Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1784
Peer-review started: December 20, 2022
First decision: February 8, 2023
Revised: February 14, 2023
Accepted: June 11, 2023
Article in press: June 11, 2023
Published online: August 27, 2023
Processing time: 248 Days and 2.9 Hours
Choledochal cysts (CC) are cystic dilatations of the biliary tract, usually diagnosed during childhood, with an estimated incidence in the general population of 1:100000. Complications related to CC include rupture, biliary obstruction, and cholangitis. Maternal CC in pregnancy are rarely reported, and there are no guidelines on optimal management.
To systematically review maternal CC diagnosed during pregnancy or post
A literature search of cases and case series of maternal CC in pregnancy and postpartum was conducted using MEDLINE/PubMed, Web of Science, Google Scholar, and Embase. There were no restrictions on language or publication year. Databases were lastly accessed on September 1, 2022.
Overall, 71 publications met the inclusion criteria, reporting 97 cases. Eighty-eight cases were diagnosed during pregnancy and nine in the puerperium. The most common symptoms were abdominal pain (81.2%) and jaundice (60.4%). Inter
Although rare, maternal CC in pregnancy should be included in the evaluation of jaundice with upper abdominal pain. Symptomatology and clinical course are variable, and treatment may range from an expectative approach to emergent surgical CC treatment and urgent CS. While most cases were managed by conservative measures or drainage procedures, CC > 15 cm and progressive cholangitis carry the risk of CC rupture and septic complications, which may increase the rates of unfavorable maternal and fetal outcomes. Therefore, such cases require specific surgical and obstetric interventions.
Core Tip: Although rare, maternal choledochal cysts (CC) in pregnancy should be included in the evaluation of jaundice with upper abdominal pain. Symptomatology and clinical course are variable, and treatment may range from an expectative approach to emergent surgical treatment of CC and urgent cesarean section. While most cases were managed by conservative measures or drainage procedures, CC > 15 cm and progressive cholangitis carry the risk of CC rupture and septic complications, which may increase the rates of unfavorable maternal and fetal outcomes. Therefore, such cases require specific surgical and obstetric interventions.
- Citation: Augustin G, Romic I, Miličić I, Mikuš M, Herman M. Maternal choledochal cysts in pregnancy: A systematic review of case reports and case series. World J Gastrointest Surg 2023; 15(8): 1784-1798
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1784.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1784
Choledochal cysts (CC) are cystic dilatations of the biliary tract, usually diagnosed during childhood (80%)[1,2]. Todani's classification is based on anatomical characteristics, and it is the most widely used classification method, which divides CC into five types[3]. Type I (cystic dilatation of the common bile duct) is the most common (90%)[4,5]. The incidence significantly varies between the East and the West[1,4]. The estimated incidence in the general population is 1:100000-1:150000 in Western countries, while in the East, it is much higher (up to 1:1000), with a male/female ratio of 1:3-4[1,6]. Although cholecystitis or choledocholithiasis is more commonly associated with upper right abdominal pain or jaundice, CC should be considered in the differential diagnosis because the treatment approach is different. CC may present with a triad of abdominal pain, jaundice, and a palpable right upper quadrant abdominal mass. The presentation of CC depends not only on size but on morphology, the presence of sludge/stones, and the status of the remaining biliary tract. When cholangitis develops, the Charcot triad may be present (jaundice, fever with rigors, and right upper quadrant abdominal pain). CC may result in severe complications such as biliary obstruction, cholangitis, or cyst rupture. Furthermore, CC are considered a premalignant condition, another reason for surgical treatment, even in asymptomatic patients[4,7]. Gomes et al[8] made the first report on CC in 1723.
Many theories on the pathogenesis of CC were offered. Some proposed that congenital dysplasia arises from early embryonic development, where dysplasia of the primordial common bile duct (CBD) leads to CBD dilation[9]. Another theory is based on a congenital weakness of the bile duct wall that allows cysts to form[10,11]. Proponents of the CBD obstruction theory believe that CC form due to congenital or acquired obstruction of the ducts[12]. A so-called dual theory states that cyst formation is due to the weakness of the bile duct wall and obstruction of the ducts[13]. However, the most widely accepted theory, the common channel theory, states that the anomalous pancreaticobiliary junction leads to the formation of an abnormally long common channel, which permits reflux of the activated proteolytic pancreatic enzymes, biliary tract wall weakness and, eventually, cystic dilatation[14].
CC treatment in the general population depends on symptomatology and CC features. Most cases require cyst excision, cholecystectomy, and Roux-en-Y hepaticojejunostomy (HJ)[6,14]. Urgent indications are cyst rupture, intraluminal biliary bleeding, or severe cholangitis[12,15]. Todani's classification determines surgical treatment. Types I and IV should be completely excised, in addition to cholecystectomy and bilioenteric continuity formation. Type II is usually treated by diverticulectomy followed by CBD closure at the diverticulum neck. Endoscopic sphincterotomy is usually sufficient for type III cysts[11,12,15]. The treatment of types IV and V is more complex, as both comprise intra- and extrahepatic components, and the intrahepatic component is difficult to treat. When the cyst adheres densely to the portal vein due to an inflammatory reaction, standard full-thickness cyst excision may not be possible. In such cases, the Lilly technique should be considered, which includes curettage or cauterization of cyst mucosa while the serosa remains attached to the portal vein. This resolves cholestasis, and at the same time, it removes the risk of malignant transformation.
Maternal CC in pregnancy are rarely reported, and no studies suggest a possible facilitating impact of pregnancy on CC development or growth. However, the pregnant uterus possibly causes biliary outflow obstruction promoting cystic dilatation, eventually leading to symptoms in prepregnancy asymptomatic individuals[16]. In addition, physiological changes during pregnancy can mask different clinical scenarios, including CC.
The effects of CC and their complications considering pregnancy outcomes have not been studied. Jaundice in pregnancy has potentially severe maternal and fetal consequences, found with intrahepatic cholestasis of pregnancy (ICP) or hemolysis, elevated liver enzymes, low platelets syndrome. Adverse fetal outcomes include preterm delivery, respiratory distress, or fetal distress. Therefore, CC with cholestasis and jaundice pose a similar risk to the mother and the fetus as ICP or other cholestatic disorders[16-18].
Due to the rarity of CC, reliable data on the risk of fetal loss and optimal CC treatment are lacking, and randomized trials or large prospective series do not exist. Therefore, we aimed to systematically review maternal CC diagnosed during pregnancy and postpartum and to propose optimal treatment strategies.
A literature search of cases and case series of maternal CC in pregnancy and postpartum was conducted. We searched MEDLINE/PubMed, Web of Science, Google Scholar, and Embase using a combination of MeSH terms: "Maternal", "choledochal cyst", "choledochocele", "pregnancy", "biliary tract disease", "puerperium", and "postpartum." Puerperium was evaluated up to the 6th postpartum week.
The search was limited to human studies published until September 2022, without language or country restrictions. Non-English articles were translated and analyzed with the help of medical scientists proficient in the languages mentioned above. We evaluated articles and references from these articles to identify additional cases. Cases with maternal CC treated and diagnosed before pregnancy were excluded. All potentially relevant articles were reviewed by three investigators (AA, GA, and IR), and all disagreements were settled by discussion.
Data included demographic data (maternal age, parity, and gestational weeks), clinical presentation (pain, jaundice, palpable mass, and fever), laboratory data (total bilirubin and liver enzymes), radiological examinations [abdominal ultrasound, abdominal magnetic resonance imaging (MRI), cyst dimensions, and cyst type according to Todani's classification], mode of treatment and delivery, maternal and fetal outcomes. The risk of bias or study quality was not assessed, as all articles were case reports or small case series. Bilirubin (μmol/L) was the only laboratory parameter sufficiently reported to be included in the analysis. If mentioned, the highest diameter of the CC and bilirubin value were used in the calculations. Pregnancies were analyzed through gestational months and trimesters; the first trimester counts from week zero to the end of the 13th week, the second from the 14th to the 27th week, and the third from the 28th to term.
Results of descriptive analyses are reported as numbers and proportions in percentages. Inferential statistics were executed on the different numbers of patients depending on the availability of analyzed variables. Categorical variables were analyzed using the chi-square test, and results are reported as odds ratios (OR) with 95%CI. A Pearson's correlation analysis was used for an association between continuous parameters (age, cyst size, bilirubin level, and parity). A P value less than 0.05 was considered statistically significant. Statistical analyses were performed via the SPSS version 26 (SPSS, Chicago, IL, United States).
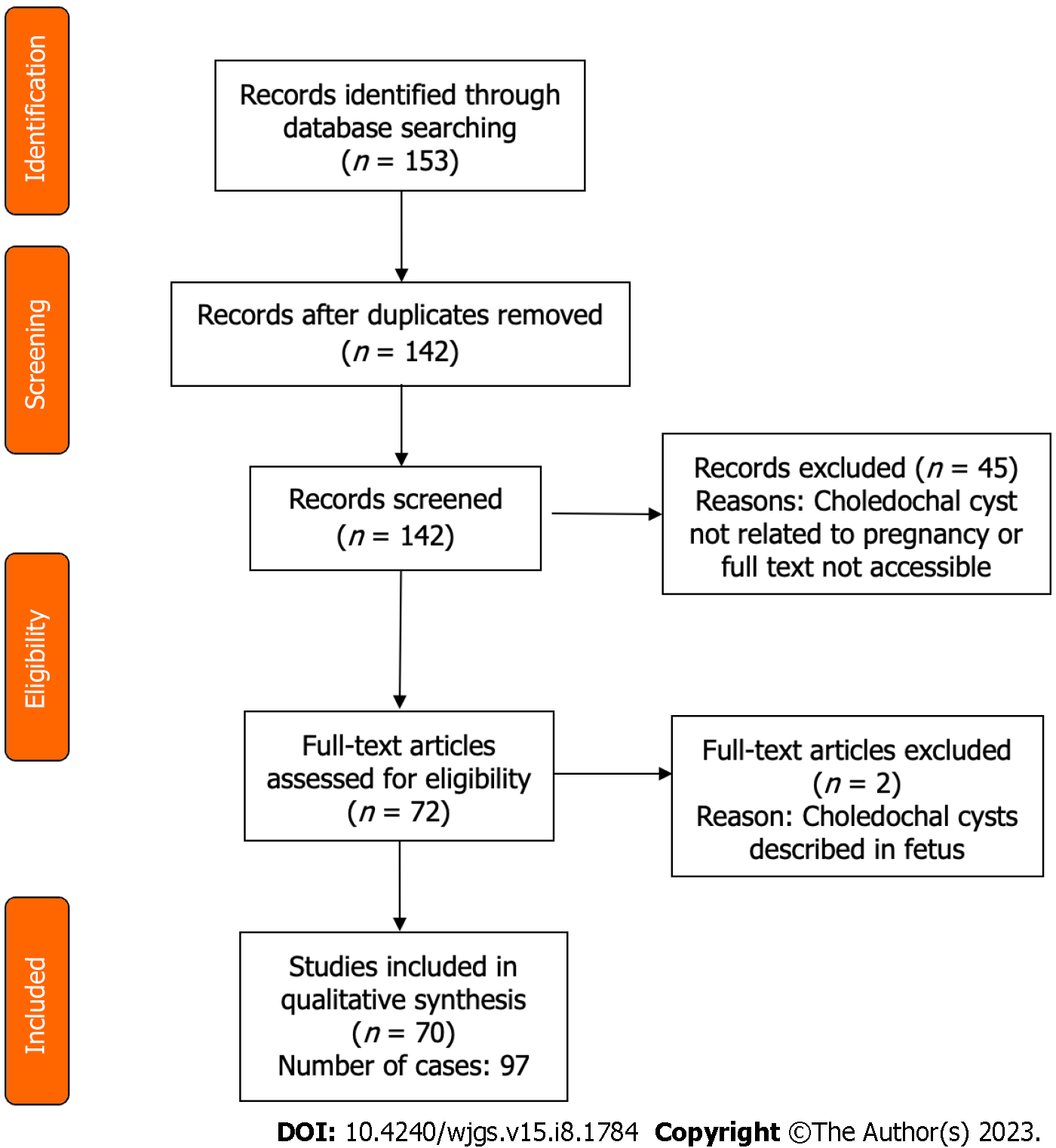
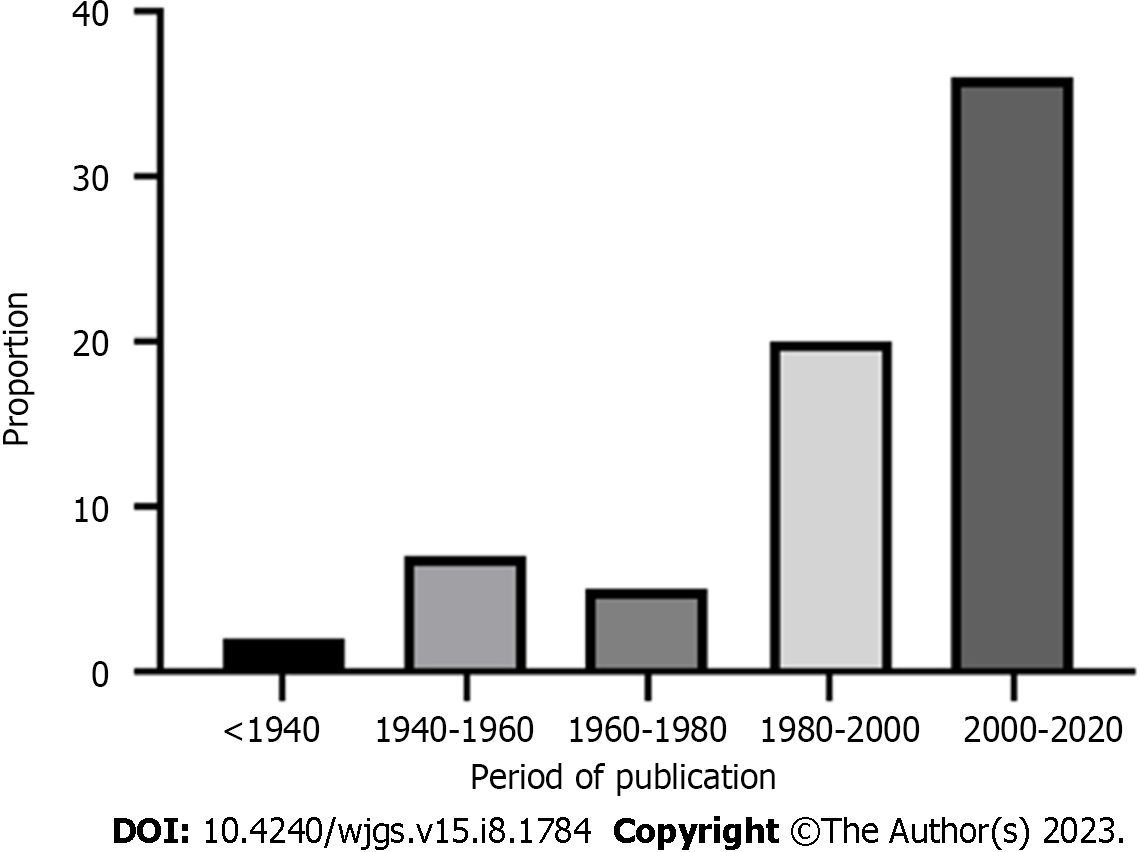
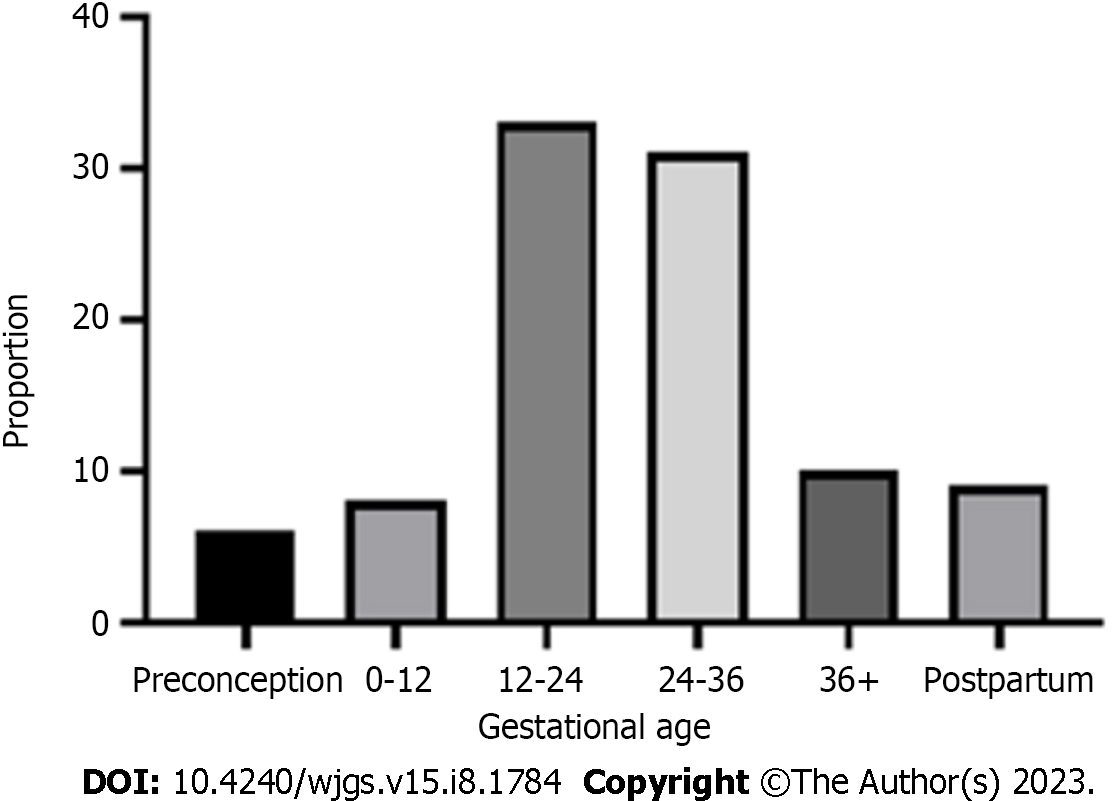
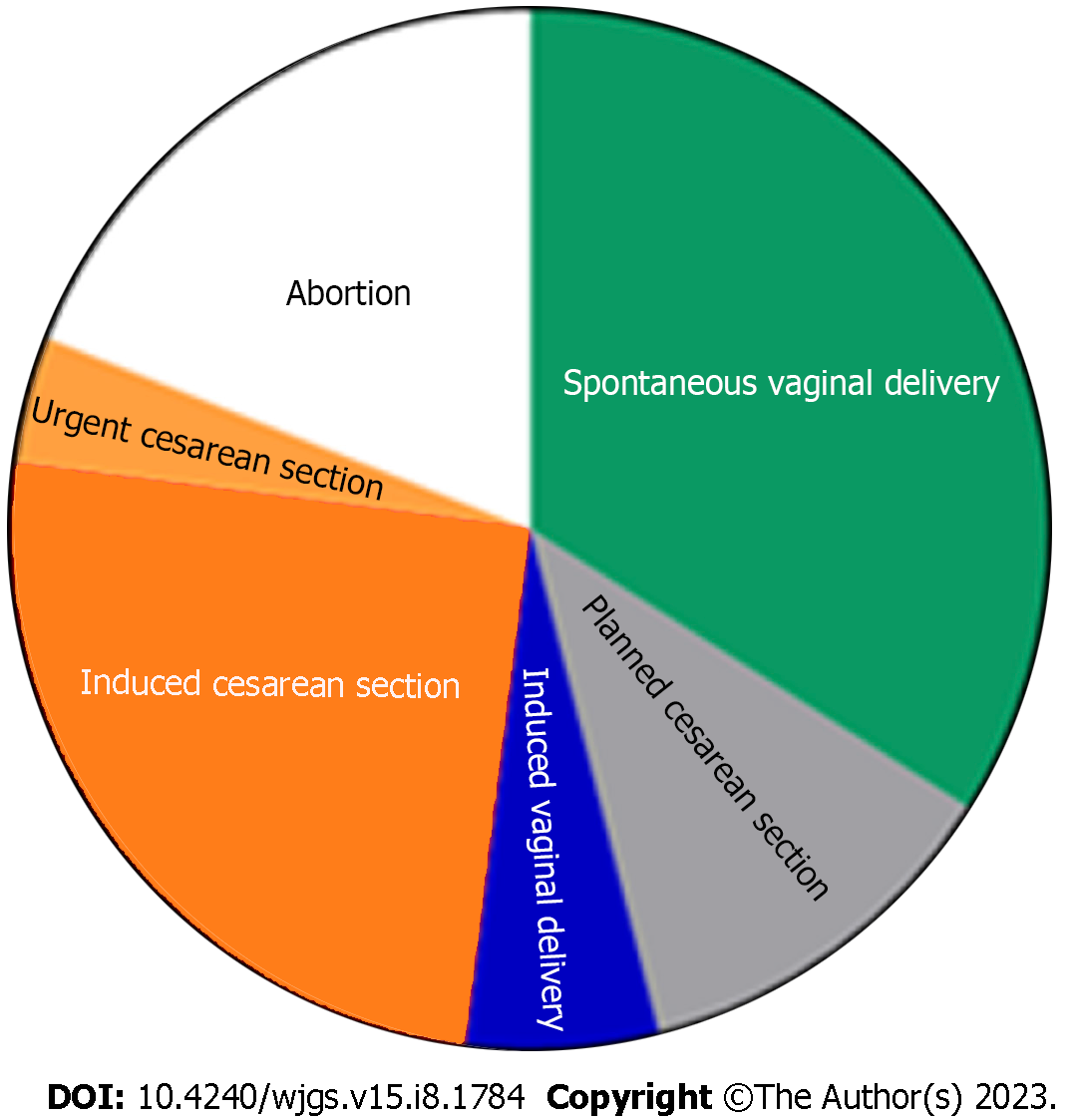
The PRISMA flow diagram (Figure 1) shows a total of 153 screened articles, of which 71 met the inclusion criteria with a total of 97 patients. The earliest report was published in 1932, and the most recent was in 2020. Half of the studies were published in the last 2 decades (Figure 2). Most cases were from Asia (n = 35), followed by Europe (n = 23) and North America (Table 1). There were ten non-English articles: Two in Chinese, two in Japanese, one in Korean, three in German, one in French, and one in Spanish. Complete patient, cyst, and treatment characteristics were described in 55% of cases. All cases reported the mother's age, maternal outcome, and gestational age at delivery (Figure 3). Type of delivery was reported in 91%, and fetal outcome in 21.6% of cases.
| Category | n (%) |
| Type of study | |
| Case reports | 56 (78.8) |
| Two cases | 8 (11.2) |
| Case series (> 2) | 7 (9.8) |
| Country | |
| United States | 21 (29.6) |
| India | 9 (12.7) |
| Japan | 8 (11.3) |
| United Kingdom | 7 (9.9) |
| China | 7 (9.9) |
| South Korea | 3 (4.2) |
| Mexico | 3 (4.2) |
| Other | 13 (18.3) |
| West vs East | 38 vs 33 (46.5 vs 53.5) |
| Year of publication | |
| < 1940 | 2 (2.1) |
| 1940-1960 | 8 (8.2) |
| 1960-1980 | 5 (5.2) |
| 1980-2000 | 20 (20.6) |
| 2000-2020 | 36 (37.1) |
| Data completion | |
| Age | 97 (100) |
| Gestational age | 97 (100) |
| Cyst size | 67 (69) |
| Cyst type | 80 (81.4) |
| Bilirubin levels | 70 (72.1) |
| Maternal outcome | 97 (100) |
| Fetal outcome | 21 (21.6) |
Patient characteristics are summarized in Table 2. The mean age was 25.3 years (range 15-40 years), and 62/83 (74.6%) patients with reported data were primigravidas. Parity was reported in 83 cases-62 women (74.6) were nulliparous, and seven had more than one previous pregnancy. Among all, eight (8.2%) were diagnosed in the first, 33 (34.8%) in the second, 40 (41.2%) in the third trimester, and nine (9.3%) in the postpartum period. In six (6.1%) cases, CC was known before conception (Caroli disease).
| Category | Results | Number of cases with reported variable |
| Age | 25.3 ± 4.03 | 97 |
| Gestational age at cyst discovery | 26.1 ± 8.2 | 96 |
| Parity | 1.37 ± 0.76 | 83 |
| 1 | 62 (74.6) | |
| 2 | 13 (15.6) | |
| > 2 | 8 (9.6) | |
| Cyst size (cm) | 12.5 ± 6.4 | 67 |
| 0-5 | 6 (8.9) | |
| 5-10 | 20 (29.8) | |
| 10-15 | 19 (28.3) | |
| 15-20 | 16 (23.8) | |
| > 20 | 6 (8.9) | |
| Bilirubin (mmol/L) | 83.2 ± 68.4 | 71 |
| Cyst type | 12.5 ± 6.4 | 84 |
| I | 62 (73.8) | |
| IV | 15 (17.8) | |
| V | 6 (7.1) | |
| Diagnostic method | 93 | |
| US | 58 (62.3) | |
| MRI | 18 (19.3) | |
| ERCP | 2 (2.1) | |
| CT | 2 (2.1) | |
| Intraoperatively | 13 (13.9) | |
| Reached gestational age (wk) | 97 | |
| 0-12 | 2 (2.2) | |
| 12-24 | 7 (7.2) | |
| 24-36 | 43 (44.3) | |
| > 36 | 45 (46.3) |
The most common symptom was epigastric/right upper quadrant pain in 78 (80%) patients (Table 3). Jaundice was present in 58 (59%) and fever in 30 (30.1%). CC triad was described in 41 (50.5%) and the Charcot triad in 28 (28.8%) patients. Eight patients were asymptomatic, and six of them were diagnosed with Caroli disease before pregnancy. Most CC were diagnosed using ultrasound (n = 58) followed by MRI (n = 18) and CT (n = 2). In two cases, endoscopic retrograde cholangiopancreatography (ERCP) was employed as a diagnostic and therapeutic tool. Data were lacking for the rest of the patients, or the diagnosis was established intraoperatively.
| Symptom | n (%) |
| Pain | 78 (81.2) |
| Jaundice | 58 (60.4) |
| Fever | 33 (34.3) |
| Nausea/vomiting | 36 (37.5) |
| Abdominal mass | 42 (43.7) |
| Asymptomatic | 8 (8.3) |
| Charcot triad (fever, jaundice, pain) | 28 (29.1) |
| Triad of choledochal cyst symptoms (abdominal mass, jaundice, pain) | 41 (42.7) |
In 28 patients (28.8%), imaging did not show dilation of intrahepatic ducts, while 22 (22.6%) had dilation. One patient had changes consistent with primary sclerosing cholangitis (PSC), while data on bile duct morphology were missing for 47 (48.4%) patients.
Due to missing data, only bilirubin level was included in the analyses. It was reported in 70 (72.1%) cases. The level was 83.2 ± 80.4 mmol/L (range 5-426 mmol/L).
According to Todani's classification, cyst type was mentioned in 79 (81.4%) cases. The type was defined from published radiologic images in an additional five cases. Out of 84 cases, 56 (66.6%) were type I, 15 (17.8%) type IV, and 6 (7.1%) type V. In one case, the cyst was described as "transitory type", not included in Todani's classification. It may indicate cyst dilation of the CBD, which resolved after biliary drainage. The cyst size was described in 67 (69%) cases with a mean size of 12.5 cm (range 1.8-40 cm). The cyst was ruptured at presentation in six cases during pregnancy and one case postpartum. Two patients with ruptured CC died, resulting in a mortality rate of ruptured CC in pregnancy of 33%.
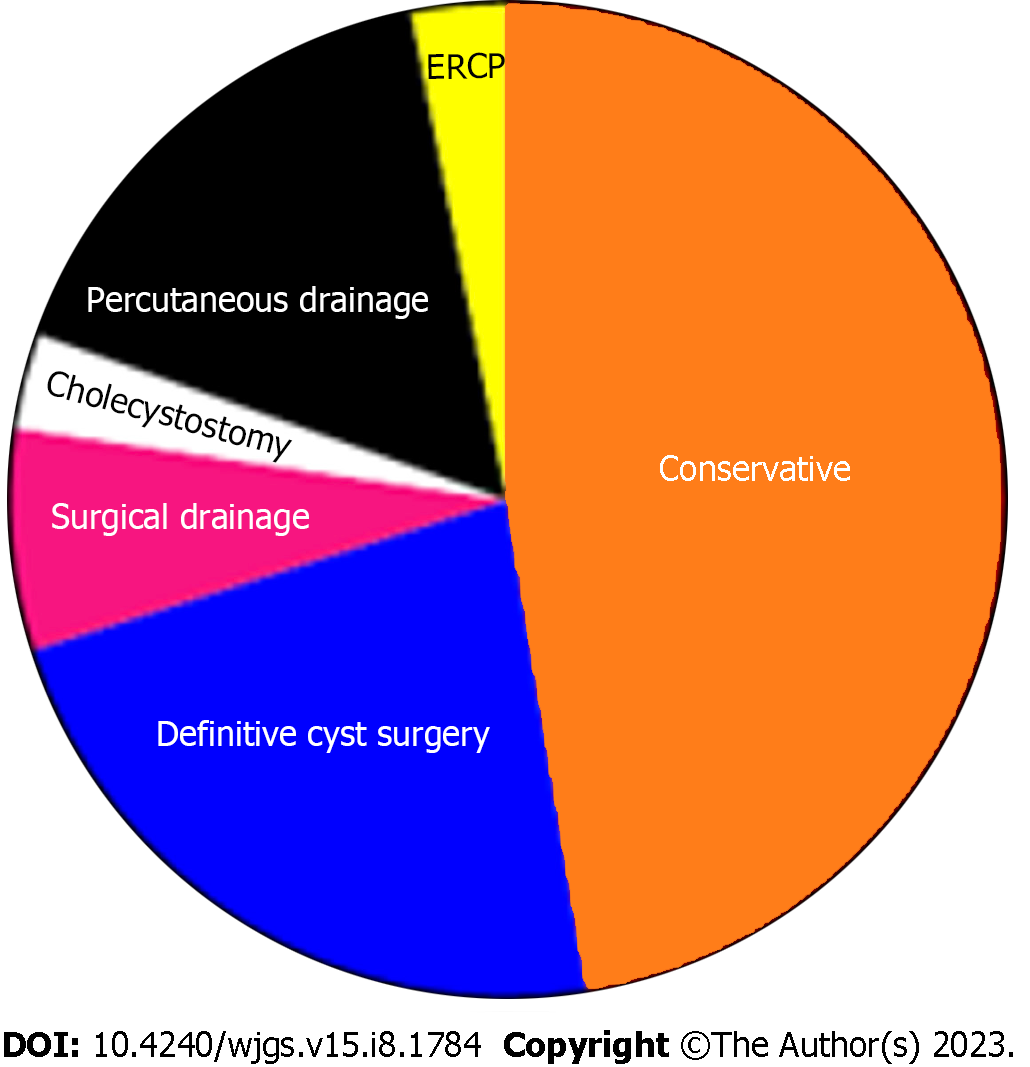
The treatment approach was divided into four categories (Table 4), according to the management of CC and the management of pregnancy (Figure 4). Among those with radiologic, endoscopic, or surgical interventions, the main indication was cholangitis, followed by cyst rupture and progressive jaundice (Table 5 and Figure 5). The indication for urgent CC surgery was cyst rupture or CC-related bleeding in 11 cases. Outside these indications, the reasons for cesarean section (CS) were severe anemia (2%) and intense abdominal pain (3%). In seven cases, no signs of fetal compromise were present, but CS was performed to prevent potential complications of CC. In 29 (29.9%) patients, the treatment was expectant for both the cyst and pregnancy. These patients were primarily treated with antibiotics, bed rest, and intravenous fluids as a bridge therapy to the anticipated labor. Urgent CS was done in 24 (25%) patients, with indications shown in Table 5. Intervention for both the CC and pregnancy was done in 16 patients, and in four patients, cyst drainage/resection was performed simultaneously with CS.
| Category | Management | |||
| Choledochal cyst | Expectative | Intervention | Expectative | Intervention |
| Pregnancy | Expectative | Expectative | Intervention | Intervention |
| Cases, n (%) | 29 (29.8) | 35 (36.0) | 17 (17.5) | 16 (16.4) |
| Category | n | % |
| CC treatment | ||
| Conservative | 46 | 47.9 |
| Intervention | 51 | 53.1 |
| Surgical resection and reconstruction | 22 | 22.9 |
| Percutaneous drainage | 16 | 16.6 |
| Surgical drainage | 7 | 7.2 |
| Cholecystostomy | 3 | 3.1 |
| ERCP | 3 | 3.1 |
| Pregnancy | ||
| Expectative approach | 67 | 69.7 |
| Vaginal labor | 35 | 36.4 |
| Planned Cesarean section | 12 | 12.5 |
| Spontaneous abortion | 2 | 2 |
| Induced vaginal labor | 3 | 3.1 |
| NR | 15 | 15.6 |
| Intervention | 33 | 34.3 |
| Cesarean section | 24 | 25 |
| Induced vaginal labor | 3 | 3.1 |
| Induced abortion/curettage | 2 | 2 |
| NR | 4 | 4.1 |
| Indications for CC management | ||
| Cholangitis | 22 | 22.9 |
| Jaundice | 9 | 9.3 |
| Rupture | 6 | 5.2 |
| Sepsis | 4 | 4.1 |
| Pylorus obstruction | 2 | 3.1 |
| Bleeding | 2 | 2 |
| NR | 9 | 9.3 |
| Indications for pregnancy management | ||
| CC complications | 11 | 11.4 |
| Fetal compromise | 6 | 6.2 |
| Severe anemia | 2 | 2 |
| Placental abruption | 2 | 2 |
| Preeclampsia | 5 | 5.2 |
| Prevention of potential CC complications | 7 | 7.2 |
The maternal outcome was mentioned in all cases. In 90 (91.7%) patients, recovery after delivery and operation was uneventful, while seven (7.2%) died during or after pregnancy (Table 6).
| Outcomes | n (%) |
| Maternal mortality | 7 (7.2)/6 (6.1)1 |
| Peritonitis, cyst rupture | 3 |
| Surgery complications | 2 |
| Not specified | 2 |
| Fetal mortality | 8 (8.2) |
| Spontaneous abortion | 2 |
| Induced abortion | 2 |
| Stillborns | 2 |
| Newborn deaths | 2 |
Three cases of maternal death were published before 1950, and all were diagnosed during surgery for CC rupture/peritonitis. Martínez-Ordaz reported two patients who died postoperatively. The first patient died of postoperative bleeding at 9 wk of gestation following emergent surgery (HJ) for a giant CC (20 cm) with cholangitis. The second patient died after upper digestive tract bleeding and ischaemic perforation in the gastric fundus and hemoperitoneum following elective Roux-en-Y HJ made 4 wk postpartum. The cause of death was not defined. The third patient, in the 7th month of pregnancy, was successfully treated for CC rupture by surgical drainage and ERCP. Still, the patient died of "some unknown cause". Another patient underwent postpartum hepaticoduodenostomy (HD) and died after 1 year from peritonitis of unknown cause (excluded from the mortality because the period is outside of puerperium). The fifth patient, at 20 wk of gestation, with typical CC symptoms and imminent abortion, underwent cyst resection and reconstruction and died from multiorgan failure and cholangitis postoperatively. In 1932, Zinninger reported a patient at 4 mo of gestation with cholangitis and CC diagnosed intraoperatively. The cyst was emptied, the anterior wall of the cyst was excised, and the defect was closed with sutures. The patient died 11 d after the operation from multiorgan failure due to progressive sepsis.
No complications related to labor were reported in patients who received interventional treatment of CC with the continuation of pregnancy. Regarding the postpartum treatment of patients who did not have definitive CC treatment during pregnancy (n = 76, 78.3%), surgical management was reported in 54 (71.0%), conservative in 8 (10.5%), while in 14 (18.4%) cases, further postpartum treatment was not specified. In surgically treated patients, after delivery, Roux-en-Y HJ was done in most cases (90.7%), followed by cystogastrostomy (5.5%) and cystoduodenostomy (3.7%). The average gestational age of pregnancy termination (induced and spontaneous) was 33.6 wk (range 9-40 wk). Fetal outcomes were reported in 21 (21.6%) cases. However, we presumed that the authors would mention fetal or newborn death, so the above mentioned fetal mortality could be reliable. From described cases, four children died postpartum, and two were stillborn. There were two spontaneous abortions (in the 8th and 20th gestational weeks) and two elective abortions (in the 20th and 14th weeks) due to cholangitis and progressive septic complications.
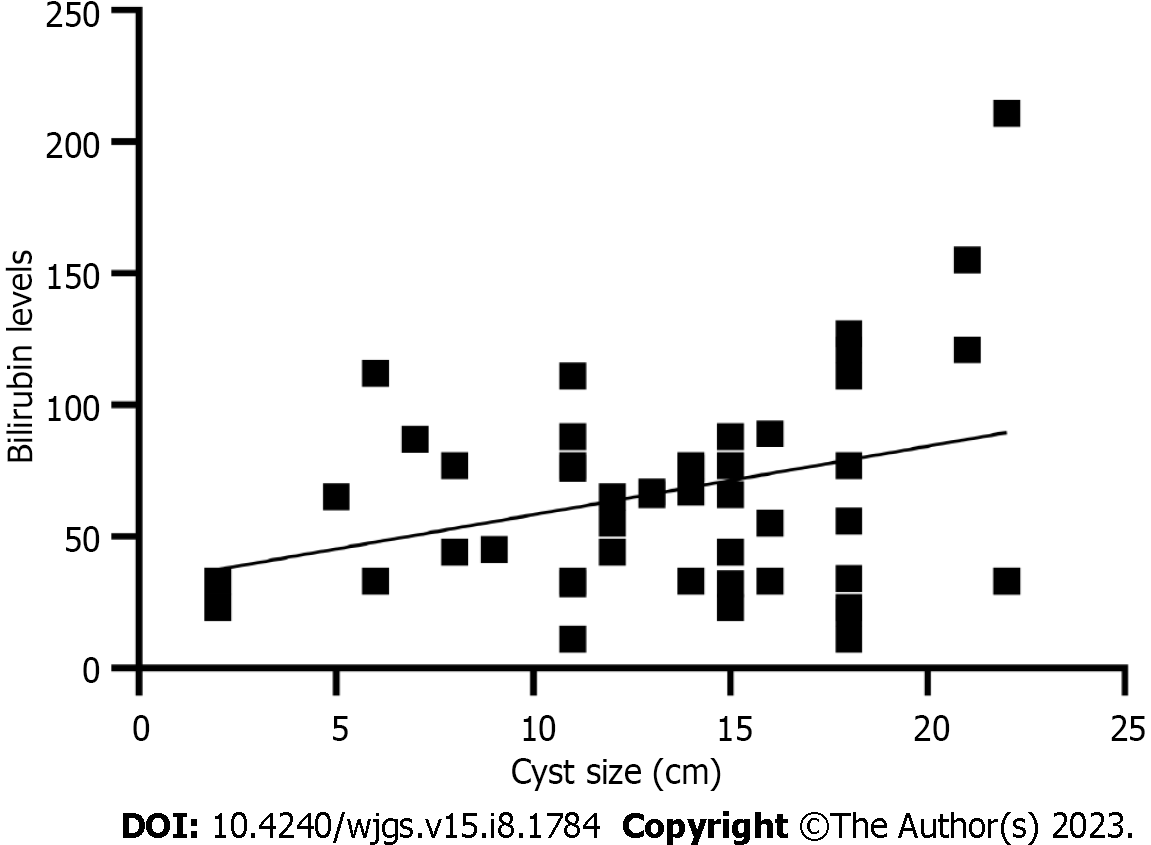
Both cyst dimension and bilirubin level were described in 51 patients with a weakly positive, statistically non-significant linear relationship (r = 0.32, P < 0.001) (Figure 6). We found no relationship between age and cyst dimension (r = 0.06, P = 0.7) for 67 patients with available data. Similarly, no correlation was found between gestational age at cyst discovery and cyst size (r = 0.11, P = 0.54).
Cyst size is not necessarily related to the severity of symptoms, but cysts larger than 15 cm had a significantly higher likelihood of treatment intervention during pregnancy. Similarly, the Charcot triad was associated with a high likelihood of CC intervention (OR = 4.3; P = 0,006), induced labor (OR = 5.4; P = 0.028), and CC triad (OR = 2.88; P = 0.017). Other analyzed factors were not associated with the odds of interventional treatment (Table 7).
| Parameter | OR | P value | 95%CI | Z statistic |
| Presence of the Charcot triad | 4.3 | 0.006 | 1.57-11.1 | 3.35 |
| Presence of the CC triad | 2.88 | 0.017 | 1.20-6.92 | 2.37 |
| Bilirubin (≥ 80 mmol/L vs < 80 mmol/L) | 1.37 | 0.78 | 1.42-15.20 | 0.84 |
| CC size (≥ 15 cm vs < 15 cm) | 4.6 | 0.011 | 0.28-3.29 | 3.72 |
| CC type (I vs IV) | 0.96 | 0.97 | 0.34-1.80 | 0.15 |
| Parity (nulliparous vs multiparous) | 0.79 | 0.57 | 0.42-2.45 | 0.55 |
| Gestational age (≥ 6 mo vs < 6 mo) | 1.01 | 0.96 | 0.41-1.66 | 0.04 |
Our study confirms the extreme rarity of maternal CC during pregnancy since less than 100 cases were collected. However, many cases of CC in pregnancy may not have been recognized or were asymptomatic, so the incidence is probably underestimated. Our collection of patients is the largest so far, and this study will contribute to a more precise estimation of the incidence of CC in pregnancy. Articles were systematically screened and reviewed, and in only two cases, we could not extract necessary data since we did not get a response from one author who presented a case in a Slovak journal[19], and we could not adequately translate a case from Alizadeh et al[20] due to the Arabic alphabet and language.
Similarities and differences between CC in the general and pregnant populations exist. First, the distribution of CC types appears similar. In pregnancy, type I is the most common (73.8%), followed by type IV, comparable to the general population, where type I ranges between 80%-90%. Types II and III have not been reported, while Caroli disease occurred in six cases, corresponding to an incidence of 7.1%, several times higher than that in the general population (1%-2%)[2,3,21]. Four cases of Caroli disease were managed expectantly, and elective CS was performed in two cases, indicating a better prognosis than that in newly diagnosed symptomatic CC type I or IV. However, a better prognosis of Caroli disease in pregnancy may be attributed to a milder clinical course than other CC types.
Second, the CC size differed compared to that in the general population. In our study, more than two-thirds of CC could be classified as giant since the largest diameter was > 10 cm. The mean reported size in the general population is 3-4 cm, rarely exceeding 9 cm[4,11,15]. Hormonal changes and the compressive effect of the gravid uterus on CC with consequent cyst enlargement could be causative. To confirm this, serial sonographic exams and measurements of CC size in pregnant women in future studies are mandatory. Pregnancy may trigger CC symptoms in previously asymptomatic cases due to increasing CC size, which, along with physiological changes during pregnancy, may precipitate symptoms related to CC, such as jaundice, abdominal pain, or abdominal mass. This hypothesis is supported by the fact that almost 91% of CC from our study were discovered in the 2nd or 3rd trimester when the gravid uterus reaches the upper abdomen[9]. There is no data on the incidence of CC triad in the general population for females. However, a typical CC triad in more than 40% of women in our study indicates that the CC triad could be several-fold higher in pregnant women than in the general population (10%-20%)[11,12]. The incidence of CC rupture in our study was 7/97 (7.1 %), which is higher than that in the general population (1%-2%)[10]. Cyst rupture was seen in six cases during pregnancy and one postpartum. Most (5/7) were reported in studies before 1990. Today, the wide availability of ultrasound, more frequent biochemical laboratory testing, and closer monitoring of pregnant women should maximally reduce the rate of severe consequences of CC. No CC rupture was noted during spontaneous or induced vaginal labor. Therefore, it remains debatable if vaginal delivery increases the risk of CC rupture.
Nausea and vomiting were reported in 37.5% of cases, comparable to previous studies[6,11,22]. Interpretation of these symptoms is difficult as it is a common consequence of hormonal changes during pregnancy. CC resulting in duodenal or pyloric compression were an indication for surgery in three cases. Abdominal distension or mass may be more prominent than that in nonpregnant patients with CC. However, the women may neglect this symptom as it may be attributed to a normal pregnancy. The pain in the upper abdomen or right subcostal area should result in evaluating potential causes other than physiological pressure from the growing uterus. ICP, acute fatty liver of pregnancy, or other cholestatic disorders may contribute to the complexity of CC diagnosis and treatment (3% of pregnant women are affected by liver disorders during pregnancy[16,23]). Hence, other liver and biliary diseases, especially cholestatic, may lead to earlier or more pronounced jaundice in CC patients or could be the only cause of cholestasis unrelated to an asymptomatic pregnant CC patient.
Due to the risk of malignancy, complete excision with biliodigestive reconstruction is the procedure of choice for nonpregnant patients with type I, II, or IV CC. Type III cysts may be managed by ERCP or endoscopic resection[5,24,25]. Our results show that Roux-en-Y HJ was a predominant operation in patients requiring cyst resection and reconstruction (92%). In two cases, HD was reported, and hepaticogastrostomy in one[26-28].
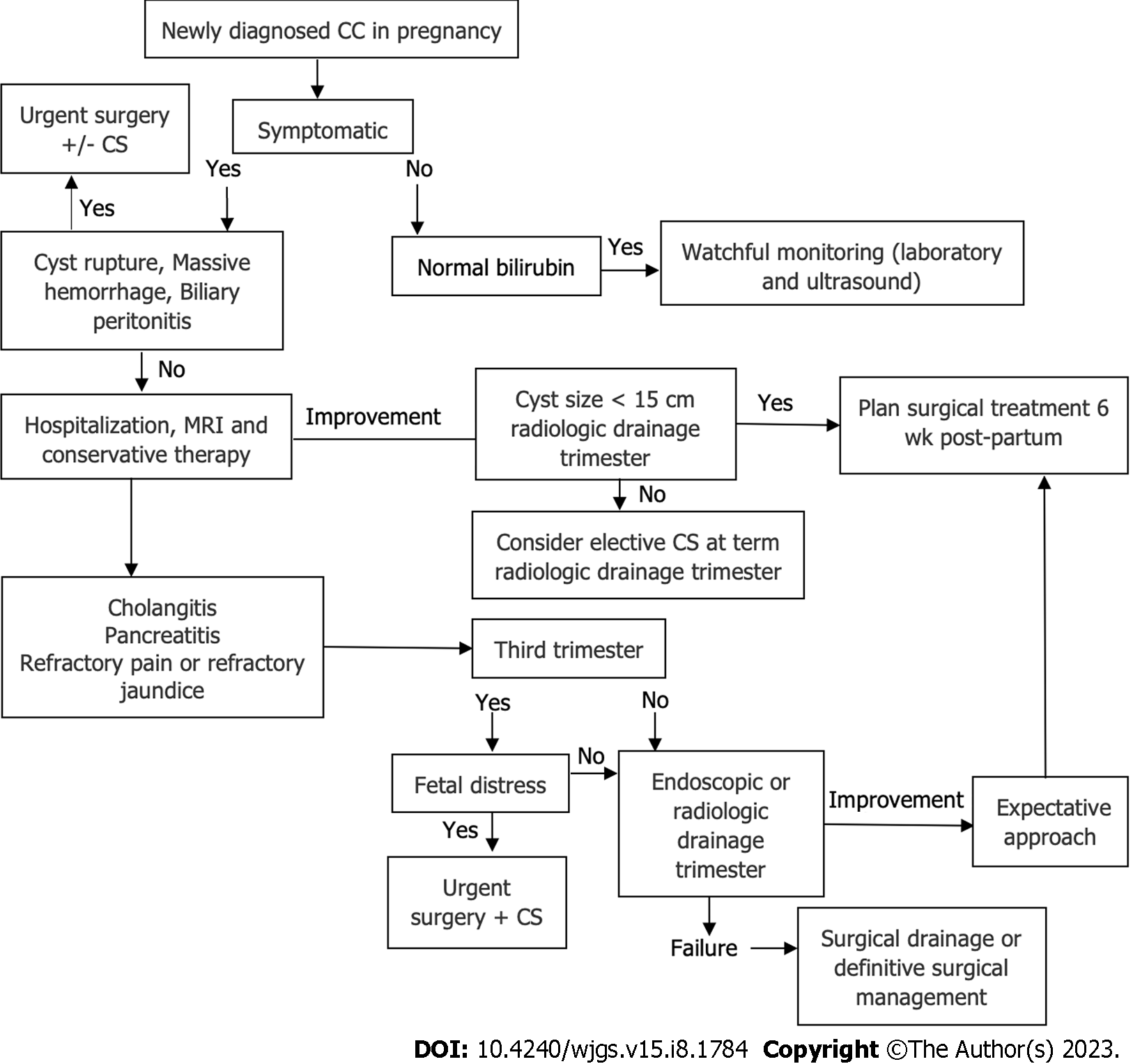
The variety of reported treatment approaches indicates no standardized management for such a clinical condition. Treatment was based on previous knowledge and experience related to CC in nonpregnant adults and general obstetric principles. The application of novel and minimally invasive percutaneous or endoscopic methods depended on the institutional availability of these procedures and the year of publication. Since this is a retrospective analysis, no control groups were available, and reliable comparative analysis of treatment approaches was not possible. However, by analyzing the severity of the clinical presentation, chosen medical approach, and outcomes, we constructed an algorithm that may help to guide clinical decisions (Figure 7).
The treatment in asymptomatic patients or patients with smaller CC without cholangitis or gastric outlet obstruction includes observation until term, with planned postpartum CC management. Almost half cases (47.3%) did not require any intervention for CC. Even when the labor was induced, in most cases, it was indicated more as a precaution than urgent obstetric indication, reported in only 39%. A prerequisite for conservative treatment is the absence of surgical or obstetric urgencies. It includes antibiotic therapy for septic complications, hospitalization, and more frequent follow-ups after discharge to prevent delayed identification of fetal compromise or CC rupture.
The decisive clinical characteristics that guided the treatment approach were the presence of cholangitis, cyst size, and to a lower degree, the presence of CC triad (Table 7). Parity, the mother's age, or jaundice alone did not affect the treatment choice. In cases of symptomatic CC, a conservative approach should be initiated, comprising analgesia, antibiotics, dietary changes, and ursodeoxycholic acid (in Caroli disease). Further steps depend on the course of the disease.
In only 22.9% of cases, open surgery, cyst excision, and reconstruction were done during pregnancy. The majority (81.0%) were operated on in the first trimester (54.5%). Possibly, most authors adopted a more conservative approach, particularly in the 2nd and 3rd trimesters. This is due to: (1) The minimization of the risk of complications from major surgery; and (2) the availability of less invasive methods for preventing CC complications.
In patients who require intervention, the minimally invasive methods have a high success rate. Only 3/22 (13.6%) cases required surgery for CC complications after unsuccessful percutaneous drainage/ERCP. Percutaneous cyst drainage may resolve symptoms, decrease the risk of cholangitis, and allow further intrauterine fetal development until viable gestational age[8,29,30]. On the other side, such procedures may carry a risk of pregnancy complications, although no severe complications of percutaneous drainage were reported in the present study. It remains unclear whether endoscopic or radiologic drainage should be performed for asymptomatic or mildly symptomatic cysts during the 3rd trimester since such procedures may trigger premature labor, while commonly, symptoms resolve on conservative measures.
Surgical urgencies (cyst rupture/peritonitis, massive bleeding, or severe cholangitis) mandate immediate "damage control" procedures such as internal/external cyst drainage, T-drainage, or cholecystostomy. Synchronous CS and cyst excision plus HJ were done in only two cases in the earlier study period. Therefore, synchronous surgical and obstetric procedures should be done exceptionally.
In conservatively treated women, the timing of surgery after the delivery was 6-8 wk postpartum in most cases. No cases of CC complications during this "waiting" period were reported, so we consider this to be a sufficient recovery time for the patient without increased CC complications related to delayed management.
The treatment of Caroli disease generally differs from that of other CC types in the general popu
Before the wide clinical usage of transabdominal ultrasound, CC during pregnancy was diagnosed intraoperatively. Since 1976, radiologic methods have enabled preoperative CC diagnosis. MRI became the common diagnostic method for pregnancy disorders in developed countries after 2000[38,39]. Consequently, cases from the first half of the 20th century were mostly diagnosed intraoperatively or at autopsy. Although emergent MRI is still not widespread, we strongly recommend MRI for newly sonographically detected CCs, as MRI defines the exact size and location of the cyst, stones, concomitant biliary anomalies, and compressive effect on surrounding organs, including the uterus.
The maternal mortality from CC was 7.2 %, but the accurate mortality appears lower since, in only four cases, a direct association with CC was confirmed. Publication bias (not reporting unfavorable outcomes) may have affected final mortality rates. In addition, in five/seven fatal cases, important variables (bilirubin levels and cyst size) were not reported. Therefore, inferential analysis related to predictive factors for maternal mortality was not appropriate in this setting, particularly if we consider the study design that covered a long period (1932-2020) which is the main reason for heterogeneous diagnostic and therapeutic approaches. Clearly, in earlier studies, the diagnosis was commonly made intraoperatively. Radiologic, endoscopic, and intensive care procedures improved significantly over time[35,40,41]. Consequently, fetal and maternal mortality rates and complications are incomparable to results from more recent studies. Instead, we presented shortly every single case of maternal mortality in the Results section and used multivariate analysis of factors that contributed to treatment choice.
There are several limitations to the study. The first is missing data. In many cases, laboratory data and fetal outcome information are sparse. Indications for CS were not mentioned in many cases, as well as information on clinical presentation and cyst pathology. However, the most important CC characteristics and maternal outcomes were reported at acceptable rates. The second limitation is the inability to collect all cases due to language or unavailable full text. Third, a more thorough comparative statistical analysis could not be conducted regarding the design of the study, long study period, lack of data, and different treatment standards.
CC in pregnancy present a great therapeutic dilemma and an under-represented topic in the literature. Our results might contribute to understanding the behavior of CC in pregnancy. Although maternal death was rare, it can occur and was reported predominantly in studies before 1990. Without cholangitis or urgent surgical CC complications, premature labor and fetal loss are uncommon. Treatment should be tailored to the individual patient and primarily based on gestational age, the risk of septic complications, and CC rupture. Careful monitoring and early recognition of CC complications are crucial for favorable maternal and fetal outcomes. CS is indicated when fetal distress, surgical emergencies, progressive cholangitis, or refractory symptoms related to CC exist. In other cases, conservative or minimally invasive CC treatment and an expectant obstetric approach should be the preferred initial approach.
No systematic data on choledochal cysts (CC) in pregnancy exist.
Due to the rarity, no guidelines exist for the diagnostic workup and treatment and obstetric strategy for CC in pregnancy.
To collect the most published case reports on CC in pregnancy.
Descriptive statistics of available patient and disease data.
Cholangitis, CC > 15 cm, and bilirubin levels > 80 mmol/L were associated with a higher likelihood of urgent cesarean section (CS) and surgical intervention for CC. Bilirubin levels positively correlated with CC size. There was no correlation between age and cyst dimension, gestational age at cyst discovery, and CC size.
Although rare, maternal CC in pregnancy should be included in the evaluation of jaundice with upper abdominal pain. Symptomatology and clinical course are variable. Treatment may range from an expectative approach to emergent surgical CC treatment and urgent CS. While most cases were managed by conservative measures or drainage procedures, CC > 15 cm and progressive cholangitis carry the risk of CC rupture and septic complications, which may increase the rates of unfavorable maternal and fetal outcomes.
All cases of CC in pregnancy should be published to understand better the epidemiology, etiology, specific diagnostic imaging methods, and treatment strategy of this extremely rare condition.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: World Society of Emergency Surgery.
Specialty type: Surgery
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cooper KM, United States; Kumar S, India S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang XD
| 1. | Stain SC, Guthrie CR, Yellin AE, Donovan AJ. Choledochal cyst in the adult. Ann Surg. 1995;222:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | O'Neill JA Jr. Choledochal cyst. Curr Probl Surg. 1992;29:361-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 89] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 836] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 4. | Wiseman K, Buczkowski AK, Chung SW, Francoeur J, Schaeffer D, Scudamore CH. Epidemiology, presentation, diagnosis, and outcomes of choledochal cysts in adults in an urban environment. Am J Surg. 2005;189:527-31; discussion 531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Singham J, Schaeffer D, Yoshida E, Scudamore C. Choledochal cysts: analysis of disease pattern and optimal treatment in adult and paediatric patients. HPB (Oxford). 2007;9:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 6. | Hopkins NF, Benjamin IS, Thompson MH, Williamson RC. Complications of choledochal cysts in adulthood. Ann R Coll Surg Engl. 1990;72:229-235. [PubMed] |
| 7. | Guan H, Chen R, Li D, Hoosen R, Xie S, Chen C, Jin S. Potential Risk Factors and Prognostic Evaluation of Malignant Changes Following Congenital Choledochal Cyst: a Retrospective Analysis. Indian J Surg. 2020;82:835-840. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Gomes C, Tivnan P, McAneny D, Tseng JF, Tkacz J, Sachs TE. Choledochal Cyst or Benign Biliary Dilation: Is Resection Always Necessary? J Gastrointest Surg. 2021;25:2353-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Kronfli R, Davenport M. Insights into the pathophysiology and classification of type 4 choledochal malformation. J Pediatr Surg. 2020;55:2642-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Kato T. Etiological relationships between choledochal cyst and anomalous junction of the pancreaticobiliary ductal system. Keio J Med. 1989;38:136-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Lee KF, Lai EC, Lai PB. Adult choledochal cyst. Asian J Surg. 2005;28:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Lavenson RS. Cyst of the common bile duct. Am J Med Sci. 1909;137:563-570. |
| 13. | Kato T. Experimental studies on pathogenesis of idiopathic dilatation of the biliary duct. J Jpn Soc Pediatr Surg. 1972;7:533-548. |
| 14. | Babbitt DP. [Congenital choledochal cysts: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb]. Ann Radiol (Paris). 1969;12:231-240. [PubMed] |
| 15. | Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 367] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 16. | Hiatt JR, Hiatt JC, Williams RA, Klein SR. Biliary disease in pregnancy: strategy for surgical management. Am J Surg. 1986;151:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Maringhini A, Ciambra M, Baccelliere P, Raimondo M, Orlando A, Tinè F, Grasso R, Randazzo MA, Barresi L, Gullo D, Musico M, Pagliaro L. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med. 1993;119:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Fulton IC, Douglas JG, Hutchon DJ, Beckett GJ. Is normal pregnancy cholestatic? Clin Chim Acta. 1983;130:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Bakos E, Mlyncek M, Dubaj M, Durcanský D, Bakos M, Korcek J, Jankovic T. [Choledochal cyst complication of pregnancy]. Rozhl Chir. 2007;86:236-240. [PubMed] |
| 20. | Alizadeh SA, Ahrari KH, Alizadeh MR. A case report on the choledochal cyst in pregnancy. J Shahid Sadoughi Univ Med Sci. 2014;22:1586-1591. |
| 21. | Liu Y, Yao X, Li S, Liu W, Liu L, Liu J. Comparison of therapeutic effects of laparoscopic and open operation for congenital choledochal cysts in adults. Gastroenterol Res Pract. 2014;2014:670260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Shi W, Yang AM. Caroli disease: an update on pathogenesis. Chin Med J (Engl). 2021;134:2844-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Yuan H, Dong G, Zhang N, Sun X, Zhao H. Minimally invasive strategy for type I choledochal cyst in adult: combination of laparoscopy and choledochoscopy. Surg Endosc. 2021;35:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Alatise OI, Owojuyigbe AM, Omisore AD, Ndububa DA, Aburime E, Dua KS, Asombang AW. Endoscopic management and clinical outcomes of obstructive jaundice. Niger Postgrad Med J. 2020;27:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Ullah AO, Amin MR. Study of Operative Events and Time Requirement of Hepaticoduodenostomy for the Treatment of Type I Choledochal Cyst- the Experience at BSMMU Hospital. Mymensingh Med J. 2023;32:454-458. [PubMed] |
| 26. | Ackermann T, Spilias D. Type VI choledochal cyst: a rare entity. ANZ J Surg. 2020;90:E215-E216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Zheng X, Gu W, Xia H, Huang X, Liang B, Yang T, Yang S, Zeng J, Dong J. Surgical treatment of type IV-A choledochal cyst in a single institution: children vs. adults. J Pediatr Surg. 2013;48:2061-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Cerwenka H. Bile duct cyst in adults: interventional treatment, resection, or transplantation? World J Gastroenterol. 2013;19:5207-5211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Neumayer L, Marcaccio M, Visser B; Members of the Evidence-Based Reviews in Surgery Group. Management of biliary tract disease during pregnancy. J Am Coll Surg. 2010;210:367-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | de Vries JS, de Vries S, Aronson DC, Bosman DK, Rauws EA, Bosma A, Heij HA, Gouma DJ, van Gulik TM. Choledochal cysts: age of presentation, symptoms, and late complications related to Todani's classification. J Pediatr Surg. 2002;37:1568-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Palma J, Reyes H, Ribalta J, Hernández I, Sandoval L, Almuna R, Liepins J, Lira F, Sedano M, Silva O, Tohá D, Silva JJ. Ursodeoxycholic acid in the treatment of cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 164] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Engstrom JL. Measurement of fundal height. J Obstet Gynecol Neonatal Nurs. 1988;17:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Gong L, Qu Q, Xiang X, Wang J. Clinical analysis of 221 cases of adult choledochal cysts. Am Surg. 2012;78:414-418. [PubMed] |
| 34. | Bhavsar MS, Vora HB, Giriyappa VH. Choledochal cysts : a review of literature. Saudi J Gastroenterol. 2012;18:230-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Jordan PH Jr, Goss JA Jr, Rosenberg WR, Woods KL. Some considerations for management of choledochal cysts. Am J Surg. 2004;187:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Kim JW, Moon SH, Park DH, Lee SS, Seo DW, Kim MH, Lee SK. Course of choledochal cysts according to the type of treatment. Scand J Gastroenterol. 2010;45:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Stipsanelli E, Valsamaki P, Tsiouris S, Arka A, Papathanasiou G, Ptohis N, Lahanis S, Papantoniou V, Zerva C. Spontaneous rupture of a type IVA choledochal cyst in a young adult during radiological imaging. World J Gastroenterol. 2006;12:982-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Ulas M, Polat E, Karaman K, Dalgic T, Ercan M, Ozer I, Teke Z, Ozogul YB, Bostanci EB, Parlak E, Akoglu M. Management of choledochal cysts in adults: a retrospective analysis of 23 patients. Hepatogastroenterology. 2012;59:1155-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | O'Neill JA Jr, Templeton JM Jr, Schnaufer L, Bishop HC, Ziegler MM, Ross AJ 3rd. Recent experience with choledochal cyst. Ann Surg. 1987;205:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Todani T, Watanabe Y, Toki A, Urushihara N, Sato Y. Reoperation for congenital choledochal cyst. Ann Surg. 1988;207:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Soares KC, Arnaoutakis DJ, Kamel I, Rastegar N, Anders R, Maithel S, Pawlik TM. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg. 2014;219:1167-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |