Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1761
Peer-review started: March 5, 2023
First decision: April 13, 2023
Revised: May 2, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: August 27, 2023
Processing time: 173 Days and 3.3 Hours
Reflux esophagitis is a common postoperative complication of proximal gastrectomy. There is an urgent need for a safer method of performing esophageal-gastric anastomosis that reduces the risk of reflux after proximal gastrectomy. We hypothesize that a novel technique termed esophagogastric asymmetric anastomosis (EGAA) can prevent postoperative reflux in a safe and feasible manner.
To observe a novel method of EGAA to prevent postoperative reflux.
Initially, we employed a thermal stress computer to simulate and analyze gastric peristalsis at the site of an esophagogastric asymmetric anastomosis. This was done in order to better understand the anti-reflux function and mechanism. Next, we performed digestive tract reconstruction using the EGAA technique in 13 patients who had undergone laparoscopic proximal gastrectomy. Post-surgery, we monitored the structure and function of the reconstruction through imaging exams and gastroscopy. Finally, the patients were followed up to assess the efficacy of the anti-reflux effects.
Our simulation experiments have demonstrated that the clockwise contraction caused by gastric peristalsis and the expansion of the gastric fundus caused by the increase of intragastric pressure could significantly tighten the anastomotic stoma, providing a means to prevent the reverse flow of gastric fluids. Thirteen patients with esophagogastric junction tumors underwent laparoscopic proximal gastrectomy, with a mean operation time of 304.2 ± 44.3 min. After the operation, the upper gastroenterography in supine/low head positions showed that eight patients exhibited no gastroesophageal reflux, three had mild reflux, and two had obvious reflux. The abdominal computed tomography examination showed a valve-like structure at the anastomosis. During follow-up, gastroscopy revealed a closed valve-like form at the anastomosis site without stenosis or signs of reflux esophagitis in 11 patients. Only two patients showed gastroesophageal reflux symptoms and mild reflux esophagitis and were treated with proton pump inhibitor therapy.
EGAA is a feasible and safe surgical method, with an excellent anti-reflux effect after proximal gastrectomy.
Core Tip: Reflux esophagitis is a common postoperative complication after proximal gastrectomy that can seriously affect the quality of life of these patients. We studied the novel surgical procedure termed esophagogastric asymmetric anastomosis (EGAA) as a potential solution to this post-surgery complication. Post-operatively, the results of upper gastroenterography showed no signs of gastroesophageal reflux while abdominal computed tomography examination findings showed a valve-like structure at the anastomosis. During follow-up, gastroscopy results revealed a closed valve-like form at the anastomosis site without stenosis or signs of reflux esophagitis. Our data suggest that EGAA is a feasible and safe procedure with excellent anti-reflux outcomes after proximal gastrectomy.
- Citation: Pang LQ, Zhang J, Shi F, Pang C, Zhang CW, Liu YL, Zhao Y, Qian Y, Li XW, Kong D, Wu SN, Zhou JF, Xie CX, Chen S. Anti-reflux effects of a novel esophagogastric asymmetric anastomosis technique after laparoscopic proximal gastrectomy. World J Gastrointest Surg 2023; 15(8): 1761-1773
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1761.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1761
Gastric cancer is a common malignant tumor of the gastrointestinal tract, with over 1 million new cases and 769000 deaths globally in 2020. It ranks fifth in terms of incidence and fourth in terms of mortality among malignant tumors[1]. Interestingly, the proportion of proximal gastric cancer (including upper gastric cancer and adenocarcinoma of the esophagogastric junction) has been on the rise for the past 40 years[2-5].
For early esophagogastric junction tumors, esophageal-residual gastric anastomosis after proximal gastrectomy is an effective method to reconstruct the digestive tract. Compared to total gastrectomy, this method maintains normal anatomy and functionality of the stomach and duodenum, and improves nutritional status and quality of life of patients post-operatively[6,7].
However, the loss of anti-reflux function due to surgical resection of the cardia, the decline in gastric peristalsis due to vagotomy, and the emptying delay caused by preserved pylorus can lead to several postoperative complications such as intractable reflux esophagitis, anastomotic stomatitis and stenosis, Barrett esophagus, and esophageal cell carcinoma[8-12], impacting quality of life post-surgery.
To address reflux esophagitis, several different gastrointestinal reconstruction modalities have been developed over the years. The primary anti-reflux surgical modalities in current use involve several common strategies[13-16]: (1) Extending the distance to reduce reflux using jejunal inter-positioning or double tract reconstruction; (2) reducing discharge resistance to reduce reflux, such as pyloric molding; and (3) in the esophageal-residual gastric anastomosis, improving anti-reflux function by rebuilding the structure of the gastric base and anastomotic opening using approaches such as the double-flap technique (Kamikawa anastomosis) or removing most of the stomach to reduce gastric acid secretion, via gastric tube reconstruction.
However, each of these techniques has its own limitations in terms of insufficient effectiveness of anti-reflux in the supine position, inadequate storage function or delayed emptying of the residual stomach, inadequate flow of food through the duodenum, and complexity of surgical approach[17-20].
Esophagogastrostomy is considered the simplest reconstruction method used after proximal gastrectomy because it requires only one anastomosis, allowing easy postoperative endoscopic surveillance. A survey of 145 Japanese medical institutions showed that esophagogastric anastomosis was chosen for gastrointestinal reconstruction in approximately 50% of medical institutions[21,22]. However, it is not widely accepted because of severe postoperative complications such as reflux esophagitis and anastomotic stricture[11,12].
To improve the procedure of esophagogastric anastomosis to prevent reflux after proximal gastrectomy, we designed and implemented a novel reconstruction technique called esophagogastric asymmetric anastomosis (EGAA) based on years of clinical experience. Additionally, we established the EGAA mode to study the anti-reflux theory mechanism of asymmetric anastomosis aided by computer simulation technology using finite element analysis.
Herein, we present the technical details of EGAA and elaborate on its short-term outcomes after laparoscopic anti-reflux surgery.
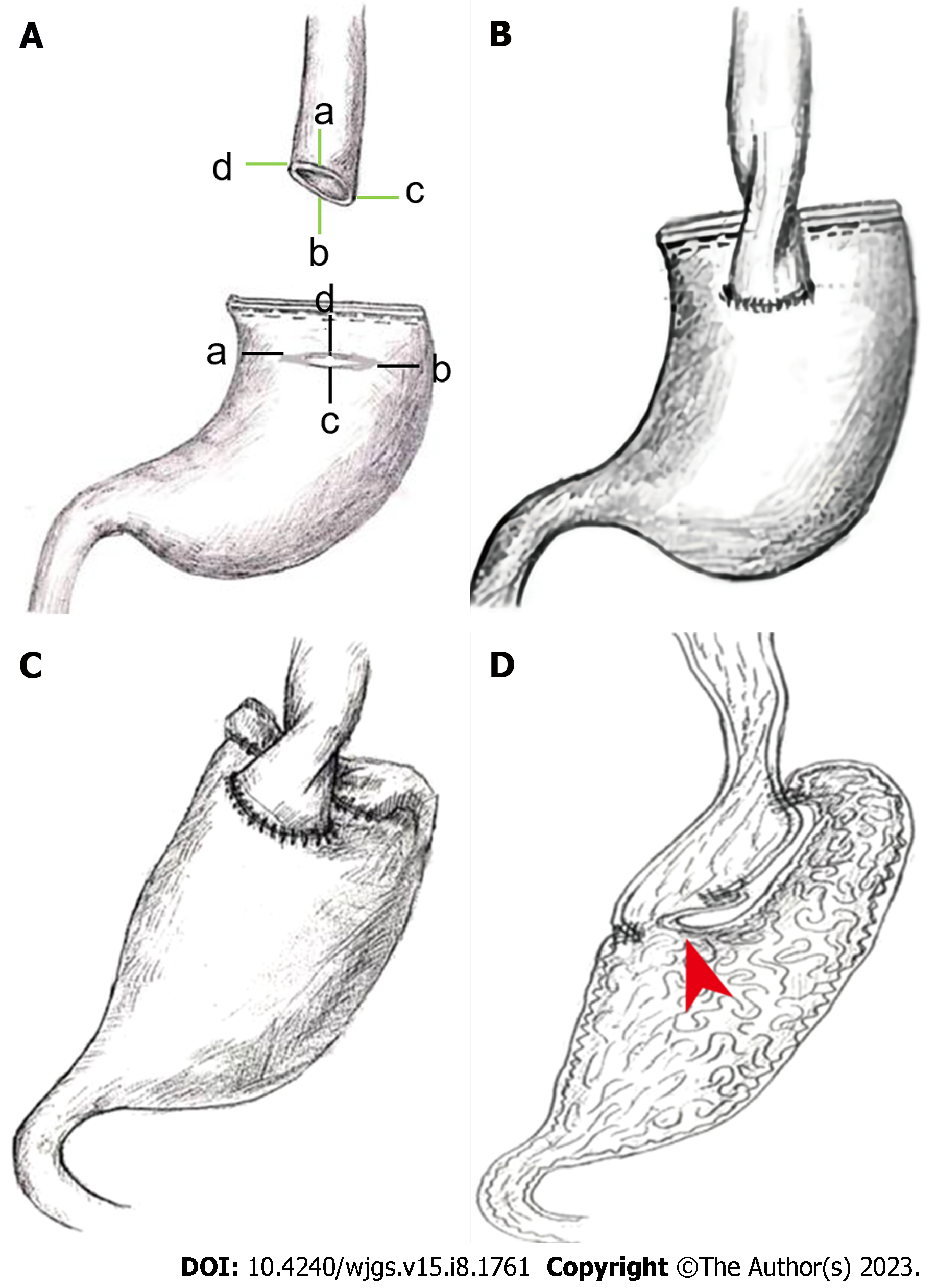
There are four critical features of the EGAA method: The asymmetric cut of the lower esophagus, the asymmetry of the esophageal diameter in relation to the incision length in the anterior wall of the residual stomach, asymmetrical torsion of the esophagus with respect to the residual stomach, and asymmetrical suturing of the seromuscular layer of the residual stomach with the esophagus.
First, because of the oblique cut of the lower esophagus, the length of the anterior wall of the esophagus at the anastomotic site is approximately 1.5 cm longer than that of the posterior wall, forming a "door and block" frame with the folded stomach parts to prevent reflux and leakage after anastomosis (Figure 1A and D).
Second, a transverse incision was made in the anterior wall of the stomach about 3.5 cm from the proximal end of the residual stomach. The length of the incision is approximately 3.2-3.5 cm that is greater than the diameter of the esophagus but equal to the length of the distal oblique incision in the flat state of the esophagus (i.e., half of the circumference of the distal oblique incision).
This design facilitates the closure of the lower end of the esophagus and helps to prevent stenosis (Figure 1A). The following formula was applied to improve the grasp of the criteria for the size of the anterior gastric wall incision:
Criterion length of gastric incision size (cm) ≈ 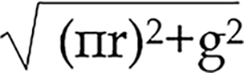 (where r is the esophageal radius and g is the gap between the front and rear lengths of the esophageal wall).
(where r is the esophageal radius and g is the gap between the front and rear lengths of the esophageal wall).
Third, the esophagus and the residual stomach are asymmetrical on the sagittal surface after suturing the lower segment of the esophagus, which has undergone a 90-degree anticlockwise torsion, to the anterior incision of the residual stomach in an end-to-side anastomosis (Figure 1B). Both the distal esophagus and anastomotic sites tended to close in a resting state.
Finally, in the posterior wall of the anastomosis, the edge of the gastric stump (about 3.0 cm from the anastomosis) is sutured to the seromuscular layer of the esophagus (0.5-1.0 cm from the anastomosis) to form a flap-like structure by folding the wall of the partial residual stomach in the gastric lumen.
The criteria for this procedure were as follows: Except for the gastric margin of approximately 0.3 cm for the anastomosis, the distance between the gastric stump margin and the suture site of the posterior esophageal wall must be greater than 2.5 cm. The length of the valve-like structure formed by the folded gastric wall must be longer than 1.0 cm to ensure the effectiveness of closure.
In the anterior wall of the anastomosis, the seromuscular layer of the residual stomach and esophagus was sutured, pushing the anterior lip toward the posterior lip of the anastomosis and increasing the tendency for anastomotic closure in synergy with asymmetrical suturing of the posterior wall of the anastomosis (Figure 1C and D).
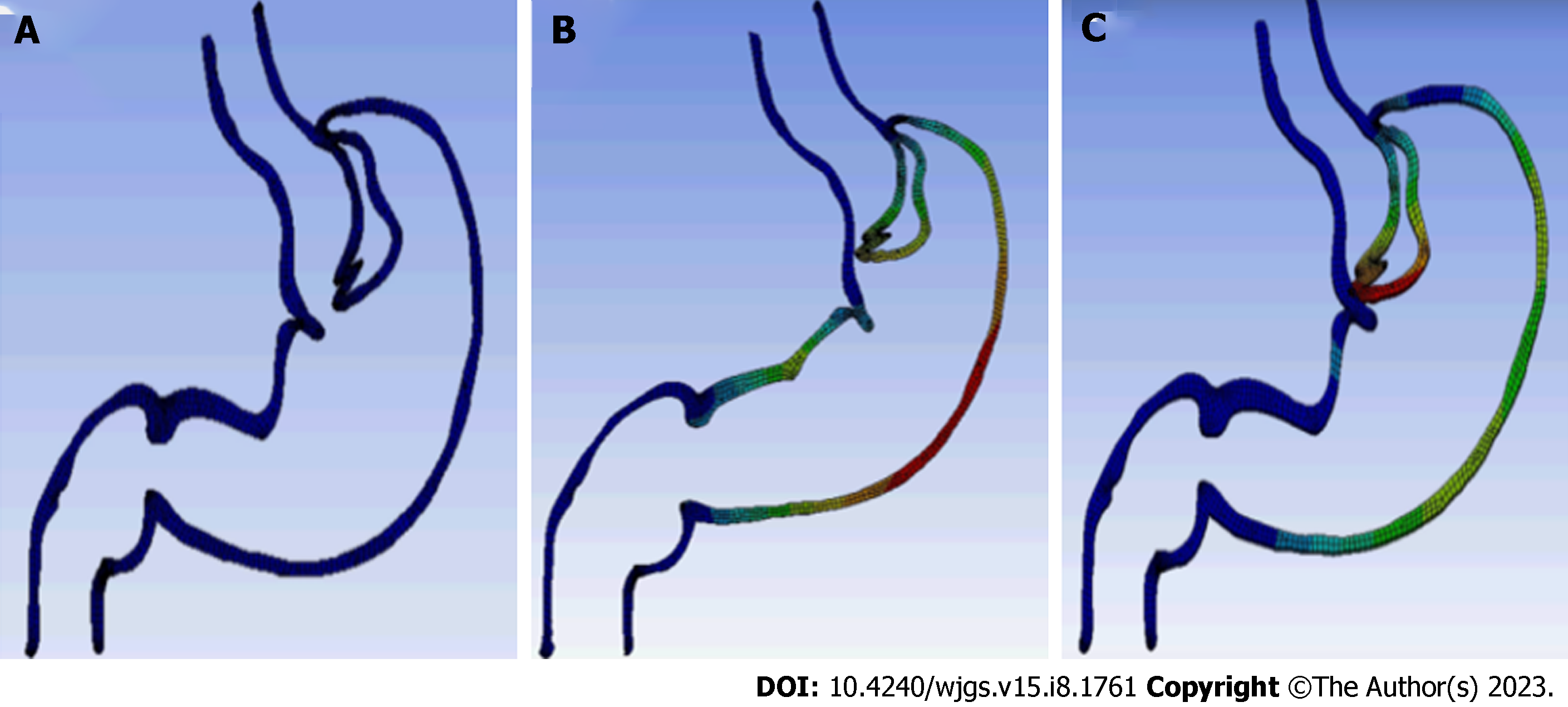
To determine whether the procedure for asymmetric anastomosis of the esophagus and stomach could achieve the expected outcome theoretically, simulations were performed according to the procedure for EGAA (Figure 1D). The contraction movement of the stomach was modeled by applying thermal strain. Rubber materials were used to simulate the elastic behavior of the stomach and esophagus. Additionally, the esophageal and duodenal ends are restricted as displacement boundary conditions, and the pressure inside the stomach was also considered.
Adobe Illustrator was used to draw the curve of the gastric section (Figure 2A). The gastric curve was then transferred into SolidWorks to generate the geometric model, and this was then used to perform the finite element simulation using the ANSYS Workbench.
Thirteen patients (9 males and 4 females) were recruited for laparoscopic proximal gastrectomy (LPG) with EGAA at the Affiliated Huaian No. 1 People's Hospital of Nanjing Medical University between September 2021 and March 2023. One patient had a gastric stromal tumor. The other 12 patients had tumors that were histologically confirmed as adenocarcinoma of esophagogastric junction (AEG). The age range was 57–78 years (66.3 ± 7.0), with body mass index (BMI, kg/m2) ranging from 21.3-32.4 (24.5 ± 3.0).
Preoperative diagnosis and evaluation included endoscopy, upper gastrointestinal series, and computed tomography (CT). Tumor stages were classified according to the International Anti-Cancer Alliance TNM staging system[23], and lymph node stations were numbered according to the definition of the Japanese Gastric Cancer Association[24]. Surgical complications were classified according to the Craven-Tindo classification[25]. Endoscopic evaluation of esophagitis was performed using the Los Angeles classification[26].
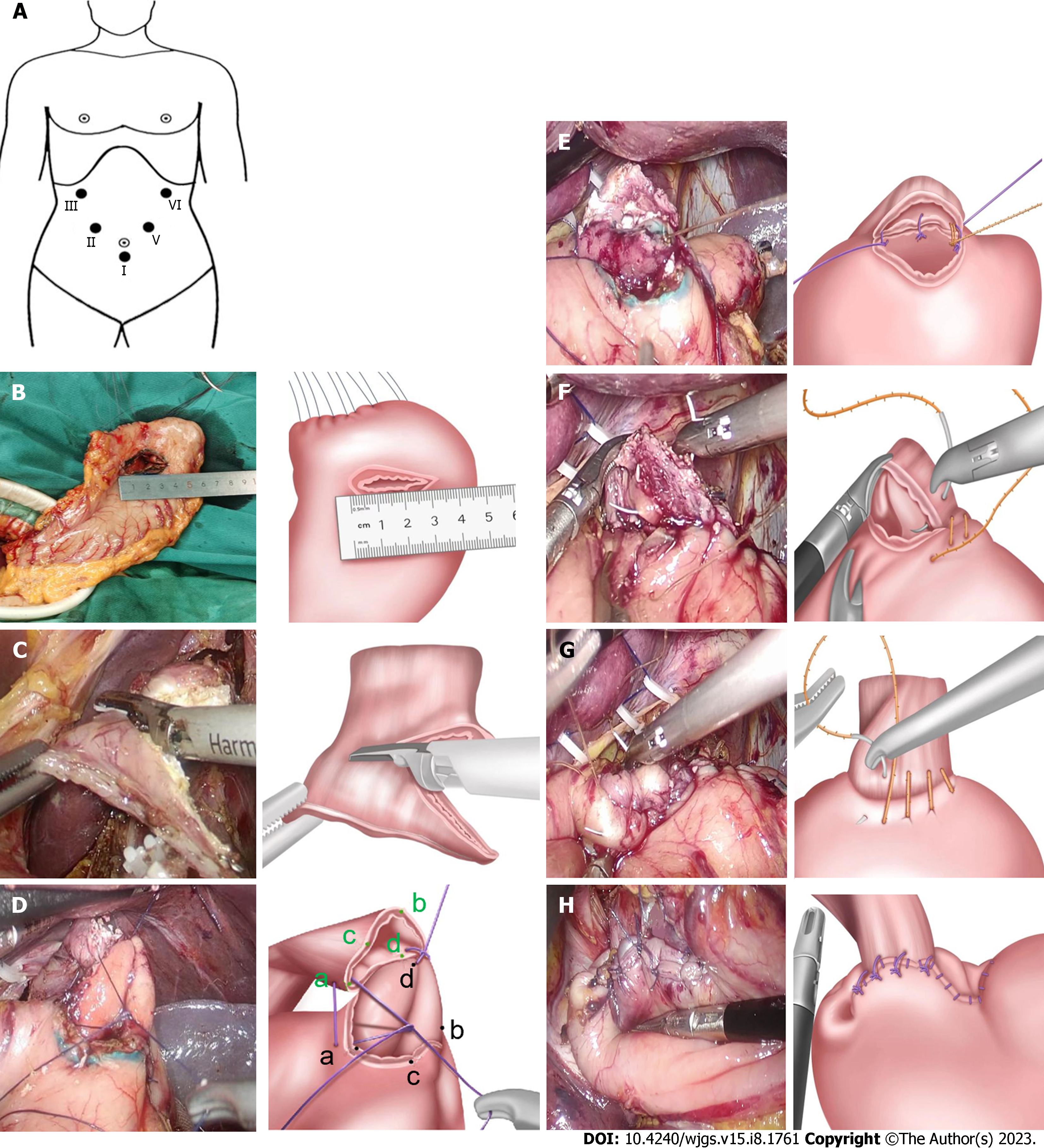
Mobilization and transection of the stomach and lymphadenectomy: Under general anesthesia, patients were placed in the reverse Trendelenburg position with their legs apart. The surgeon and the first assistant were positioned on the right and left sides of the patient, respectively. After pneumoperitoneum was established using an open technique at the umbilicus and maintained at approximately 13-15 mmHg abdominal pressure, an electro-laparoscope was introduced through the 12-mm umbilical trocar before placing the remaining four working trocars (Figure 3A).
Omentectomy was performed along the superior edge of the transverse colon. The right parts of the omentum were dissected from the mesocolon around the transition zone of lymph node (LN) stations 4d–6, and the right gastroepiploic vessels were preserved.
The origin of the left gastroepiploic vessel (LGEV) was divided and ligated using hemo-clips. Dissection of the short gastric vessels (SVG) was continued along the spleen up to the esophagogastric junction before performing lymphadenectomy, including LNs 4sb and 4sa along the LGEV and SVG. The stomach was then elevated and the peritoneum along the superior edge of the pancreas was mobilized. LNs along the left gastric artery (No. 7), common hepatic artery (No. 8a), celiac artery (No. 9), and proximal splenic artery (No. 11p) were retrieved. The root of the left gastric artery in the coronary vein was clipped and divided. The esophagogastric junction was mobilized. After complete exposure of the abdominal esophagus with the division of the anterior and posterior vagal trunks, LPG was completed by transection of the esophagus 2 cm proximal to the tumor with a 45-mm endoscopic linear stapler. The right pericardial (No. 1), left pericardial (No. 2), lesser curvature (No. 3), and lower thoracic para-esophageal nodes (No. 110) were completely retrieved using this procedure.
Proximal gastrectomy with an assisted abdominal incision: A small incision was made in the upper abdomen, and the stomach was exteriorized and stapled using a 60-mm linear cutting suture about 4 cm from the distal end of the tumor. The proximal margin of the specimen was examined pathologically when necessary. At the anterior wall 3.5 cm from the proximal end of the residual stomach, the transverse incision was marked and cut approximately 3.2-3.5 cm (Figure 3B).
Procedure of laparoscopic-assisted EGAA: The residual stomach was placed back into the abdominal cavity, the incision was temporarily closed, and pneumoperitoneum was re-established. A laparoscopic-assisted EGAA (hand suture) was performed following the critical features outlined previously.
The distal end of the esophagus was incised with an oblique short right and long left margin, with a difference of approximately 1.5 cm between the two sides (Figure 3C).
With a longitudinal anticlockwise torsion of the esophagus at 90°, an end-to-side anastomosis was performed between the cut end of the esophagus and the residual stomach incision.
First, one full-thickness intermittent suture was performed between the right side (point d) of the lower esophagus and the middle point (point d) of the posterior wall in gastric incision. Then, the right point of the gastric wall incision (point a) was sutured to the middle point (point a) of the anterior wall in the lower esophagus, and the left point (point b) of the gastric incision was sutured to the middle point (point b) of the posterior wall in the lower esophagus (Figure 3D). After this three-stitch full-layer suture was used to complete the positioning suture of the posterior wall of the EGAA, the points a/b/d of the esophageal and gastric wall incisions were aligned in the posterior wall of the anastomosis (Figure 1A), and the lower esophageal segment was rotated anticlockwise at 90°. The whole muscle layer was continuously sutured in the posterior and anterior walls of the anastomosis with ETHICON SXMD1B405 (tensile strength size 3-0) (Figure 3E and F), and the anterior wall of the anastomosis was further strengthened by a suture in the seromuscular layer (Figure 3G).
Then, 50 mL saline with 2 mL methylene blue was injected through the gastric tube (dimensioning 35 cm) to confirm that no anastomotic leak occurred.
The cut end of the residual stomach (approximately 3.0 cm from the anastomosis) was sutured with 3-5 stitchs to the posterior wall of the esophagus at a site 0.5-1.0 cm from the anastomosis in the seromuscular layer (Figure 3H). The residual stomach was sutured fixedly to the bilat with 3-5 stitchs eral diaphragmatic feet.
This study used clinicopathological, surgical, and follow-up data. All patients were counseled about the operative procedure, including the potential merits and disadvantages of our approach and the uncertainty of clinical outcomes. All patients were in stable condition and written informed consent was obtained from each patient prior to the procedure. All experimental and surgical procedures of the study were approved by the ethics committee of The Affiliated Huaian No. 1 People's Hospital of Nanjing Medical University and strictly adhered to the guidelines of the Helsinki Declaration of 1964 and its latest amendments.
Gastric peristalsis was simulated using the thermal-strain method. After decreasing the temperature, the model size was reduced by 30% in a clockwise direction. However, the suture of the incisal gastric margin and the posterior esophageal wall caused the valve-like folded gastric wall and posterior lower esophageal wall to move to the upper left, tightening of the anastomotic stoma (Figure 2B, Video 1).
To simulate the dilation of the reconstructed gastric fundus under gastric peristalsis and intragastric pressure, we applied different pressures to the gastric fundus, gastric body and antrum. During gastric peristalsis and contraction, the gas and liquid contents in the gastric cavity were observed to flow to the proximal end, increasing the pressure and expanding the gastric fundus gradually. According to the simulation results, because of the increased pressure in the gastric cavity and the expansion of the gastric fundus, the folded gastric wall and the esophageal wall moved to the left, tightening the anastomotic stoma (Figure 2C).
Following the simulations, EGAA surgeries were performed. All procedures were completed with a mean operation time of 304.2 ± 44.3 min, and the mean blood loss was 88.5 ± 46.3 mL. No intraoperative complications, conversions, or operative mortality was observed in the 13 patients. Two postoperative complications (minor grade II anastomotic leakage, cured conservatively) occurred, and patients recovered (median postoperative hospital stay: 19.2 ± 11.7 d). Patient background and surgical outcome are shown in Table 1.
| Case | Age | Gender | Preoperative BMI | Operation method | Siewert type | Operation time (min) | Anastomosis time (min) | Blood loss (mL) | Final TNM | Final stage1 | Discharge (POD) | Operative morbidity | Reflux esophagitis | Postoperative BMI | Symptom of reflux | Postoperative follow-up (mo) |
| 1 | 68 | F | 32.4 | LPG | II | 340 | 45 | 200 | T2N0M0 | IB | 12 | None | Class LA-B | 33.5 | + | 19 |
| 2 | 57 | F | 27 | LPG | III | 360 | 55 | 100 | T1N0M0 | IA | 17 | None | Class LA-B | 23.2 | + | 18 |
| 3 | 65 | M | 22.5 | LPG | II | 315 | 50 | 150 | T1N0M0 | IA | 12 | None | None | 23.7 | None | 18 |
| 4 | 64 | M | 22.1 | LPG | II | 260 | 40 | 50 | T1N0M0 | IA | 10 | None | None | 22.1 | None | 18 |
| 5 | 67 | M | 24.6 | LPG | II | 290 | 45 | 50 | T1N0M0 | IA | 11 | None | None | 24.9 | None | 12 |
| 6 | 63 | M | 25.1 | LPG | II | 270 | 45 | 50 | T3N1M0 | IIB | 45 | Grade II Anastomotic leakage | None | 25.1 | None | 9 |
| 7 | 77 | M | 26.1 | LPG | II | 320 | 50 | 100 | T3N0M0 | IIA | 14 | None | None | 24.4 | None | 9 |
| 8 | 71 | F | 21.3 | LPG | II | 280 | 45 | 100 | T1N0M0 | IA | 18 | None | None | 22.6 | None | 9 |
| 9 | 66 | M | 23.5 | LPG | I | 200 | 35 | 100 | T1N0M0 | IA | 15 | None | None | 23.5 | None | 7 |
| 10 | 57 | F | 25.2 | LPG | II | 350 | 50 | 100 | T3N0M0 | IIA | 11 | None | None | 22.3 | None | 5 |
| 11 | 57 | M | 21.5 | LPG | II | 330 | 40 | 50 | T2N1M0 | IIB | 43 | Grade II Anastomotic leakage | None | 20.3 | None | 4 |
| 12 | 72 | M | 24.4 | LPG | II | 340 | 45 | 50 | T1N0M0 | IA | 18 | None | None | 24.4 | None | 3 |
| 13 | 78 | M | 22.9 | LPG | II | 300 | 40 | 50 | T1N0M0 | IA | 24 | None | None | 22.9 | None | 2 |
| mean ± SD | 66.3 ± 6.993 | 24.51 ± 2.953 | 304.2 ± 44.34 | 45.00 ± 5.401 | 88.46 ± 46.34 | 19.23 ± 11.66 | 24.07 ± 3.121 | 10.23 ± 6.207 |
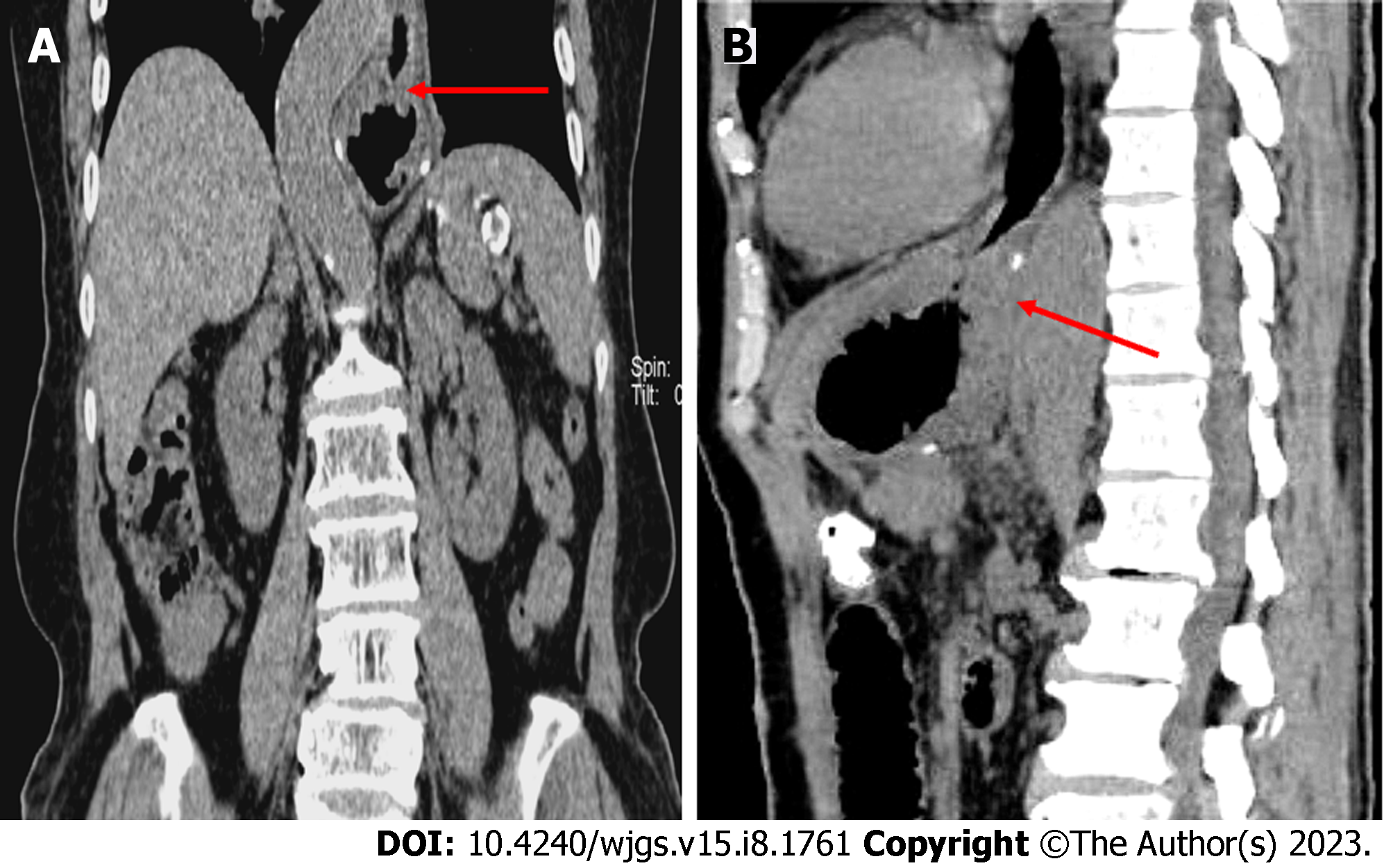
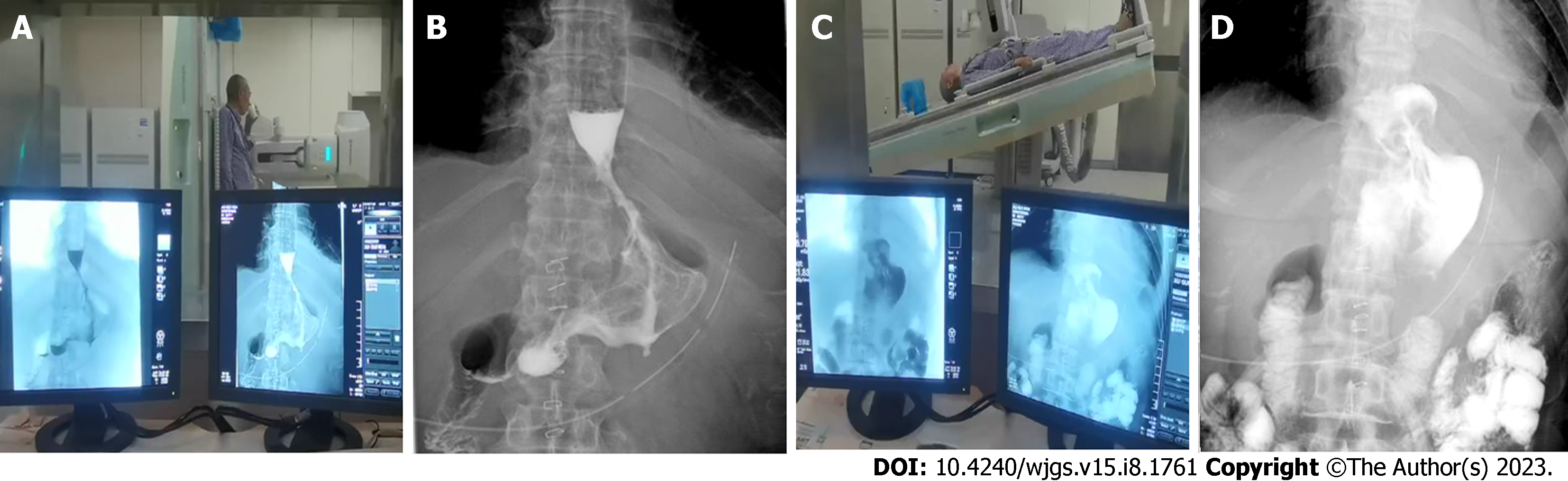
One week post-surgery, abdominal CT examination (oral CO2 powder) revealed that 11 patients had valve-like structures, inflatable stomach cavity and closed anastomosis (Figure 4). Upper gastroenterography showed good residual gastric excretion, no anastomotic leakage/stenosis, and no reflux (all patients, upright position). In supine/low head positions, eight patients exhibited no gastroesophageal reflux, three had mild reflux, and two had obvious reflux (Figure 5, Video 2).
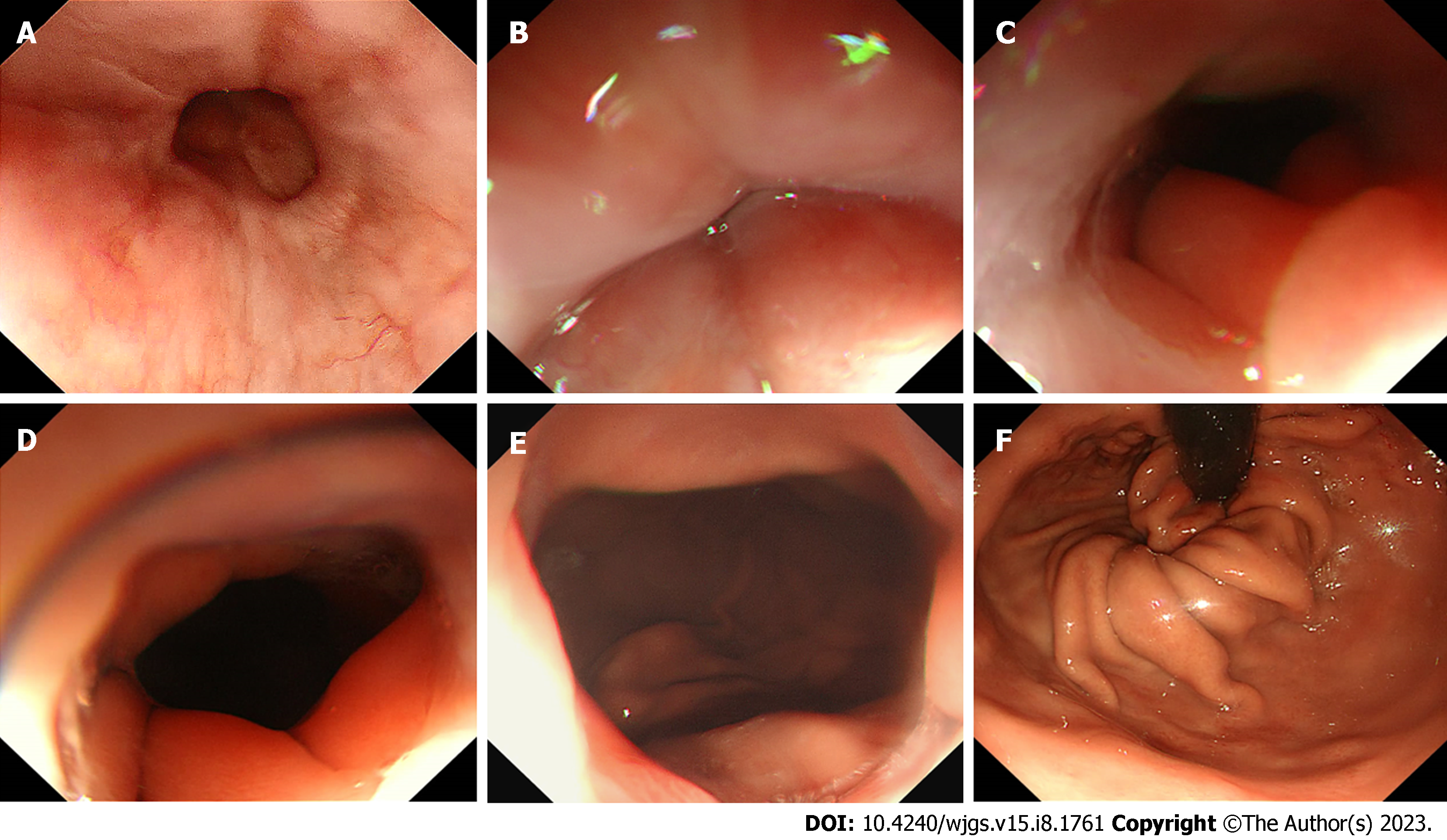
Gastroscopy during postoperative follow-up (2 to 19 mo) revealed a closed valve-like structure at the anastomosis site in 11 patients. Additionally, good extensibility and gastric residual discharge were observed, with no signs of stenosis or apparent reflux esophagitis. Only two patients exhibited an uncharacteristic valve-like structure and reflux esophagitis (class LA-B, Los Angeles classification). Inverted gastroscope revealed a reconstructed gastric base (all patients), with 11 patients showing good coverage by the gastric mucosal valve-like structures at site of anastomosis. Follow-up endoscopic findings in representative cases are shown in Figure 6 and Video 3).
In the average 10.2 ± 6.2 mo of questionnaire follow-up, 11 patients recovered well with no symptoms such as stomach distension, heartburn, and dysphagia. Two patients exhibited gastroesophageal reflux (controlled by proton pump inhibitors, gastric dynamic drugs, and functional exercise). No recurrence or fatalities occurred during the median 10.2-month follow-up period (range, 2-19 mo).
By last follow-up, of the 13 EGAA surgery patients, four lost weight (one developed fatty diarrhea post-cholecystectomy 6 month post-surgery). Five patients showed no change in body weight and four showed weight gain. The specific changes in BMI are shown in Table 1.
Esophagogastrostomy is considered the simplest and most convenient reconstruction procedure following proximal gastrectomy, as it preserves digestion and absorption. However, it is associated with a high incidence of reflux esophagitis and anastomotic stenosis, which significantly impact the patient's quality of life[21,27].
There are three problems to be addressed for anastomosis stoma of the esophagus and stomach to alleviate postoperative complications such as reflux and anastomosis stenosis[28-30]: (1) Maintaining closed state of anastomosis stoma and reducing gastric fluid reflux in the supine position; (2) maintaining gastric cavity tension to prevent weakness and gastric retention; and (3) prevent anastomosis narrowing.
To overcome these problems, we designed a four-asymmetric suture technique to reconstruct the gastric fundus and form a valve shape by folding part of the residual stomach wall at the posterior lip of the anastomosis in the gastric cavity. To improve study design processes and confirm a specific anti-reflux function, a computer simulation was applied using finite element analysis.
The anti-reflux effect of asymmetric anastomosis was further evaluated by imaging and endoscopy post-operatively. Abdominal CT examination (oral CO2) showed circular expansion of the residual stomach cavity, and a valve-like tightly closed stoma at the site of anastomosis. The CO2 did not leak easily, confirming the "one-way valve" function. Upper gastroenterography showed good efficiency of stomach discharge or excretion to the small intestine, no gastric weakness, and no reflux even when most of the patients reached 15° in the supine and head lowered position, indicating an anti-reflux effect of the reconstructed anastomosis. Digestive endoscopy showed that the anastomosis was not narrowed, softened, or extended and had no mucus lake in the gastric cavity. The reconstructed gastric base and valve-like structures were observed via an inverted gastroscope and revealed the gastric mucosa wrapped around the mirror body wall.
The results of our computer simulation and theoretical analysis further support the findings of the clinical examination. The computer simulation results demonstrate that during the peristaltic state, the folded stomach wall and the esophageal wall move in a clockwise forward motion, primarily due to the suture fixation of the residue and the rear wall of the esophagus. This movement leads to further tightening of the anastomosis stoma. Additionally, the expansion of the gastric cavity through increased peristaltic pressure applies pressure on the lower esophageal tissue, resulting in an enhanced anti-reflux effect.
Based on the above results, we speculate that in the resting state, the anastomotic stoma and lower esophageal end are closed by four synergistic actions: longitudinal torsion of the lower section of the esophagus, asymmetric size of the esophageal diameter and residual gastric wall incision, suture of the seromuscular layer in the anterior wall, and the valve-like structure at the site of the posterior wall of the anastomosis stoma, acting as an anti-reflux function. Meanwhile, the joint effect of the pylorus can maintain a certain pressure in the stomach cavity, promote gastric emptying, and reduce the symptoms of fullness and discomfort caused by stomach retention.
Three functional changes may occur in the state of gastric peristalsis: (1) With gastric contraction and an increase in gastric cavity pressure, gastric reflux to the esophagus occurs, pushing the valve-like stoma and further closing the anastomotic site. This valve-like structure functions as a unidirectional valve that is not prone to slip and cause reflux and leakage. However, while eating, the peristaltic pressure of the proximal esophagus and esophageal expansion by food clumps make the valve-like structure move distally but does not affect the passage of food; and (2) With peristalsis carried out from proximal to distal, the relaxation of the reconstructed gastric fundus and the subsequent passive expansion also have a certain anti-reflux effect on the compression of the lower end of the esophagus. According to computer simulation results, when the stomach contracts and shrinks, the valve-like structure moves to the upper left side, tightening the anastomotic stoma.
During follow-up, the majority of patients showed satisfactory recovery outcomes, except for two early EGAA patients who developed complications of reflux esophagitis, characterized by acid reflux and belching. This suggests that the four different asymmetric suture techniques might not have met the design standards due to lack of practical experience in the early stage of attempting the surgery, leading to incomplete closure of the valve-like structure, a half-folded gastric wall, and poor anti-reflux effect. However, with experience and improved surgical techniques, subsequent EGAA procedures were successful in achieving good anti-reflux effects, as observed in 11 patients. Notably, in the last 8 patients, gastroenterography revealed no reflux even in the supine and low head positions.
Finally, despite the advantages of our new approach, we acknowledge the limitations of the present study. As this was a single-center study with a small number of patients, more objective comparisons in multicenter trials are required to validate the procedure. Moreover, as a new technique, this requires a learning curve for surgeons to gain the necessary skills before satisfactory results can be achieved.
In this clinical study, we designed the valve-like structure and anti-reflux function of the EGAA. The surgical procedures we adopted adhered to basic medical principles as well as being relatively easy to master. The clinical outcomes of 13 patients indicated that this surgical technique is practical, safe, and reliable. However, it is necessary to further investigate its long-term anti-reflux effectiveness with more patients and randomized controlled studies.
The direction of future research will be focused on investigating the long-term effectiveness of anti-reflux measures with larger patient populations and randomized controlled studies.
The implementation of esophagogastric asymmetric anastomosis (EGAA) proved to be a secure and viable procedure, yielding outstanding anti-reflux results following proximal gastrectomy.
The objective of this research was to investigate the effectiveness of the EGAA technique in preventing reflux after proximal gastrectomy that represents a new approach to anti-reflux surgery.
First, we utilized a thermal stress computer simulation to replicate gastric peristalsis at the EGAA site. This was conducted to gain a deeper understanding of the mechanism and efficacy of the anti-reflux function. Subsequently, we performed digestive tract reconstruction on 13 patients who had undergone laparoscopic proximal gastrectomy using the EGAA technique. We closely monitored the structural and functional changes of the reconstruction through imaging exams and gastroscopy after the surgery. Lastly, we conducted follow-up assessments on the patients to determine the effectiveness of the anti-reflux effects.
The research findings suggest that the valve-like reconstructed structure at the site of EGAA was effective in preventing gastroesophageal reflux in patients who underwent the procedure. However, further studies are needed to evaluate the long-term efficacy and safety of this technique.
The limitations of current anti-reflux surgical techniques have led to the development of novel methods to prevent postoperative reflux. The EGAA technique is designed to address the shortcomings of conventional techniques by utilizing computer simulation technology to study the anti-reflux mechanism of asymmetric anastomosis. Solving these problems and improving the effectiveness of anti-reflux techniques will have significant implications for the future of gastrointestinal reconstruction and postoperative patient outcomes.
Our study highlights the increasing proportion of proximal gastric cancer over the past few decades that has resulted in reflux esophagitis becoming a common postoperative complication after proximal gastrectomy. There is a need for a safer method of performing esophageal-gastric anastomosis to reduce the risk of reflux and other complications for patients undergoing this surgery. Esophageal-residual gastric anastomosis after proximal gastrectomy is an effective way to reconstruct the digestive tract, but the loss of anti-reflux function can lead to several postoperative complications, affecting the quality of life of the patient. The significance of this study lies in finding ways to reduce these complications and improve the outcomes for patients.
We would like to thank Xu C and Xu R for the technical assistance in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shida A, Japan; Viswanath YK, United Kingdom S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Liu K, Yang K, Zhang W, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16:131-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 4. | Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, Shimoda T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Rosa F, Quero G, Fiorillo C, Bissolati M, Cipollari C, Rausei S, Chiari D, Ruspi L, de Manzoni G, Costamagna G, Doglietto GB, Alfieri S. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG). Gastric Cancer. 2018;21:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y, Nakada K. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Yanes M, Santoni G, Maret-Ouda J, Ness-Jensen E, Färkkilä M, Lynge E, Pukkala E, Romundstad P, Tryggvadóttir L, Euler-Chelpin MV, Lagergren J. Laryngeal and Pharyngeal Squamous Cell Carcinoma After Antireflux Surgery in the 5 Nordic Countries. Ann Surg. 2022;276:e79-e85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Berlth F, Lorenz F, Kleinert R, Langhammer N, Hadzijusufovic E, Chon SH. [GERD and Barett: Natural Course of One Disease - Update Diagnostics and Therapy]. Ther Umsch. 2022;79:151-158. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Nishi T, Makuuchi H, Ozawa S, Shimada H, Chino O. The Present Status and Future of Barrett's Esophageal Adenocarcinoma in Japan. Digestion. 2019;99:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Chen XF, Zhang B, Chen ZX, Hu JK, Dai B, Wang F, Yang HX, Chen JP. Gastric tube reconstruction reduces postoperative gastroesophageal reflux in adenocarcinoma of esophagogastric junction. Dig Dis Sci. 2012;57:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Yoo CH, Sohn BH, Han WK, Pae WK. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004;36:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Aikou T, Natsugoe S, Shimazu H, Nishi M. Antrum preserving double tract method for reconstruction following proximal gastrectomy. Jpn J Surg. 1988;18:114-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 14. | Kameyama J, Ishida H, Yasaku Y, Suzuki A, Kuzu H, Tsukamoto M. Proximal gastrectomy reconstructed by interposition of a jejunal pouch. Surgical technique. Eur J Surg. 1993;159:491-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S, Shirakawa Y, Fujiwara T. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg. 2016;223:e7-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 16. | Shiraishi N, Hirose R, Morimoto A, Kawano K, Adachi Y, Kitano S. Gastric tube reconstruction prevented esophageal reflux after proximal gastrectomy. Gastric Cancer. 1998;1:78-79. [PubMed] [DOI] [Full Text] |
| 17. | Shoji Y, Nunobe S, Ida S, Kumagai K, Ohashi M, Sano T, Hiki N. Surgical outcomes and risk assessment for anastomotic complications after laparoscopic proximal gastrectomy with double-flap technique for upper-third gastric cancer. Gastric Cancer. 2019;22:1036-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 18. | Kuroda S, Choda Y, Otsuka S, Ueyama S, Tanaka N, Muraoka A, Hato S, Kimura T, Tanakaya K, Kikuchi S, Tanabe S, Noma K, Nishizaki M, Kagawa S, Shirakawa Y, Kamikawa Y, Fujiwara T. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study). Ann Gastroenterol Surg. 2019;3:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 19. | Aihara R, Mochiki E, Ohno T, Yanai M, Toyomasu Y, Ogata K, Ando H, Asao T, Kuwano H. Laparoscopy-assisted proximal gastrectomy with gastric tube reconstruction for early gastric cancer. Surg Endosc. 2010;24:2343-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Nomura E, Isozaki H, Fujii K, Toyoda M, Niki M, Sako S, Mabuchi H, Nishiguchi K, Tanigawa N. Postoperative evaluation of function-preserving gastrectomy for early gastric cancer. Hepatogastroenterology. 2003;50:2246-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Kumagai K, Shimizu K, Yokoyama N, Aida S, Arima S, Aikou T; Japanese Society for the Study of Postoperative Morbidity after Gastrectomy. Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan. Surg Today. 2012;42:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Aizawa M, Yabusaki H, Nakada K, Matsuki A, Bamba T, Nakagawa S. Simple modifications of conventional esophagogastrostomy after proximal gastrectomy adequately reduces the postoperative reflux esophagitis: a retrospective analysis of posterolateral fundoplication. Langenbecks Arch Surg. 2022;407:3153-3160. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 954] [Article Influence: 318.0] [Reference Citation Analysis (0)] |
| 24. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 960] [Reference Citation Analysis (0)] |
| 25. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8593] [Article Influence: 537.1] [Reference Citation Analysis (0)] |
| 26. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1653] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 27. | Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today. 2016;46:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Aizawa M, Ishida M, Kodera Y, Kanazawa T, Fukushima R, Akashi Y, Yoshimura F, Ota S, Oshio A, Nakada K. A comparison of the effects of anti-reflux procedures during esophagogastrostomy after proximal gastrectomy on the postoperative quality of life. Surg Today. 2023;53:182-191. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Tao K, Dong J, He S, Xu Y, Yang F, Han G, Abe M, Zong L. Surgical Strategies for Siewert Type II Esophagogastric Junction Carcinomas: A Randomized Controlled Trial. Front Oncol. 2022;12:852594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 30. | Zhang H, Sun Z, Xu HM, Shan JX, Wang SB, Chen JQ. Improved quality of life in patients with gastric cancer after esophagogastrostomy reconstruction. World J Gastroenterol. 2009;15:3183-3190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |