Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.1978
Peer-review started: May 31, 2023
First decision: June 14, 2023
Revised: June 25, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: September 27, 2023
Processing time: 114 Days and 10.4 Hours
Patients with colorectal cancer (CRC) are prone to stress ulcer after laparoscopic surgery. The analysis of risk factors for stress ulcer (SU) in patients with CRC is important to reduce mortality and improve patient prognosis.
To identify risk factors for SU after laparoscopic surgery for CRC, and develop a nomogram model to predict the risk of SU in these patients.
METHODS
The clinical data of 135 patients with CRC who underwent laparoscopic surgery between November 2021 and June 2022 were reviewed retrospectively. They were divided into two categories depending on the presence of SUs: The SU group (n = 23) and the non-SU group (n = 112). Univariate analysis and multivariate logistic regression analysis were used to screen for factors associated with postoperative SU in patients undergoing laparoscopic surgery, and a risk factor-based nomogram model was built based on these risk factors. By plotting the model's receiver operating characteristic (ROC) curve and calibration curve, a Hosmer-Lemeshow goodness of fit test was performed.
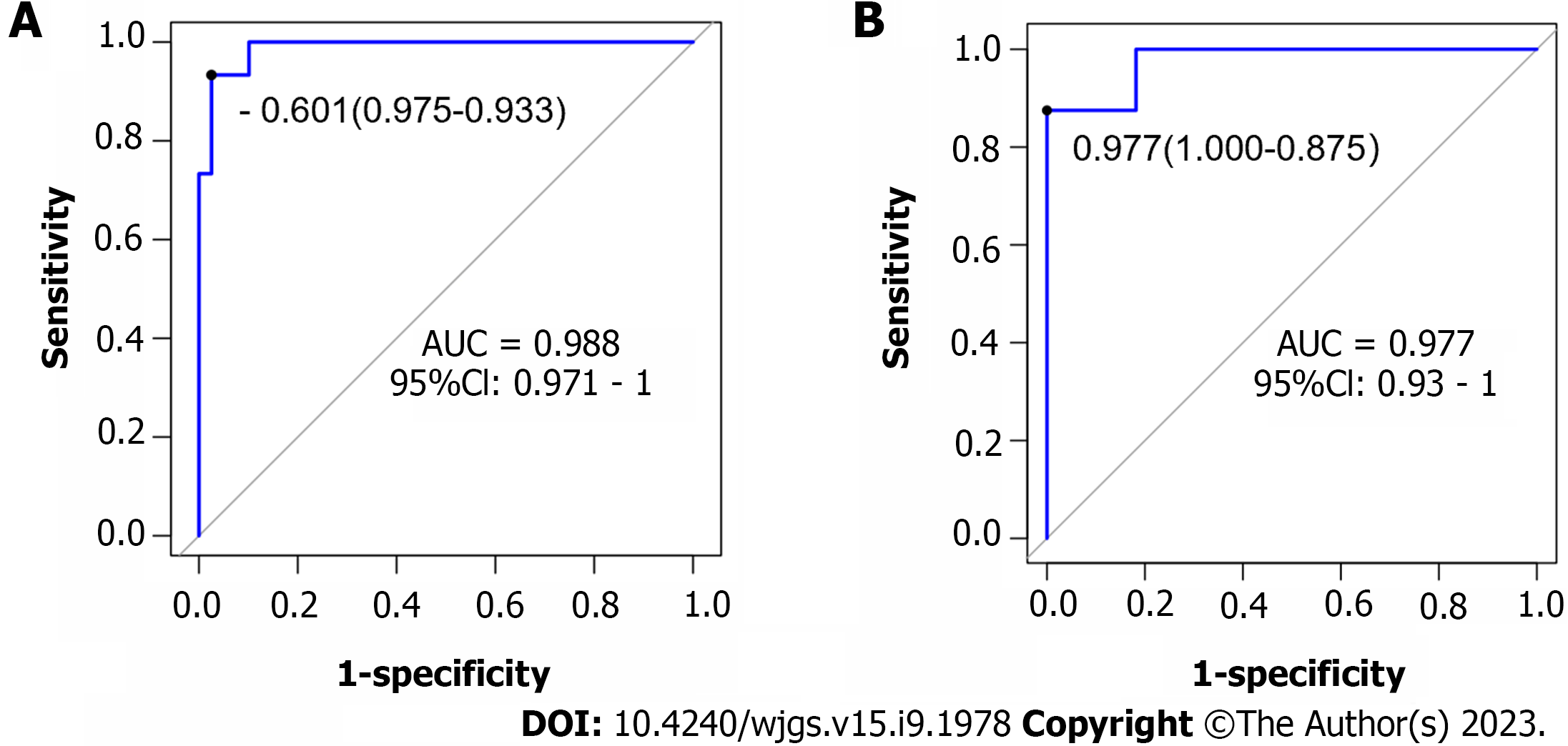
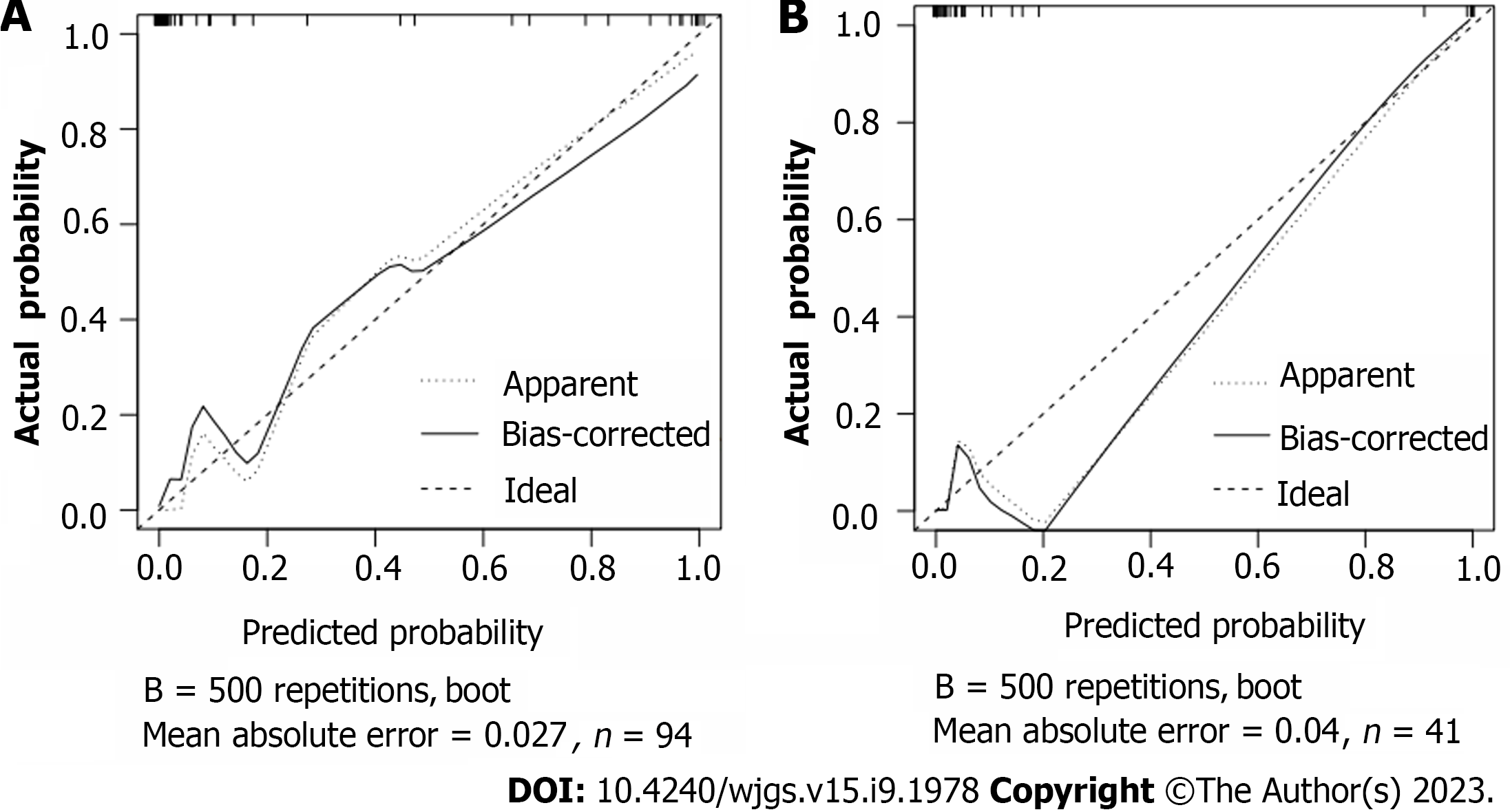
Among the 135 patients with CRC, 23 patients had postoperative SU, with an incidence of 17.04%. The SU group had higher levels of heat shock protein (HSP) 70, HSP90, and gastrin (GAS) than the non-SU group. Age, lymph node metastasis, HSP70, HSP90, and GAS levels were statistically different between the two groups, but other indicators were not statistically different. Logistic regression analysis showed that age ≥ 65 years, lymph node metastasis, and increased levels of HSP70, HSP90 and GAS were all risk factors for postoperative SU in patients with CRC (P < 0.05). According to these five risk factors, the area under the ROC curve for the nomogram model was 0.988 (95%CI: 0.971-1.0); the calibration curve demonstrated excellent agreement between predicted and actual probabilities, and the Hosmer-Lemeshow goodness of fit test revealed that the difference was not statistically significant (χ2 = 0.753, P = 0.999), suggesting that the nomogram model had good discrimination, calibration, and stability.
Patients with CRC aged ≥ 65 years, with lymph node metastasis and elevated HSP70, HSP90, GAS levels, are prone to post-laparoscopic surgery SU. Our nomogram model shows good predictive value.
Core Tip: Colorectal cancer (CRC) can cause hematochezia, dizziness, abdominal pain, diarrhea, constipation and other symptoms. We evaluated 135 patients who underwent laparoscopic surgery for CRC, identified 17 risk factors for post-surgery stress ulcer (SU), and established a nomogram model to predict the risk of SU in these patients. This model is useful for clinical prevention of postoperative SU in patients with CRC.
- Citation: Yu DM, Wu CX, Sun JY, Xue H, Yuwen Z, Feng JX. Prediction model of stress ulcer after laparoscopic surgery for colorectal cancer established by machine learning algorithm. World J Gastrointest Surg 2023; 15(9): 1978-1985
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/1978.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.1978
Colorectal cancer (CRC) is a common malignant tumor with an increasing incidence, which seriously threatens people's health[1]. It is easily overlooked by patients as the early symptoms are not obvious. As the cancer progresses, patients may experience abdominal pain, constipation and diarrhea, and in the advanced stage, they may experience general symptoms such as anemia and weight loss[2]. CRC kills over 900000 individuals worldwide each year, which makes it the fourth most lethal cancer[3]. Due to the advantages of low trauma, aesthetic criteria, and rapid postoperative recovery, laparoscopic radical dissection of CRC has emerged as the primary treatment for this disease[4,5]. However, CO2 pneumoperitoneum, hemodynamic changes, local immune dysfunction and other reasons may lead to changes in neuroendocrine metabolism in patients, causing a significant stress response[6,7]. Stress ulcer (SU) is one of the more typical postoperative complications in patients with CRC. The main clinical manifestations are acute mucosal erosion and ulceration, which eventually progress to hemorrhage and intestinal perforation, leading to a major physiological and psychological impact on the patient[8]. Severe bleeding from a SU can result in longer hospital stays and an elevated risk of death. Therefore, analyzing and preventing SU risk factors in CRC patients can effectively reduce mortality, accurately forecast CRC predisposition, and provide targeted treatment for high-risk groups. In the present study, we examined the clinical information of 135 CRC patients admitted to Hebei Traditional Chinese Medicine Hospital, examined the risk factors for the growth of SU after CRC surgery, and built a nomogram model to provide a foundation for the early prevention and risk reduction of SU after CRC surgery.
All 135 patients with CRC who underwent laparoscopic surgery at the Hebei Traditional Chinese Medicine Hospital between November 2021 and June 2022 were chosen for this retrospective study. The patients consisted of 80 males and 55 females, ranging in age from 22 to 85 years, with a mean age of (55.16 ± 13.18) years. Inclusion criteria were: (1) Meet the diagnostic criteria for CRC in the “Chinese protocol of diagnosis and treatment of colorectal cancer (2020 edition)”[9] and confirmed by pathology; (2) All patients underwent laparoscopic surgery; and (3) Complete clinical data. Exclusion criteria were: (1) Presence of hematological diseases; (2) Recent peptic ulcer; (3) Recent gastrointestinal surgery or invasive gastrointestinal examination; and (4) Other trauma, bleeding, etc. leading to blood in the digestive tract.
The patients were divided into the SU (23 cases) and non-SU (112 cases) group according to whether they developed a SU after surgery, with an SU incidence of 17.04%. SU was diagnosed if the patient met one of the following parameters: (1) Spitting or gastric extract showed visible bright red or coffee colored liquid; (2) Tar colored stool, black stool or fecal occult blood test was positive; and (3) Microscopic examination revealed that the patient's gastric mucosa showed patchy or punctate bloody lesions, ulceration or erosion[10].
Clinical data collected included gender, age, smoking history, drinking history, liver and kidney function, lymph node status, etc. Laboratory examinations included routine blood and biochemical blood indicators including C-reactive protein, tumor necrosis factor α, interleukin (IL)-6, IL-8, heat shock protein (HSP) 70, HSP90, gastrin (GAS), hemoglobin, albumin, fasting blood glucose at admission, serum potassium, etc.
SPSS26.0 statistical software was used for data processing and analysis. The χ2 test was applied to compare the two groups. Data on counts are presented as [n (%)]. The Shapiro-Wilk test was used to determine normalcy. The two independent samples t-test was used to analyze measurement data with a normal distribution. The non-normal distribution measurement data were reported as the median and interquartile range [M (P25, P75)], and the Mann-Whitney U test was employed. The risk factors for SU after CRC surgery were evaluated using univariate and multivariate logistic regression analysis, and the risk factors were imported into R software to develop a nomogram model to predict the risk of SU following CRC surgery. To assess the model's discrimination, a receiver operating characteristic (ROC) curve was created and the area under the curve (AUC) was determined. An AUC ≥ 90% was excellent, 70%-89% was good, 50%-69% was moderate, and 0.05 indicated good stability. P ≤ 0.05 was regarded as statistically significant.
The differences in age, lymph node metastases, HSP70, HSP90, and GAS levels between the two groups were statistically significant, while the other indicators were not. The SU group had higher levels of HSP70, HSP90, and GAS than the non-SU group (Table 1).
| Variables | Non-SU group (n = 112) | SU group (n = 23) | χ2/t | P value |
| Sex, n (%) | 0.086 | 0.769 | ||
| Male | 67 (59.82) | 13 (56.52) | ||
| Female | 45 (40.18) | 10 (43.48) | ||
| Age (yr), n (%) | 15.062 | < 0.001 | ||
| < 65 | 80 (71.43) | 6 (26.09) | ||
| ≥ 65 | 32 (28.57) | 17 (73.91) | ||
| Drinking history, n (%) | 2.280 | 0.131 | ||
| Yes | 46 (41.07) | 14 (60.87) | ||
| No | 66 (58.93) | 9 (39.13) | ||
| Smoking history, n (%) | 0.513 | 0.474 | ||
| Yes | 61 (54.46) | 15 (65.23) | ||
| No | 51 (45.54) | 8 (34.78) | ||
| Liver and kidney dysfunction, n (%) | 2.035 | 0.088 | ||
| Yes | 21 (18.75) | 8 (34.78) | ||
| No | 91 (81.25) | 15 (65.22) | ||
| Lymph node metastasis, n (%) | 14.316 | < 0.001 | ||
| Yes | 33 (29.46) | 17 (73.91) | ||
| No | 79 (70.54) | 6 (26.09) | ||
| Postoperative albumin decreased, n (%) | 0.263 | 0.608 | ||
| < 50 | 28 (25.00) | 4 (17.39) | ||
| ≥ 50 | 84 (75.00) | 19 (82.61) | ||
| Serum potassium (mmol/L) | 3.71 ± 0.58 | 3.73 ± 0.61 | -0.154 | 0.878 |
| Hemoglobin (g/L) | 121.28 ± 16.39 | 115.20 ± 18.52 | 0.115 | 0.115 |
| Fasting blood glucose (mmol/L) | 10.03 ± 4.54 | 8.15 ± 3.62 | 1.866 | 0.064 |
| CRP (mg/L) | 72.37 ± 12.09 | 75.32 ± 13.47 | 1.045 | 0.298 |
| TNF-α (pg/mL) | 149.00 ± 18.37 | 151.75 ± 21.41 | 0.635 | 0.526 |
| IL-6 (pg/mL) | 14.46 ± 2.28 | 15.02 ± 3.27 | 0.990 | 0.324 |
| IL-8 (pg/mL) | 18.43 ± 3.45 | 19.82 ± 3.04 | 1.793 | 0.075 |
| HSP70 | 2.32 ± 0.56 | 3.37 ± 0.81 | -5.876 | < 0.001 |
| HSP90 | 119.42 ± 17.81 | 159.35 ± 27.37 | -6.712 | < 0.001 |
| GAS | 121.92 ± 29.39 | 146.82 ± 35.36 | -3.571 | < 0.001 |
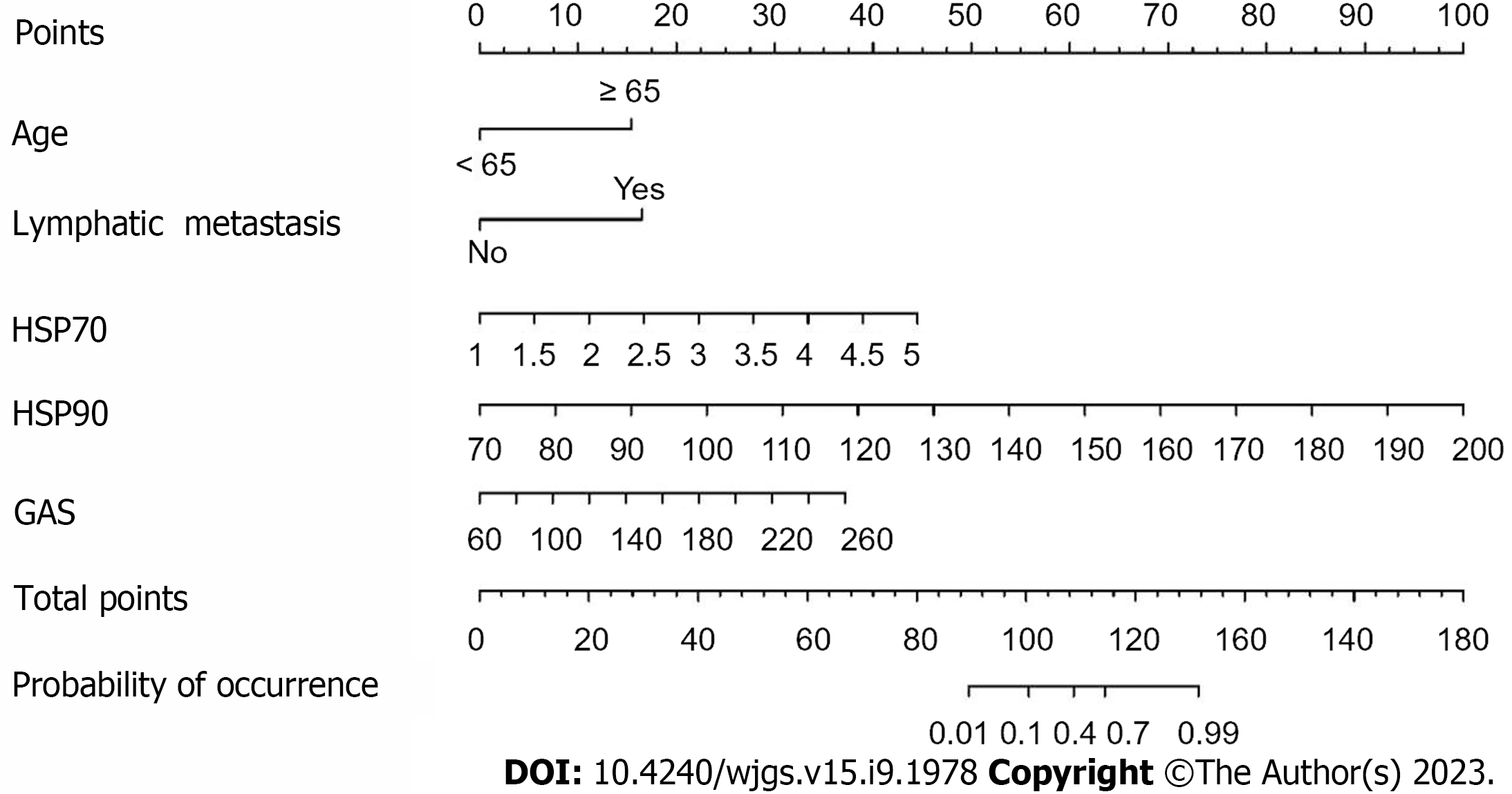
Univariate analysis was employed to identify statistically significant variables among the 17 factors related to CRC, including gender, age, drinking history, smoking history, abnormal liver and kidney function, lymph node metastasis, postoperative albumin decline and other laboratory indicators, to assign them (Age: < 65 years = 1, ≥ 65 years = 2; lymph node metastasis: yes = 1, no = 0), and regression analysis was carried out using a multivariate model. The results indicated that age ≥ 65 years, lymph node metastasis, and increased levels of HSP70, HSP90 and GAS were all independent risk factors for the development of SU in CRC patients after surgery (P < 0.05) (Table 2).
| Variables | β | SE | Wald | P value | OR | 95%CI |
| Age | 3.301 | 1.505 | 4.811 | 0.028 | 27.146 | 1.421-518.593 |
| Lymph node metastasis | 3.462 | 1.721 | 4.048 | 0.044 | 31.869 | 1.093-928.858 |
| HSP70 | 2.496 | 1.132 | 4.857 | 0.028 | 12.129 | 1.318-928.858 |
| HSP90 | 0.169 | 0.061 | 7.758 | 0.005 | 1.184 | 1.051-1.333 |
| GAS | 0.041 | 0.018 | 5.510 | 0.019 | 1.042 | 1.007-1.079 |
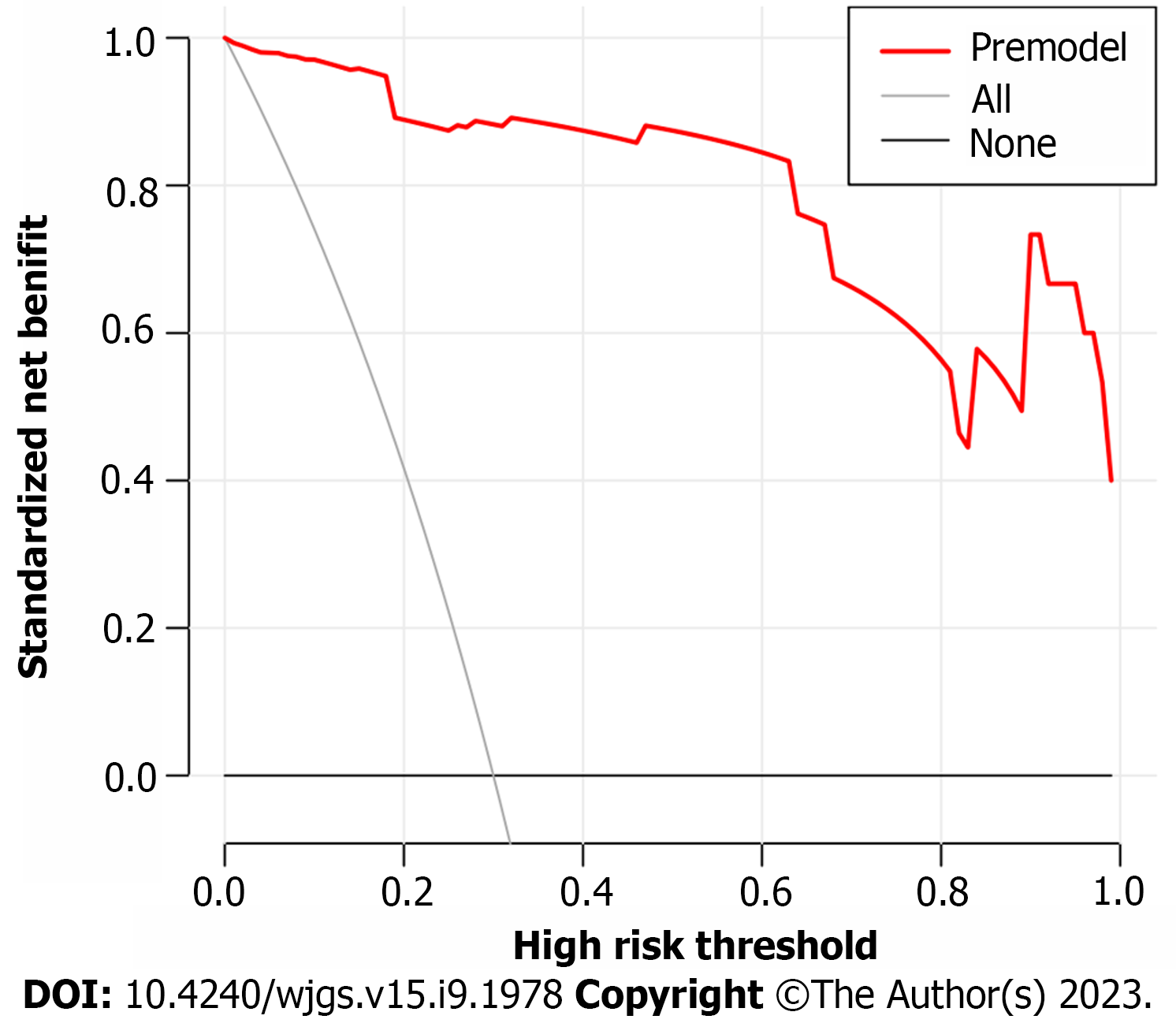
Based on the independent risk factors for postoperative SU in CRC patients screened out in multivariate analysis, the risk prediction model of postoperative SU in patients was established by R statistical software. The individual scores for each risk factor were obtained from the scale at the top of the nomogram for that factor, and the scores for all risk factors were added together to obtain a total score to obtain the incidence of SU in the corresponding patient. A higher total score indicated a greater likelihood of developing a SU (Figure 1). The AUC of the ROC was 0.988 (95%CI: 0.971-1.0), indicating that this nomogram model discriminated well (Figure 2). When the Youden index was 0.908, the related sensitivity and specificity were 93.3% and 97.5%, respectively. The training and validation set calibration curves suggested that the simulated and actual curves essentially followed the same trend (Figure 3), suggesting that prediction of the probability of postoperative SU in patients with CRC obtained by the nomogram model had good consistency with the actual probability. The Hosmer-Lemeshow goodness of fit test revealed no statistically significant change (χ2 = 0.753, P = 0.999), indicating that the model was well calibrated and stable. The decision curve analysis (DCA) is based on continuous potential risk thresholds, and the net benefit of risk-stratifying patients illustrates the model's clinical value. The prediction model's decision curves revealed that the model trended away from extreme curves with a high net benefit and clinical practicability (Figure 4).
SU is one of the postoperative complications of CRC. It occurs when the human body is subjected to various major injuries or psychological diseases, causing acute gastrointestinal mucosal erosion, ulcer and other lesions. Severe cases can be complicated by gastrointestinal bleeding or even perforation, leading to aggravation and deterioration of the original disease and increased mortality[11]. Analyzing the risk factors for SU in patients with CRC is of great significance for the prevention and prognosis of SU after CRC surgery.
Serum HSP70 and HSP90 are highly conserved stress proteins in the heat shock protein family. They have anti-inflammatory and anti-oxidation effects and can affect cell stability and the stress response[12]. Studies have found that HSP70 and HSP90 Levels are closely linked to SU risk[13,14]. When gastric mucosal cells are stimulated by trauma factors, the cell protein configuration changes, which can induce cell inflammatory response and activate HSP70 and HSP90. With the increase in HSP70 and HSP90 content, this promotes the synthesis and folding of proteins in gastric mucosal cells, thereby reducing the damage caused by the stress response to mucosal cells and exerting a role in gastric mucosal protection[15]. According to our findings, patients in the SU group had greater serum levels of HSP70 and HSP90 than patients in the non-SU group. It is suggested that the higher the levels of HSP70 and HSP90, the more severe the postoperative stress response in patients with CRC, the greater the compensatory increase in HSP70 and HSP90, the more serious the damage caused by stress response to gastric mucosal cells, and the higher the risk of SU. Therefore, serum levels of HSP70 and HSP90 are indicators of the likelihood of developing a SU in individuals who undergo laparoscopic surgery for CRC.
GAS is a potent hormone that promotes gastric acid secretion. The increase in GAS level may be related to sympathetic nerve excitation caused by trauma, continuous contraction of gastric mucosal blood vessels, vagus nerve choline fiber excitation caused by increased intracranial pressure, use of a strong dose of dehydrating agent and catabolic disorder[16]. Studies have found that when the level of serum GAS in critically ill patients increases, it will increase gastric acid secretion, resulting in transient small intestinal dysfunction and decreased gastric emptying capacity and gastric pyloric sphincter tension. Food reflux from the small intestine stimulates GAS secretion[17]. According to the study findings, patients with SUs had higher serum GAS levels than patients without SUs. The results of regression analysis showed that GAS level was a risk factor for SU, which was consistent with the results reported in the literature[12]. In this study, it was concluded that the increase in serum GAS level correlated with disease severity, and that the severity of the patient's condition was correlated with the intensity of the stress response and stomach mucosal damage, which could be utilized as a predictor of the likelihood of developing a SU.
The results of this study suggest that age ≥ 65 years and lymph node metastasis are risk factors for the development of SU following CRC surgery. This may be the reason why elderly patients are prone to SU, and may be related to stress changes such as relatively low physical resistance and decreased postoperative self-regulation ability. Lymphatic metastasis is a common feature of advanced cancer. Surgical treatment of advanced rectal cancer often involves a long operation time and complicated procedures. Lymph node dissection leads to increased surgical trauma, stress response, acid-base imbalance and further acidosis. At the same time, the increase in oxygen free radicals increases the risk of SU bleeding[18].
In this study, 17 factors that may affect postoperative SU in patients with CRC were investigated by univariate analysis combined with multivariate logistic regression analysis. To construct a nomogram model, five independent risk factors identified in the logistic analysis were entered into R software. The nomogram model performed well in terms of discrimination, as indicated by the AUC of 0.988 (95%CI: 0.971-1.0), and this performance was further confirmed in the validation set. In addition, the calibration curves also demonstrated the good consistency of the nomogram model. No significant difference was found by the Hosmer-Lemeshow goodness of fit test (χ2 = 0.753, P = 0.999), indicating a stable and well-calibrated model. However, these results do not fully explain whether the nomogram model can be applied in clinical practice. Therefore, further analysis by DCA was carried out and the results of the prediction model DCA showed that the model was far from the extreme curve and the net benefit rate was high, showing that the nomogram had good clinical applicability. In this retrospective study with a small number of affecting factors, the findings may be biased as it was not a multi-center, large-sample epidemiological survey. In the future, a more reasonable and larger-sample prospective randomized controlled clinical trial will be designed to further improve the model's predictive value.
Age ≥ 65 years, lymph node metastasis, and elevated HSP70, HSP90, and GAS are independent risk factors for postoperative SU in patients with CRC. The nomogram model constructed accordingly had high clinical application value, calibration, and stability. It is helpful for clinicians to take targeted measures to reduce the incidence of postoperative SU in patients with CRC.
Colorectal cancer (CRC) is a complex multifactorial disease, usually manifested as hematochezia, abdominal pain, diarrhea, and constipation.
Patients with rectal cancer are prone to stress ulcer (SU) after laparoscopic surgery.
This study aimed to investigate the risk factors for SU in patients with CRC after laparoscopic surgery, and construct a risk prediction nomogram model with clinical value based on these risk factors.
This study was a retrospective analysis of the clinical data of 135 patients with CRC who underwent laparoscopic surgery from November 2021 to June 2022. Risk factors for the development of postoperative SU were screened by univariate and multivariate regression analyses, and nomogram models were constructed based on these risk factors.
Among the 135 patients with CRC, 23 patients had postoperative SU, with an incidence of 17.04%.
By comparing other studies, we found that most scholars emphasize the advantages of laparoscopic treatment of CRC, but there is a lack of research on its disadvantages. This study proposes that laparoscopic treatment of CRC is prone to SU, further analyzes its influencing factors, and establishes a predictive model with clinical value. This study proposed a new prediction model of SU after laparoscopic surgery in patients with CRC.
Future research should be based on clinical observation, incorporating more possible influencing factors, and establishing a more practical predictive model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fielding GA, United States; Surve A, United States S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Alçın G, Şanlı Y, Yeğen G, Kaytan Sağlam E, Çermik TF. The Impact of Primary Tumor and Locoregional Metastatic Lymph Node SUV(max) on Predicting Survival in Patients with Rectal Cancer. Mol Imaging Radionucl Ther. 2020;29:65-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3029] [Article Influence: 504.8] [Reference Citation Analysis (3)] |
| 3. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1586] [Article Influence: 264.3] [Reference Citation Analysis (2)] |
| 4. | Chen Y, Xi D, Zhang Q. Laparoscopic Radical Resection versus Routine Surgery for Colorectal Cancer. Comput Math Methods Med. 2022;2022:4899555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Nakagawa K, Watanabe J, Ota M, Suwa Y, Suzuki S, Suwa H, Momiyama M, Ishibe A, Saigusa Y, Yamanaka T, Kunisaki C, Endo I. Efficacy and safety of enoxaparin for preventing venous thromboembolic events after laparoscopic colorectal cancer surgery: a randomized-controlled trial (YCOG 1404). Surg Today. 2020;50:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Liu R, Qin H, Wang M, Li K, Zhao G. Transversus abdominis plane block with general anesthesia blunts the perioperative stress response in patients undergoing radical gastrectomy. BMC Anesthesiol. 2019;19:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Balagué Ponz C, Trias M. Laparoscopic surgery and surgical infection. J Chemother. 2001;13 Spec No 1:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Mohamed WA, Schaalan MF, Ramadan B. The expression profiling of circulating miR-204, miR-182, and lncRNA H19 as novel potential biomarkers for the progression of peptic ulcer to gastric cancer. J Cell Biochem. 2019;120:13464-13477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | National Health Commission of the People's Republic of China. [Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition)]. Zhonghua Wai Ke Za Zhi. 2020;58:561-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 10. | Siddiqui AH, Farooq U, Siddiqui F. Curling Ulcer. 2023 Apr 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 11. | Pallan A, Dedelaite M, Mirajkar N, Newman PA, Plowright J, Ashraf S. Postoperative complications of colorectal cancer. Clin Radiol. 2021;76:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Szyller J, Kozakiewicz M, Siermontowski P, Kaczerska D. Oxidative Stress, HSP70/HSP90 and eNOS/iNOS Serum Levels in Professional Divers during Hyperbaric Exposition. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Khotib J, Rahmadi M, Ardianto C, Nisak K, Oktavia R, Ratnasari A, Dinintia Y, Shinta DW, Aryani T; Suharjono. Selective serotonin reuptake inhibitor fluvoxamine ameliorates stress- and NSAID-induced peptic ulcer possibly by involving Hsp70. J Basic Clin Physiol Pharmacol. 2019;30:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Hoter A, Naim HY. The Functions and Therapeutic Potential of Heat Shock Proteins in Inflammatory Bowel Disease-An Update. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Schulze A, Beliu G, Helmerich DA, Schubert J, Pearl LH, Prodromou C, Neuweiler H. Cooperation of local motions in the Hsp90 molecular chaperone ATPase mechanism. Nat Chem Biol. 2016;12:628-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Ren X, Wang Y, He Z, Liu H, Xue K. Effects of cefuroxime axetil combined with Xingpi Yanger granules on the serum gastrin, motilin, and somatostatin levels in children with upper respiratory tract infection accompanied by diarrhea: results of a randomized trial. Transl Pediatr. 2021;10:2106-2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Zhou X, Fang H, Xu J, Chen P, Hu X, Chen B, Wang H, Hu C, Xu Z. Stress ulcer prophylaxis with proton pump inhibitors or histamine 2 receptor antagonists in critically ill adults - a meta-analysis of randomized controlled trials with trial sequential analysis. BMC Gastroenterol. 2019;19:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Jin M, Frankel WL. Lymph Node Metastasis in Colorectal Cancer. Surg Oncol Clin N Am. 2018;27:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |