Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1739
Peer-review started: April 23, 2023
First decision: May 27, 2023
Revised: May 31, 2023
Accepted: July 6, 2023
Article in press: July 6, 2023
Published online: August 27, 2023
Processing time: 124 Days and 6.4 Hours
Whether patients over 85 years old with gastrointestinal cancer should undergo surgery remains controversial. We aimed to describe the changing trends of characteristics to provide more information to decision makers, and strive to find appropriate surgical plan.
To describe the changing trends of characteristics to provide more information to decision makers, and strive to find appropriate surgical plan.
A total of 218 gastric cancer (GC) patients and 563 colorectal cancer (CRC) patients who underwent surgery between 2001 and 2021 were enrolled in this retrospective analysis. Changes in clinicopathological features, surgical treatments, and survival status were analyzed longitudinally at 5-year intervals.
Only 14 GC patients underwent laparoscopic surgery where 219 CRC patients had this procedure. Cardia and esophagogastric junction cancer increased in GC patients, and the proportion of sigmoid colon cancer decreased in CRC patients. Pulmonary infection gradually became the most common postoperative complication, its incidence in period 4 reached 48.79%. However, the incidence of anastomotic leakage decreased from 26.79% to 9.38% (P < 0.01). Additionally, 30-d mortality significantly decreased from 32.14% to 9.01%. Increases were observed in 5-year overall survival (OS) in GC patients from period 1 to period 4 (18.18% vs 33.32%, respectively) and CRC patients (0 vs 36.32%, respectively). Disease-free survival (DFS) also increased in GC and CRC patients (7.14% vs 27.74% and 0 to 36.03%, respectively). The average survival time of GC patients following radial lymphadenectomy was higher than in patients that underwent limited lymphadenectomy (26 vs 22 mo, respectively), the same was seen in CRC patients (44 vs 33 mo, respectively). This advantage was particularly evident in patients with TNM I, but not in patients with TNM II/III period cancer.
The safety as well as effectiveness of surgery in ultra-elderly patients is increasing. Radical lymphadenectomy has advantages in patients with TNM I gastrointestinal cancer, but not TNM II/III.
Core Tip: The safety as well as effectiveness of surgery in ultra-elderly patients is increasing. Radical lymphadenectomy has advantages in patients with TNM I gastrointestinal cancer, but not TNM II/III.
- Citation: Chen K, Li M, Xu R, Zheng PP, Chen MD, Zhu L, Wang WB, Wang ZG. Changing trends in gastric and colorectal cancer among surgical patients over 85 years old: A multicenter retrospective study, 2001–2021. World J Gastrointest Surg 2023; 15(8): 1739-1750
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1739.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1739
Gastric cancer (GC) and colorectal cancer (CRC) are common digestive system cancers worldwide. The latest world cancer data report showed that in 2021[1], there were 1089103 new cases of GC and an expected 768793 deaths world
At present, the incidence and mortality of gastrointestinal cancer remain high. With the development of increased living standards, improved screening techniques and medical advances, all-cause mortality has declined. Consequently, most countries are facing an aging society. From 2000 to 2020, the number of people over 85 in China increased to more than 14 million, with a corresponding increase from 0.31% to 1.64%[2]. In America, the number of adults aged 85 years and older is expected to nearly triple from 6.4 million in 2016 to 19.0 million by 2060[3]. Factors that may slow or even reverse progress include high blood pressure, rising rates of diabetes, and a widening gap between the rich and poor. However, relatively little is known about the burden of gastrointestinal cancer in this special age group and most guidance related to the management of gastrointestinal cancer is based on trials undertaken in the fit, younger patient. Historically the elderly have been underrepresented in clinical trials, which frequently have a restricted inclusion to an upper age limit of 75. For this reason, knowledge about appropriate treatment methods and the complex healthcare needs of elderly cancer patients is limited.
In Japan, the life expectancy of men and women aged 85 is 8.4 and 6.2 years, respectively[4]. Therefore, the probability of diagnosis of gastrointestinal cancer in patients aged 85 and above will only increase. Due to the increase in life expectancy and progress in medical technology, patients may be more willing to undergo radical surgery. However, elderly patients inevitably experience a decline in major organ function, and postoperative complications are higher than in younger patients. These factors may lead to a significant increase in mortality. Besides, a significant number of elderly patients live in rural China, where they receive only basic medical security and the poor referral system limits access to high quality medical care. Diagnosis and treatment of gastrointestinal cancer in older individuals are often complicated by factors such as comorbidities and cognitive impairment. Many studies have investigated the outcomes of GC and CRC surgery in different age groups[3,5-9]. However, there is very little information on the oldest old.
David et al[10] studied patients over 85 years old who underwent CRC resection in a single center from 2010 to 2018. The treatment effects of 48 patients over 85 years old and 136 patients between 75 and 84 years old were compared. The results revealed no significant difference in short-term effects such as severity of complications, anesthesia score, or 30-d mortality between the two groups. However, the author did not follow up on long-term survival. A study from Japan included 134 GC patients over 85 years old seen in nine centers between 2000 and 2014 and found that surgical treatment was necessary and effective in patients with stage II[11].
In this report, we present surgical information and trends from a multicenter population aged 85 years and older diagnosed with GC and CRC in China. We also discuss some of the unique challenges affecting these patients and attempt to summarize the existing evidence on how to best optimize the treatment of patients with gastrointestinal cancer in the oldest old.
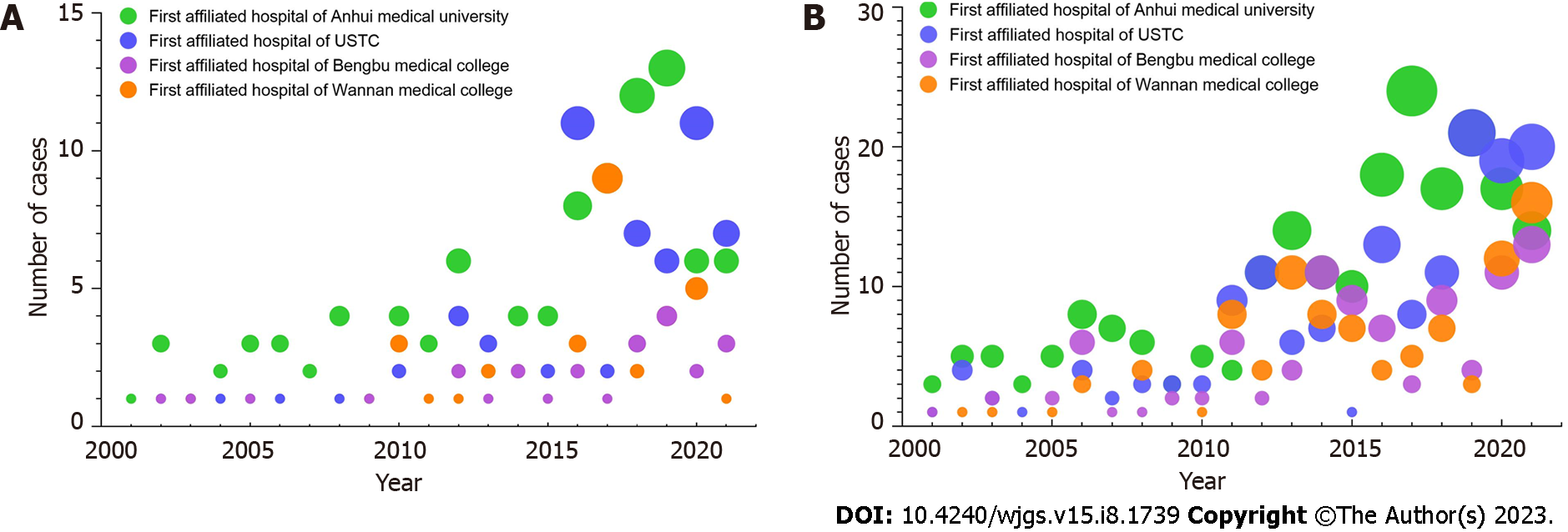
In the high incidence area of gastrointestinal cancer in China, the first affiliated hospital of Anhui Medical University, the first affiliated hospital of the University of Science and Technology of China, the first affiliated hospital of Bengbu Medical College and the first affiliated hospital of Wannan Medical College have completed more than 80000 surgeries for GC and CRC over the past 21 years. This included 781 patients over 85 years old and among these, 218 patients had GC and 563 patients had CRC.
The clinicopathological data of these patients, including age, sex, length of stay, surgical time, 30-d mortality rate after surgery, TNM period, surgical mode, pathological differentiation, tumor size, tumor location, and survival status were collected. Inclusion criteria included: pathological results clearly diagnosed GC or CRC, all patients underwent radical resection from 2001 to 2021, and all cases had complete surgical information, postoperative pathology, and follow-up data. Patients were excluded if they underwent endoscopic resection of the malignant lesion without surgical intervention, patients whose preoperative examination indicated possible distant metastasis or serious organ dysfunction, or any other special circumstances that made them not suitable for surgery.
In this study, survival time ranged from the day of surgery to the day of death (if the specific day of death was unknown, survival time was recorded as the last follow-up day). Follow-up methods included telephone follow-up and outpatient follow-up. The first follow-up was in February 2022, the second follow-up was conducted by telephone in August 2022, and the last follow-up was in January 2023.
The standard operability of each case was determined according to the Japanese guidelines for the treatment of stomach[12-14] and colorectal cancer[15-18]. Based on the clinical period and location of the cancer, each institution selects the appropriate surgical procedure and determines the scope of resection, including possible lymph node resection. Radical lymphadenectomy is applicable to cT1N0 patients who have undergone D1 or more extensive lymphadenectomy, as well as cN+ or cT2-4 patients who have undergone D2 Lymphadenectomy.
According to the Japanese Classification of Gastric Cancer, 3rd edition[19], the state of the residual tumor after surgery is also described as the R state. R0 represents no residual tumor, R1 represents a microscopic residual tumor (with a positive resection margin), and R2 represents a macroscopic residual tumor visible to the naked eye. In this study, R2 patients included patients with bypass or non-resection surgery.
Short-term outcomes included complications, operative time, blood transfusion, 30-d mortality and length of hospital stay. Postoperative complications were defined as those that arose up to 30 d postoperatively. Operative mortality was defined as death from any reason occurring less than 30 d after surgery (in or out of the hospital) and more than 30 d after surgery during the same hospitalization.
Long-term outcomes included 5-year survival [overall survival (OS) and disease-free survival (DFS)], tumor recurrence or distant metastasis, metastasis site (if distant), and postoperative chemotherapy or other treatment.
Unadjusted analysis was performed for each independent variable. Continuous variables are shown as means plus standard deviation and were compared by One Way Analysis of Variance. A P value < 0.05 was used to define statistical significance, and all analyses were performed using SPSS 19.0.
Between 2000 and 2021, more than 80000 cases of gastrointestinal cancer were captured in the database of our four high-volume centers in China. This included 786 patients over 85 years old. Among these, 218 patients had GC, five patients had small intestinal cancer, and 563 patients had CRC. Five years was considered a “period,” and this study covered a total of four periods (2001-2021). There were 64 people in the first period (18 GC, 46 CRC, 2001-2005), 107 people in the period phase (23 GC, 84 CRC, 2006-2010), 176 people in the third period (40 GC, 136 CRC, 2011–2015), and 434 people in the fourth period (137 GC, 297 CRC, 2016-2021).
Of the hospitals, the first affiliated hospital of Anhui Medical University had the highest number of patients (38.35%), where the first affiliated Hospital of Wannan Medical College had the lowest number (19.41%, Figure 1). Among all patients, the percentage of adenocarcinoma cases was 93.12% (203/218). This included four patients with squamous cell carcinoma and 11 patients with adenosquamous carcinoma). Tumor location data is shown in Table 1.
| Tumor location | Period 1 (n = 18) | Period 2 (n = 23) | Period 3 (n = 40) | Period 4 (n = 137) | |
| GC | The cardia or gastroesophageal, junction (n = 58) | 3 (16.67%) | 5 (21.74) | 16 (40.00) | 34 (24.82) |
| Stomach body (n = 50) | 5 (27.78) | 10 (43.48) | 7 (17.50) | 28 (20.44) | |
| Gastric antrum (n = 91) | 10 (55.55) | 7 (30.43) | 8 (20.00) | 66 (48.18) | |
| Whole stomach (n = 19) | 0 | 1 (4.35) | 9 (22.50) | 9 (6.56) | |
| Tumor location | Period 1 (n = 38) | Period 2 (n = 64) | Period 3 (n = 154) | Period 4 (n = 307) | |
| CRC | Ileocecal region (n = 28) | 2 (5.25) | 2 (3.13) | 3 (1.95) | 21 (6.84) |
| Ascending colon or the hepatic, curvature of the colon (n = 28) | 0 | 3 (4.69) | 7 (4.55) | 18 (5.86) | |
| Transverse colon (n = 28) | 1 (2.63) | 6 (9.37) | 10 (6.49) | 11 (3.58) | |
| Splenic or descending colon (n = 19) | 1 (2.63) | 4 (6.25) | 3 (1.95) | 11 (3.58) | |
| Sigmoid colon (n = 149) | 17 (44.75) | 23 (35.94) | 30 (19.48) | 79 (25.74) | |
| Uppermost segmzent of the rectum (n = 181) | 10 (26.32) | 16 (25.00) | 60 (38.96) | 95 (30.94) | |
| Lower segment of the rectum (n = 130) | 7 (18.42) | 10 (15.62) | 41 (26.62) | 72 (23.46) | |
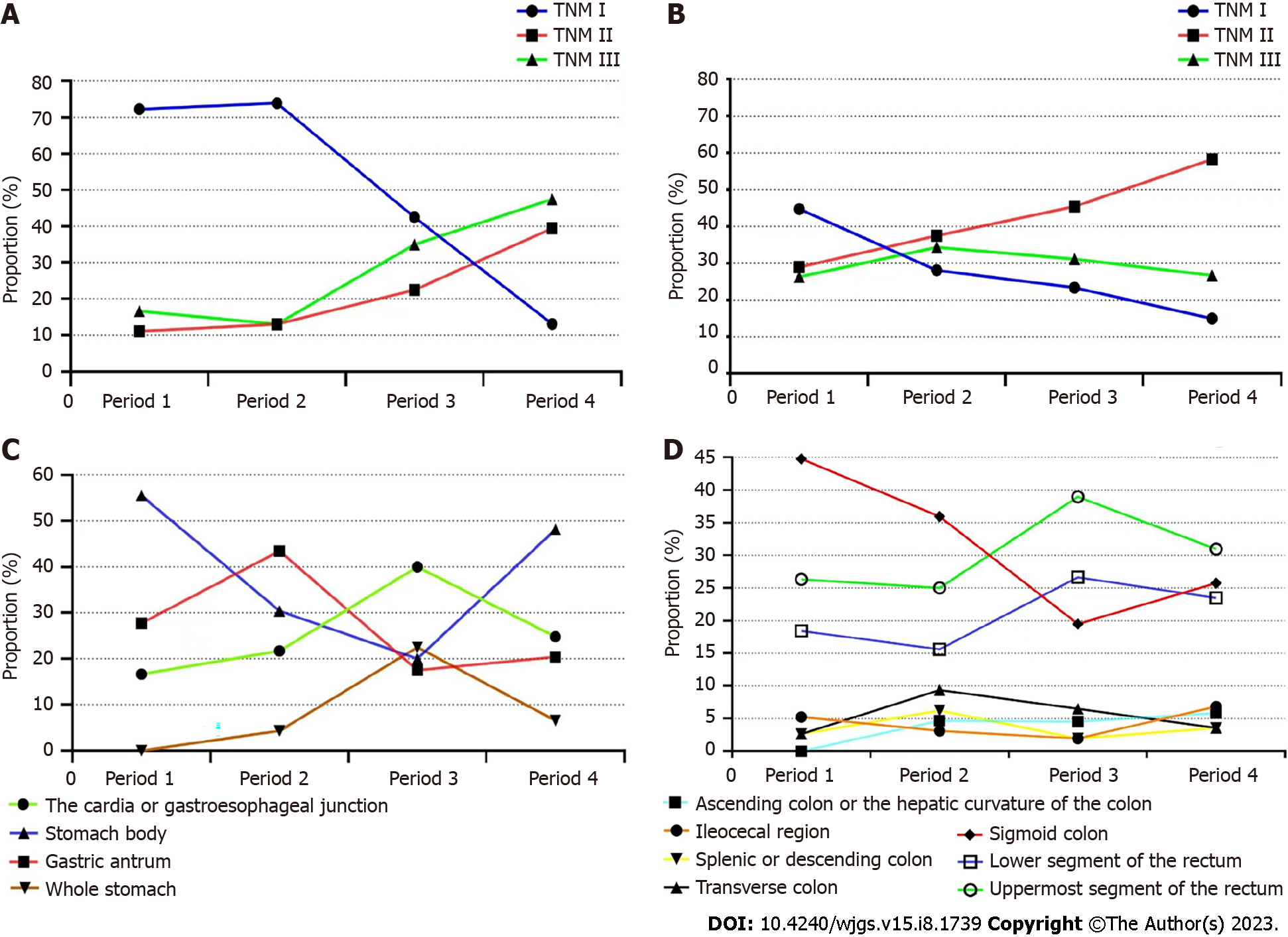
The location of the tumor has also been changing in these two decades, but this result is not sustainable. In the field of GC, the tumor at the antrum is still ahead of other parts at present, accounting for nearly 50% in period 4, while the proportion of cancer at the cardia or gastroesophageal junction reached the highest level in 2011-2015, nearly 40%, but shrunk to around 25% in 2016-2021. However, this is still significantly higher than the first stage, with a statistically significant difference (Figure 2A). In the field of CRC, rectal cancer has always accounted for a high proportion, while the proportion of sigmoid colon cancer has decreased significantly, with a statistically significant difference, especially in the third stage. The change trend of upper or lower rectal cancer is relatively close (Figure 2C).
Of the 218 super-aged patients with GC who received surgical treatment, a total of 18 patients were included in the study in period 1, 23 patients were included in period 2, and 40 and 137 patients were enrolled in period 3 and period 4, respectively. According to the postoperative pathological results, each patient was accurately phased using the TNM staging system. The percentage of TNM I stage tumors decreased from 72.23% in period 1 to 13.14% in period 4, where the percentage of TNM III tumors increased from 16.67% to 47.75% (P < 0.001, Figure 2B). In CRC patients, 44.74% of cases had TNM I stage tumors in period 1 and this decreased to 14.98% in period 4. Overall, the number of TNM II tumors increased by more than half in two decades. However, the number of TNM III remained almost unchanged (Figure 2D).
Among the 218 patients with GC who underwent surgery, the ratio of men to women was 4.32:1. This was much higher than GC patients in other age groups (2.6:1, data not shown). In the CRC group, the ratio of men to women was 3.61:1, which was higher than CRC patients in other age groups (2.49:1, data not shown). The average age of all GC cases increased from period 1 (87.12 ± 1.98 years) to period 5 (88.81 ± 2.34 years), however, the change was not statistically different. A similar trend was seen in patients with CRC. In GC patients, the largest tumor size decreased from period 1 (4.45 ± 3.09 cm) to period 4 (3.63 ± 2.74 cm). The percentage of highly differentiated tumors decreased from 45.7% in period 1 to 9.08% in period 5. However, the percentage of poorly differentiated tumors increased from 24.31% to 69.24% (P < 0.001). The percentage of pT1 tumors gradually decreased over time from 72.22% in period 1 to 13.14% in period 5 (P < 0.001, Table 2).
| GC (n = 218) | CRC (n = 563) | |
| Age (yr) | 85-95 | 85-96 |
| Sex (male/female) | 177/41 | 441/122 |
| hospital stay | 19.45 ± 7.22 | 15.61 ± 6.07 |
| (Post-operation, d) | ||
| Comorbidity | ||
| Hypertension | 194 | 510 |
| Respiratory disease | 17 | 50 |
| Diabetes mellitus | 29 | 61 |
| ASA stage | ||
| I | 11 | 25 |
| II | 144 | 416 |
| III | 55 | 103 |
| Missed | 8 | 19 |
| Procedure | ||
| Open | 204 | 304 |
| Laparoscopy | 14 | 219 |
| Lymphadenectomy | ||
| Limited | 76 | 120 |
| Radical | 142 | 443 |
| Resection | ||
| R0 | 197 | 471 |
| R1 | 21 | 92 |
| Number of resected LN | 12.78 ± 5.33 | 11.44 ± 6.17 |
| Operative time (mins) | 155.04 ± 69.52 | 129.90 ± 47.76 |
| Blood transfusion | 148 | 157 |
| Tumor size (cm) | 3.24 ± 2.03 | 4.37 ± 2.10 |
| Differentiation | ||
| High | 29 | 48 |
| Middle | 68 | 118 |
| Low | 121 | 397 |
| TNM stage | ||
| I | 65 | 73 |
| II | 68 | 384 |
| III | 85 | 106 |
In CRC patients, the largest tumor size decreased from period 1 (5.41 ± 3.07 cm) to period 4 (5.02 ± 2.76 cm). The percentage of highly differentiated tumors decreased from 35.7% in period 1 to 19.8% in period 5, where the percentage of poorly differentiated tumors increased from 34.35% to 65.22% (P < 0.001). The percentage of pT1 tumors gradually decreased over time from 55.32% in phase 1 to 10.81% in phase 5 (P < 0.001). Additionally, 65.14% of GC patients (142/218) and 78.68% of CRC patients underwent radical lymphadenectomy.
Another noteworthy change was the use of laparoscopic surgery. In the first and second periods, there was no record of laparoscopic surgery use in GC or CRC patients. However, this increased rapidly starting in period 3, especially in patients with CRC.
Of the 218 patients with GC, 204 underwent open surgery and 14 underwent laparoscopic surgery. The first case of GC laparoscopic surgery in a patient over 85 years old occurred in 2016. Roux-en-Y reconstruction gradually became the dominant mode, compared to period 1, the incidence of period 4 increased to 71.2% (P < 0.001), and the average number of harvested lymph nodes in period 4 increased significantly (P < 0.001). The rate of combined organ resection remained low across the four periods. The most common organ resection was the spleen. There were few records of patients entering the intensive care unit (ICU) after surgery in the first and second periods. However, the proportion of GC patients entering ICU after surgery in the fourth period was close to 90%.
Of the 563 CRC patients, 344 underwent open surgery and 219 underwent laparoscopic surgery. The first case of laparoscopic CRC surgery in a patient over 85 years old occurred in 2009. In the fourth period, the percentage of CRC patients undergoing laparoscopic surgery reached 49.97%, and the average number of harvested lymph nodes in the fourth period increased significantly to 17.11 (P < 0.001). The rate of combined organ resection in the fifth period increased from 12.8% to 16.4% (P < 0.001), and the percentage of patients admitted to the ICU after surgery was 61.00% in period 4.
A changing trend was also observed in postoperative complications over the past 21 years. The overall complication rate was at a relatively high level (100% in the first period) but it dropped to 84.01% in the fourth period. The main causes of death included multiple organ failure, severe infection, hemorrhage, and possible thrombosis, which are all significantly related to organ dysfunction in elderly patients. However, progress in medical technology and the optimization of advanced life support have significantly reduced perioperative mortality, as evidenced by a decrease from 32.14% in period 1 to 9.01% in period 4. Pulmonary infection gradually became the largest source of postoperative complications, rising from 16.07% in period 1 to 48.79% in period 4. We believe that the number in period 1 may be underestimated, although the observed change may be consistent with the actual trend. Further, anastomotic leakage is another common and serious complication whose incidence decreased significantly from 26.79% in period 1 to 9.38% in period 4. This change may be related to the use of staplers and progress in nutritional support (Table 3).
| Period 1 (n = 56) | Period 2 (n = 87) | Period 3 (n = 194) | Period 4 (n = 444) | P value1 | |
| Postoperative complications | 56 (100) | 80 (91.95) | 182 (93.81) | 373 (84.01) | < 0.01 |
| Pulmonary | 9 (16.07) | 28 (35.00) | 73 (40.12) | 182 (48.79) | |
| complications | |||||
| Pleural effusion | 9 (16.07) | 8 (10.00) | 25 (13.74) | 39 (10.46) | |
| Intra-abdominal infection | 6 (10.71) | 6 (7.50) | 17 (9.34) | 30 (8.04) | |
| Intra-abdominal hemorrhage | 6 (10.71) | 4 (5.00) | 11 (6.04) | 21 (5.64) | |
| Leakage | 15 (26.79) | 16 (20.00) | 28 (15.38) | 35 (9.38) | |
| Wound infection | 8 (14.30) | 9 (11.25) | 13 (7.14) | 33 (8.845) | |
| Others2 | 3 (5.35) | 9 (11.25) | 15 (8.24) | 33 (8.845) | |
| Operative mortality3 | 18 (32.14) | 17 (19.54) | 29 (14.95) | 40 (9.01) | < 0.01 |
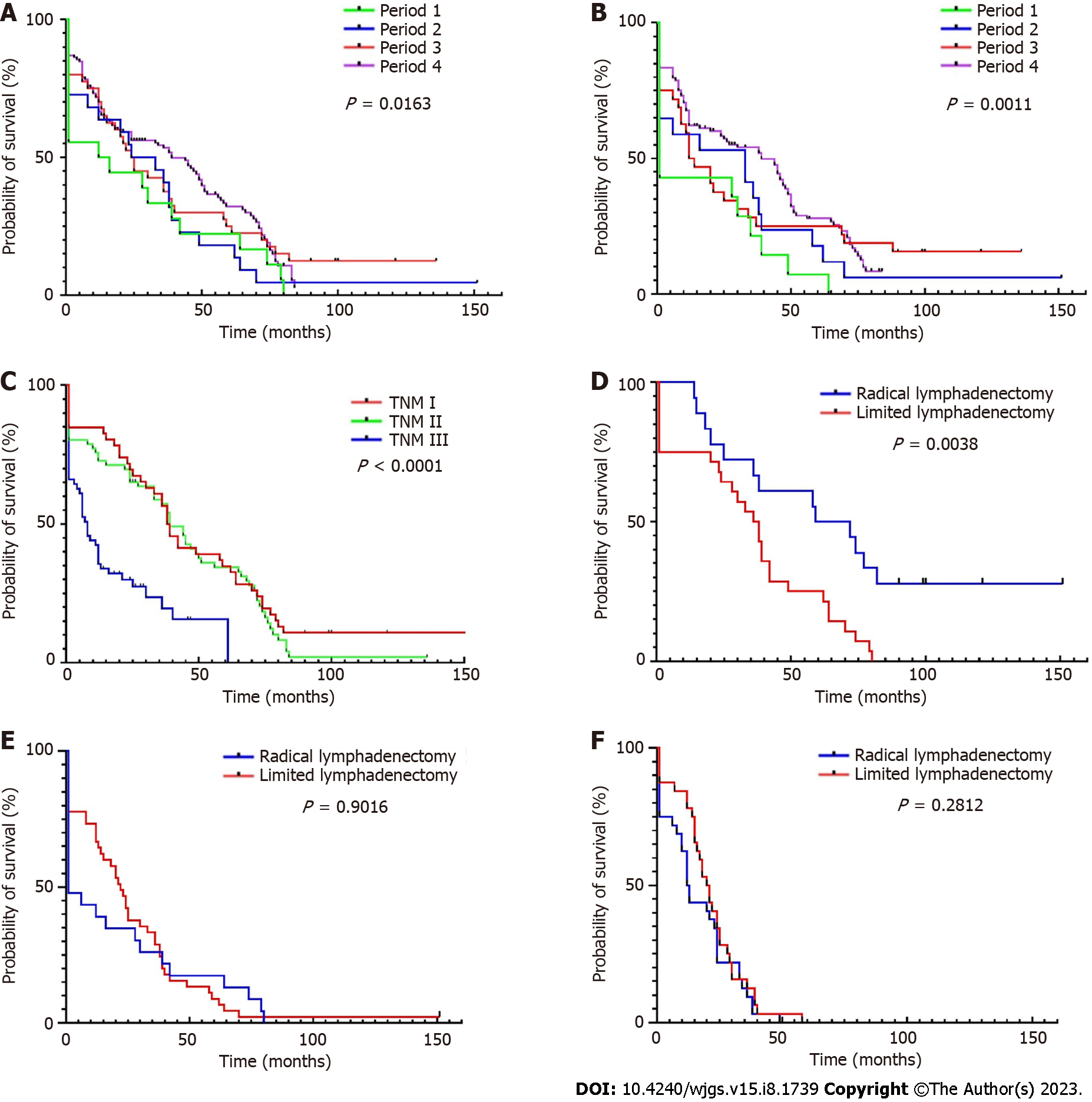
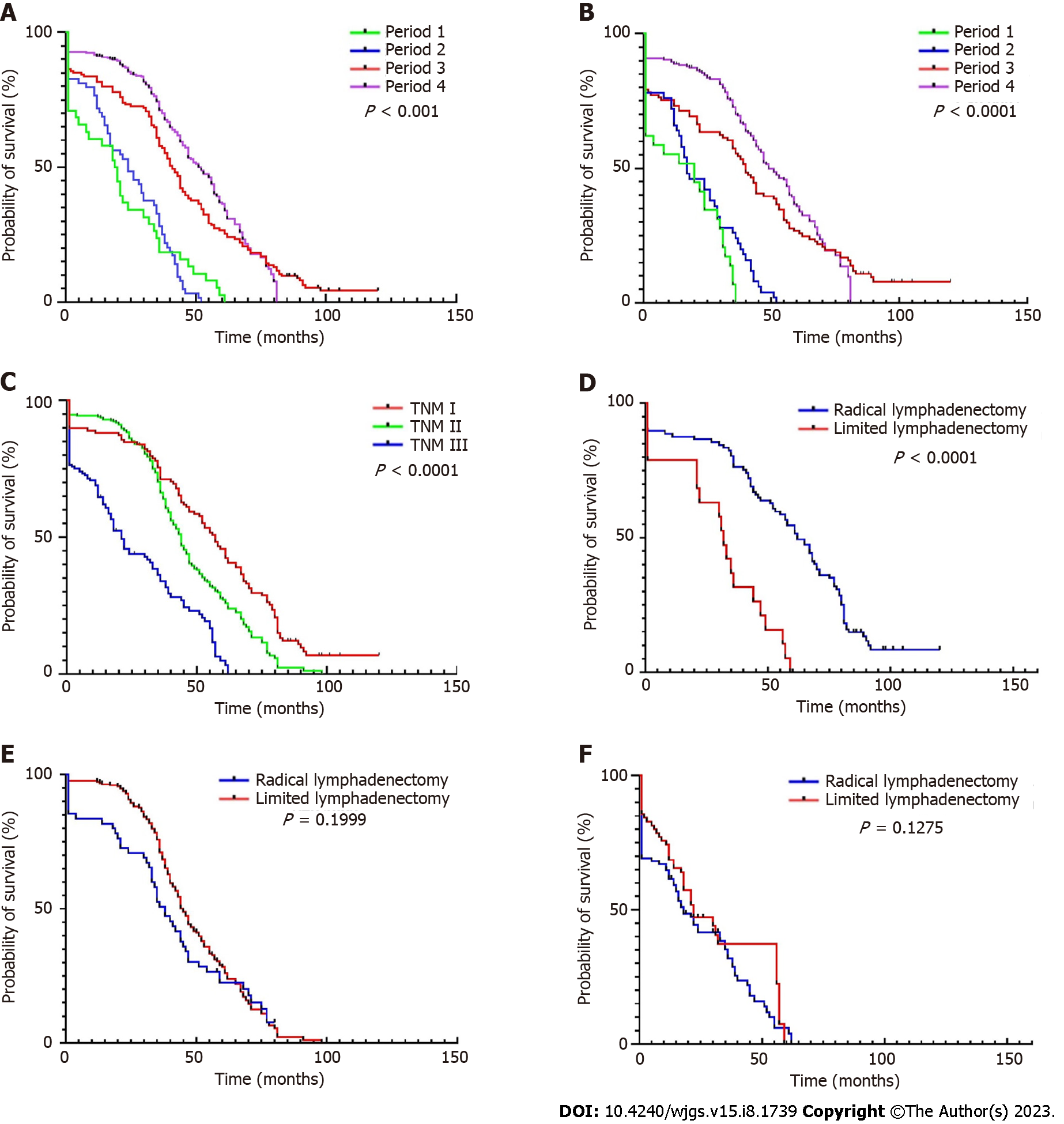
Trends in OS and DFS of patients over 85 years old was examined across the 12-264 mo of follow-up. DFS data from some patients could not be included due to the long follow-up time or uncertain causes of death, so these patients were deleted when drawing the DFS curve. In this study, the longest survival time after surgery for GC was 152 mo (TNM I, died in 2015), and the longest survival time after surgery for CRC was 121 mo (TNM I, died in 2017). The prognosis of patients with TNM I is significantly better than that of other periods. This has been confirmed many times in patients from the general age group, but is also true in the ultra-elderly group. The 5-year OS (18.18% in period 1 vs 33.32% in period 4) and DFS (7.14% vs 27.74%, respectively) in patients with GC increased (Figure 3), this was also noted in CRC (5-year OS: 0%-36.32% and 5-year DFS: 0%-36.03%). Compared to patients that underwent limited lymphadenectomy, the average survival time of patients who underwent radical lymphadenectomy for GC (22 vs 26 months for limited and radical treatments, respectively) and CRC (33 vs 44 mo, respectively) was higher (Figure 4). This advantage was particularly evident in patients with TNM I, but not in patients with TNM II/III.
Significant changes in the epidemiological characteristics and treatment of GC and CRC in elderly patients in the past two decades have been noted[20,21]. However, the incidence of gastrointestinal cancer in the elderly over 85 years old is rarely reported, especially in China. In this retrospective study, data from four top hospitals over the past 20 years were collected. Among more than 80000 patients who underwent gastrointestinal surgery, the clinicopathological data of 781 patients over 85 years old were finally selected for analysis. The goal was to describe the changing trends of various influencing factors.
GC accounted for 34.1% of all gastrointestinal cancers in this multicenter study. The prevalence of GC is higher in China than in western countries. However, due to the relatively high trauma and risk of surgery, the number of ultra-elderly patients receiving radical surgery for GC in the first two periods was very low. Starting from the third period, these numbers increased rapidly. A study included 2914 oldest old patients from 2006 to 2015 demonstrated that the oldest old patients with period I-III GC could benefit from elective surgery[22], this is consistent with our results. Surgical trauma and time were significantly less in laparoscopic surgery than in traditional open surgery. Therefore, patients in good physical condition and health choose surgery to prolong their lives for as long as possible. It is worth noting that starting in the third period, the application rate of laparoscopic surgery in CRC patients began increasing. This is because laparoscopic technologies, including surgical robot technology, have been increasingly applied in China, and surgeons are becoming more familiar with laparoscopic surgery. Additionally, patients are embracing the advantages of laparoscopy such as the dramatic reduction in surgical incisions and in some cases, incision-free surgery (via the natural duct). The fine and individual control of the procedure and more accurate control of negative incisional margins are also advantages, but these are at the expense of longer surgical time and a high CO2 abdominal environment. However, most patients do benefit from this procedure.
Correspondingly, the proportion of people receiving laparoscopic radical gastrectomy in the elderly population remains low, which may be due to its complex anatomical position, the fact that laparoscopic radical gastrectomy may require a longer learning curve, and the need for long-term tacit cooperation of assistants. Even in the fourth period of the most rapid development of laparoscopy, only seven patients over the age of 85 had received laparoscopic radical gastrectomy for GC in this study. In addition to the above common factors, this may also be because manual surgery minimizes the surgical time, resulting in a shorter recovery time. As can be seen from the surgical time, the duration of laparoscopic radical gastrectomy in the seven cases was significantly higher than that of conventional open surgery, although no cases were transferred to open surgery. There was no statistical difference between laparoscopic and open surgical time for CRC, which may be because the advantages of laparoscopic surgery in lower rectal cancer or complex CRC surgery compensate for the long duration of traditional laparoscopic surgery. We should also note that the number of intraoperative or postoperative blood transfusions decreased significantly over the 20-year period, particularly in CRC (51.04% in period 1 to 30.17% in period 4 (P < 0.01). In addition, the number of surgical transfusions for GC also decreased significantly; the percentage of intraoperative or postoperative transfusions for GC was 100% in period 1 and 67.79% in period 4 (P < 0.01). These results indicate that a more complete system has been established in the past 20 years in terms of gastrointestinal tumor anatomy, surgical approach, and lymph node dissection scope, leading to a shorter net surgical time and better control of bleeding.
It is important to note that in this multicenter series, the proportion of TNM period I gastrointestinal tumors in surgical procedures decreased, despite an increase in the overall number of procedures. It is particularly noteworthy that this change was particularly obvious in period 4. The significant advantages of digestive endoscopy in addressing early gastrointestinal tumors in elderly patients is evident from this study. We collected the relevant endoscopic-submucosal-dissection (ESD)/endoscopic mucosal resection (EMR).
ESD/EMR information of ultra-elderly patients from the First Affiliated Hospital of Anhui Medical University and Anhui Provincial Hospital (not shown in the results). The earliest clinical cases were from 2015. The patients who underwent radical surgery for period I gastrointestinal cancer in these two hospitals during the same time-period were collected. When no metastatic lymph nodes were indicated by postoperative pathology, there was no statistically significant difference in 3-year survival between patients who underwent ESD/EMR surgery and those who underwent radical surgery, and the cost of surgery was halved.
We also noted changes in focal sites over the two decades. The incidence of cardia cancer is increasing in developed countries. Before 2010, the main site of GC in China was the antrum. However, after 2015, the main site became the junction of the stomach and esophagus. This may be due to progress in digestive endoscopy technologies, which has increased the identification of lesions at the base of the cardia stomach, consistent with the changing epidemiologic characteristics of GC. The incidence of non-cardia GC has been steadily declining over the past half century. This is associated with the eradication of Helicobacter pylori and improved food preservation technologies. Further, the incidence of colon cancer is also gradually increasing. Prior to 2010, rectal cancer accounted for more than 70% of all CRC cases. However, during 2015-2020, this trend fundamentally changed, and colon cancer now accounts for 50% of CRC. This is consistent with the epidemiological characteristics of colon cancer in Chinese society, and may be due to changes in lifestyle and diet.
Surgical resection plus D2 Lymphadenectomy is becoming the standard treatment for gastrointestinal cancers. With these changes, the number of lymph nodes harvested during surgery increased from 6.1 in period 1 to 19.2 in period 4. The dissection and examination of lymph nodes have become increasingly standardized over the last two decades. Improvement in surgical skills involves the accumulation of experience by the surgeon, the use of monopolar electrocautery and an ultrasonic scalpel during the procedure, and the adoption of standardized lymph node dissection techniques[23-25].
A limitation of this study is that it was restricted to surgical cases; patients with non-resective surgery (bypass or biopsy only) were excluded. This may have led to selection bias. Ultra-aged patients who ultimately decided to undergo surgery are generally considered to be in good health and able to complete their activities of daily life, which does not reflect the actual situation of all ultra-elderly patients. Another shortcoming is that the 5-year survival period of all patients could not be included, which may have impacted the overall survival status.
The safety as well as effectiveness of surgery in ultra-elderly patients is increasing. Radical lymphadenectomy has advantages in patients with TNM I gastrointestinal cancer, but not TNM II/III.
An increasing number of patients over 85 years old are receiving surgical treatment for gastric cancer (GC) and colorectal cancer (CRC). The number of surgical patients in 2016-2021 was nearly eight times that of patients in 2001-2005 in China. TNM stage and the number of tumor prone sites changed significantly, and the incidence of perioperative complications significantly decreased. Perioperative mortality in the last period was 71.97% lower than it was in the first period. Laparoscopic technology is increasingly used in elderly patients with CRC, but its uptake in the field of GC is slow. In patients with TNM I stage, radical rather than limited lymphadenectomy should be prioritized.
Whether patients over 85 years old with gastrointestinal cancer should undergo surgery remains controversial.
describe the changing trends of characteristics to provide more information to decision makers, and strive to find appropriate surgical plan.
Retrospective analysis.
Only 14 GC patients underwent laparoscopic surgery where 219 CRC patients had this procedure. Cardia and esophagogastric junction cancer increased in GC patients, and the proportion of sigmoid colon cancer decreased in CRC patients. Pulmonary infection gradually became the most common postoperative complication, its incidence in period 4 reached 48.79%. However, the incidence of anastomotic leakage decreased from 26.79% to 9.38% (P < 0.01). Additionally, 30-d mortality significantly decreased from 32.14% to 9.01%. Increases were observed in 5-year OS in GC patients from period 1 to period 4 (18.18% vs 33.32%, respectively) and CRC patients (0 vs 36.32%, respectively). DFS also increased in GC and CRC patients (7.14% vs 27.74% and 0 to 36.03%, respectively). The average survival time of GC patients following radial lymphadenectomy was higher than in patients that underwent limited lymphadenectomy (26 vs 22 mo, respectively), the same was seen in CRC patients (44 vs 33 mo, respectively). This advantage was particularly evident in patients with TNM I, but not in patients with TNM II/III period cancer.
The safety as well as effectiveness of surgery in ultra-elderly patients is increasing. Radical lymphadenectomy has advantages in patients with TNM I gastrointestinal cancer, but not TNM II/III.
Gastric cancer, colorectal cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu CC, Taiwan; Moshref RH, Saudi Arabia S-Editor: Ma YJ L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Zhenwu Zhai. Population Opportunities and Challenges for High Quality Development in the New Era.12 May 2021. Available from: http://www.stats.gov.cn/sj/sjjd/202302/t20230202_1896484.html. |
| 3. |
US Census Bureau.
2017 National Population Projections Tables. Main Series. Table |
| 4. | Ministry of Health, Labour and Welfare. Abridged life tables for Japan 2014. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/life/life14/index.html. |
| 5. | Zu H, Wang H, Li C, Kang Y, Xue Y. Clinico-pathological features and prognostic analysis of gastric cancer patients in different age groups. Hepatogastroenterology. 2015;62:225-230. [PubMed] |
| 6. | Ma W, Wang M, Wang K, Cao Y, Hertzmark E, Ogino S, Ng K, Willett WC, Giovannucci EL, Song M, Chan AT. Age at Initiation of Lower Gastrointestinal Endoscopy and Colorectal Cancer Risk Among US Women. JAMA Oncol. 2022;8:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Jin EH, Han K, Lee DH, Shin CM, Lim JH, Choi YJ, Yoon K. Association Between Metabolic Syndrome and the Risk of Colorectal Cancer Diagnosed Before Age 50 Years According to Tumor Location. Gastroenterology. 2022;163:637-648.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Wang S, Zheng R, Arnold M, Abnet C, Zeng H, Zhang S, Chen R, Sun K, Li L, An L, Bray F, Wei W, He J. Global and national trends in the age-specific sex ratio of esophageal cancer and gastric cancer by subtype. Int J Cancer. 2022;151:1447-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Shitara K, Doi T, Hosaka H, Thuss-Patience P, Santoro A, Longo F, Ozyilkan O, Cicin I, Park D, Zaanan A, Pericay C, Özgüroğlu M, Alsina M, Makris L, Benhadji KA, Ilson DH. Efficacy and safety of trifluridine/tipiracil in older and younger patients with metastatic gastric or gastroesophageal junction cancer: subgroup analysis of a randomized phase 3 study (TAGS). Gastric Cancer. 2022;25:586-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Takahashi Y, Yamamichi N, Kubota D, Shimamoto T, Nagao S, Sakuma N, Sakaguchi Y, Yakabi S, Tsuji Y, Wada R, Mitsushima T, Ichinose M, Fujishiro M. Risk factors for gastric cancer in Japan in the 2010s: a large, long-term observational study. Gastric Cancer. 2022;25:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Asaka M, Kobayashi M, Kudo T, Akino K, Asaka Y, Fujimori K, Kikuchi S, Kawai S, Kato M. Gastric cancer deaths by age group in Japan: Outlook on preventive measures for elderly adults. Cancer Sci. 2020;111:3845-3853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Flynn DE, Mao D, Yerkovich S, Franz R, Iswariah H, Hughes A, Shaw I, Tam D, Chandrasegaram M. Should we resect colorectal cancer in patients over the age of 85? World J Gastrointest Oncol. 2021;13:185-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Konishi H, Ichikawa D, Itoh H, Fukuda K, Kakihara N, Takemura M, Okugawa K, Uchiyama K, Nakata M, Nishi H, Kosuga T, Komatsu S, Okamoto K, Otsuji E. Surgery for gastric cancer patients of age 85 and older: Multicenter survey. World J Gastroenterol. 2017;23:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1335] [Article Influence: 333.8] [Reference Citation Analysis (2)] |
| 15. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |
| 16. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 602] [Article Influence: 301.0] [Reference Citation Analysis (2)] |
| 17. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Takiuchi H, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 18. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 490] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 19. | Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 603] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 20. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1310] [Article Influence: 262.0] [Reference Citation Analysis (1)] |
| 21. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 22. | DeSantis CE, Miller KD, Dale W, Mohile SG, Cohen HJ, Leach CR, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69:452-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 23. | Okuyama A, Higashi T. Patterns of cancer treatment in different age groups in Japan: an analysis of hospital-based cancer registry data, 2012-2015. Jpn J Clin Oncol. 2018;48:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Guo J, Yu J, Xu Z, Sun X, Zheng J. The role of surgery in patients aged 85 years or older with resectable gastric cancer: a propensity score matching analysis of the SEER database. Scand J Gastroenterol. 2020;55:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Casella F, Sansonetti A, Zanoni A, Vincenza C, Capodacqua A, Verzaro R. Radical surgery for gastric cancer in octogenarian patients. Updates Surg. 2017;69:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |