Daum S, Kobelt D, Stein U. S100A4 Is a Strong Negative Prognostic Marker and Potential Therapeutic Target in Adenocarcinoma of the Stomach and Esophagus. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Zhang CD, Takeshima H, Sekine S, Yamashita S, Liu YY, Hattori N, Abe H, Yamashita H, Fukuda M, Imamura Y, Ushiku T, Katai H, Makino H, Watanabe M, Seto Y, Ushijima T. Prediction of tissue origin of adenocarcinomas in the esophagogastric junction by DNA methylation. Gastric Cancer. 2022;25:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Jung MK, Schmidt T, Chon SH, Chevallay M, Berlth F, Akiyama J, Gutschow CA, Mönig SP. Current surgical treatment standards for esophageal and esophagogastric junction cancer. Ann N Y Acad Sci. 2020;1482:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Suhara H, Hirooka Y, Kawashima H, Ohno E, Ishikawa T, Nakamura M, Miyahara R, Ishigami M, Hashimoto S, Goto H. Transabdominal ultrasound elastography of the esophagogastric junction predicts reflux esophagitis. J Med Ultrason (2001). 2019;46:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Dai Z, Lang W, Yang H, Tian J, Sun W, Pekbay B, Lin Y, Wang M, Cui B, Yang S, Li H, Luo L, Guo H, Zhang L. Validation of EORTC QLQ-OES18 for Chinese patients with esophageal cancer. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | DeCarlo C, Manxhari C, Boitano LT, Mohebali J, Schwartz SI, Eagleton MJ, Conrad MF. Transabdominal approach associated with increased long-term laparotomy complications after open abdominal aortic aneurysm repair. J Vasc Surg. 2021;73:1603-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Trevellin E, Pirozzolo G, Fassan M, Vettor R. Prognostic value of stem cell markers in esophageal and esophagogastric junction cancer: a meta-analysis. J Cancer. 2020;11:4240-4249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 682] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 14. | van Workum F, Verstegen MHP, Klarenbeek BR, Bouwense SAW, van Berge Henegouwen MI, Daams F, Gisbertz SS, Hannink G, Haveman JW, Heisterkamp J, Jansen W, Kouwenhoven EA, van Lanschot JJB, Nieuwenhuijzen GAP, van der Peet DL, Polat F, Ubels S, Wijnhoven BPL, Rovers MM, Rosman C; ICAN collaborative research group. Intrathoracic vs Cervical Anastomosis After Totally or Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer: A Randomized Clinical Trial. JAMA Surg. 2021;156:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Lee Y, Min SH, Park KB, Park YS, Ahn SH, Park DJ, Kim HH. Long-term Outcomes of Laparoscopic Versus Open Transhiatal Approach for the Treatment of Esophagogastric Junction Cancer. J Gastric Cancer. 2019;19:62-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Berlth F, Hoelscher AH. History of Esophagogastric Junction Cancer Treatment and Current Surgical Management in Western Countries. J Gastric Cancer. 2019;19:139-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Hoshino I, Gunji H, Ishige F, Iwatate Y, Takiguchi N, Ikeda A, Soda H, Tonooka T, Sato N, Kawahara K, Nabeya Y. Surgical treatment strategy for esophagogastric junction cancers based on the tumor diameter. BMC Surg. 2019;19:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Shinohara H, Haruki S, Ohata Y, Ueda H, Arita K, Ito K, Matsumoto A, Takiguchi N. [A Case of Advanced Esophagogastric Junction Cancer Responding to Combined Modality Therapy]. Gan To Kagaku Ryoho. 2020;47:466-468. [PubMed] |
| 19. | Pericay C, Macías-Declara I, Arrazubi V, Vilà L, Marín M. Treatment in esophagogastric junction cancer: Past, present and future. Cir Esp (Engl Ed). 2019;97:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Hosoda K, Yamashita K, Tsuruta H, Moriya H, Mieno H, Ema A, Washio M, Watanabe M. Prognoses of advanced esophago-gastric junction cancer may be modified by thoracotomy and splenectomy. Oncol Lett. 2018;15:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Takada K, Yabuuchi Y, Yamamoto Y, Yoshida M, Kawata N, Takizawa K, Kishida Y, Ito S, Imai K, Hotta K, Ishiwatari H, Matsubayashi H, Kawabata T, Ono H. Predicting the depth of superficial adenocarcinoma of the esophagogastric junction. J Gastroenterol Hepatol. 2022;37:363-370. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, Anders RA, Kelly RJ. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 23. | Chen Y, Hu L, Lin H, Yu H, You J. Serum metabolomic profiling for patients with adenocarcinoma of the esophagogastric junction. Metabolomics. 2022;18:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Zheng YH, Zhao EH. Recent advances in multidisciplinary therapy for adenocarcinoma of the esophagus and esophagogastric junction. World J Gastroenterol. 2022;28:4299-4309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Tang Z, Wang Y, Liu D, Wang X, Xu C, Yu Y, Cui Y, Tang C, Li Q, Sun J, Zhang Q, Ji Y, Ma G, Li H, Shen Z, Shen K, Zheng R, Hou Z, Liu T, Wang J, Sun Y. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun. 2022;13:6807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | Rice TW, Lu M, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration Investigators. Precision Surgical Therapy for Adenocarcinoma of the Esophagus and Esophagogastric Junction. J Thorac Oncol. 2019;14:2164-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Kumar NA, Desouza A, Bhandare MS, Murugan JR, Khandelwal G, Chaudhari V, Ostwal V, Shrikhande SV. Curative resection for adenocarcinoma of the gastro-esophageal junction following neo-adjuvant chemotherapy-thoraco-abdominal vs. trans-abdominal approach. Langenbecks Arch Surg. 2021;406:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |